Pituitary stalk interruption syndrome and liver changes: From clinical features to mechanisms
Ze-Yu Wu, Yi-Ling Li, Bing Chang
Abstract Pituitary stalk interruption syndrome (PSIS) is a rare congenital abnormality characterized by thinning or disappearance of the pituitary stalk, hypoplasia of the anterior pituitary and an ectopic posterior pituitary. Although the etiology of PSIS is still unclear, gene changes and perinatal adverse events such as breech delivery may play important roles in the pathogenesis of PSIS. PSIS can cause multiple hormone deficiencies, such as growth hormone, which then cause a series of changes in the human body. On the one hand, hormone changes affect growth and development, and on the other hand, they could affect human metabolism and subsequently the liver resulting in nonalcoholic fatty liver disease (NAFLD). Under the synergistic effect of multiple mechanisms, the progression of NAFLD caused by PSIS is faster than that due to other causes. Therefore, in addition to early identification of PSIS, timely hormone replacement therapy and monitoring of relevant hormone levels, clinicians should routinely assess the liver function while managing PSIS.
Key Words: Pituitary stalk interruption syndrome; Hormone deficiency; Etiology; Liver change; Clinical characteristics; Mechanisms
INTRODUCTION
Pituitary stalk interruption syndrome (PSIS) is a rare congenital abnormality characterized by thinning or disappearance of the pituitary stalk, anterior pituitary hypoplasia and an ectopic posterior pituitary[1]. Fujisawaet al[2]first reported PSIS in 1987. The incidence of PSIS is not very clear. However, it was found that 6.8% of nonacquired growth hormone (GH) deficiency cases were due to PSIS[3]. This disease is mainly sporadic, and only 5% of the cases are familial[4]. The ratio of males to females is 2.3:1[4]. The age at diagnosis of PSIS ranges from newborn to adult. The diagnosis mainly depends on the deficiency of hormones and a typical abnormality of the pituitary gland as revealed by magnetic resonance imaging (MRI).
PSIS mainly causes changes in pituitary hormones, which can be manifested as an isolated GH deficiency or multiple pituitary hormone deficiencies. However, patients with PSIS could also have hyperprolactinemia[4,5]. A study reported growth hormone deficiency (100%), gonadotropins deficiency (97.2%), corticotrophin deficiency (88.2%) and thyrotropin deficiency (70.3%) in patients with PSIS[6]. Many patients with PSIS have more than three kinds of pituitary hormone deficiencies[5].
Changes in pituitary hormones have an important impact on the human body. The prevalence of obesity and hyperlipidemia in patients with GH deficiency is high[7]. In addition, studies have found that the prevalence of nonalcoholic fatty liver disease (NAFLD) is high in patients with hypopituitarism, and the severity of GH deficiency is positively correlated with the severity of hepatic steatosis in NAFLD[8,9]. Interestingly, NAFLD develops quickly in patients with hypothalamic dysfunction or hypopituitarism[10]. It has been suggested that pituitary dysfunction is one of the causes of NAFLD. In this review, the research progress into PSIS and the changes in the liver caused by PSIS are analyzed.
ETIOLOGY
Perinatal adverse events
The etiology of PSIS is still unclear. Perinatal adverse events may play a role in the occurrence and development of PSIS. A large number of reports have shown that there are many perinatal adverse events in patients with PSIS, such as breech delivery, hypoxia, dystocia,etc.It has been reported that 26.9% of patients with pituitary stalk dysgenesis have a traumatic birth or perinatal complications[11]. Another study found that half of the patients had a cesarean section or breech delivery and/or neonatal hypoxemia[12]. Three cases of PSIS reported by Yoo[13]all had a history of breech delivery. Wanget al[14]studied 59 cases of children with PSIS, among which 54 cases had a breech delivery. A case reported in China involved a boy with a breech delivery who had PSIS, while his brother who had a normal delivery did not, although they had the same genotype[15]. Another case also reported that breech delivery is a risk factor for PSIS[16].
Genes
Some studies have pointed out that PSIS is more likely to be caused by gene mutations. One interesting hypothesis is that breech delivery and neonatal hypoxemia may be the results of a pituitary abnormality rather than the cause[17,18]. PSIS can occur in patients with Fanconi’s anemia, a rare autosomal recessive hematological disease, suggesting that gene mutations may play a role in the pathogenesis of PSIS[19]. In addition, 48% of patients with PSIS had extrapituitary malformations[20], which also suggests a role for genetic mutations in the pathogenesis of PSIS.
Multiple genes play an important role in the development of the pituitary such asGLI2,SOX2,SOX3,HESX1,LHX3andLHX4, which are expressed in the early stage of pituitary development andPROP1andPOU1F1, which are expressed in the late stage[21]. Among these genes, many PSIS related gene mutations have been reported, including inHESX1[4,22],OTX2[23],SOX3[24],LHX4[4,25,26],PROP1[11],PROKR2[27,28],CDON[29], holoprosencephaly related geneTGIFandSHH[30],GPR161[31]andROBO1[32]. However, a mutation of a single gene may not explain the genetic background of PSIS. A study found that PSIS may have a polygenic cause[33]. McCormacket al[34]also reported a case of PSIS with double genePROKR2andWDR11mutations, which were inherited from the mother and father, respectively, and both parents were normal. The mutated PSIS genes may be different in sporadic and familial cases. Heterozygous mutations were found in 92% of the sporadic PSIS cases in a Chinese Han population, which were related to the Notch, Shh and Wnt signaling pathways, and more than one mutation was found in 83% of individuals in this cohort. However, no previously reported mutated genes related to familial PSIS such asHESX1,LHX4,OTX2,SOX3orPROP1were found[35]. Chromosome abnormalities have also been reported in patients with PSIS, including de novo 18p deletion, 2p25 duplication, 2q37 deletion and 17q21.31 microdeletion[36-38]. We searched the PubMed database and listed related literature about the gene or chromosome mutations of PSIS, as shown in Table 1.
Generally, PSIS is a disease with a complex pathogenesis. Multiple genes may be related to it, and perinatal adverse events may also play a promoting role in its pathogenesis. Hypophyseal dysfunction will have serious consequences. Therefore, for newborns with perinatal adverse events such as an abnormal birth position, neonatal hypoxia and other high-risk factors, we should be alert to the possibility of PSIS.
CLINICAL CHARACTERISTICS
The initial manifestations of PSIS are diverse, and it can progress from isolated growth hormone deficiency to multiple hormone deficiencies[47], causing multiple system symptoms. The main complaint of patients with PSIS is growth delay[20]. Therefore, PSIS should be included in the differential diagnosis of retarded growth and delayed puberty in children[48]. However, Leeet al[49]reported a case of PSIS with multiple hormone deficiencies that did not affect growth, and the specific mechanism is still unclear. Some other atypical initial manifestations also need to be kept in mind. A patient with recurrent seizures due to hyponatremia was reported, and the final diagnosis was PSIS[50]. A case of PSIS with recurrent hyponatremia was also reported in Korea[51]. Therefore, children with severe hyponatremia need to be tested for multiple pituitary hormone deficiency[52], and PSIS should be considered. Neonatal jaundice, hypoglycemia and cryptorchidism or micropenis are also signs of possible hormone deficiency[20]. In addition, it was reported that the hormone deficiencies of PSIS patients with extrapituitary manifestations are more serious[4]. However, the authors of one study had a different opinion, namely, that there is no correlation between the degree of hormone deficiency and extrapituitary malformations[20]. This may be related to the size of the study sample.
PSIS patients may have extrapituitary malformations, such as septum pellucidum loss[53], central nervous system and/or craniofacial malformations[20]. Tatsiet al[30]also reported cases of PSIS with a single central incisor.
In addition, liver changes caused by pituitary hormone deficiency are of concern. As in our case, the liver lesions are very serious and progress rapidly, so a lack of pituitary hormones should also be considered in cases of unexplained liver diseases.
PSIS AND LIVER CHANGES
PSIS causes hormone deficiency, which not only causes growth and development problems but also causes liver lesions. We experienced a patient with PSIS complicated by cirrhosis. She was a 32-year-old woman with a height of 165 cm, weight of 73 kg, body mass index (BMI) of 26.81 kg/m2, dystocia with a foot presentation, delayed growth and development and no menstruation. Cirrhosis was found due to her abdominal distension. A liver biopsy revealed that the liver tissue was divided into nodules by fibrous septum with different widths. Most of the hepatocytes in the nodules showed bullous steatosis, as shown in Figure 1. Hepatocytes around the fibrous septum were edematous, a few of which showed balloon-like changes, and Mallory body could be seen (Figure 2). MRI showed that her pituitary stalk was truncated and the posterior pituitary was ectopic (Figure 3).
The relevant hormone assay results were: Adrenocorticotropic hormone (7.20-63.30) 8:00 1.00 pg/mL, 15:00 1.06 pg/mL, 24:00 1.00 pg/mL; cortisol (64.00-327.00 PM, 171.00–536.00 AM) 8:00 11.02 nmol/L, 15:00 9.66 nmol/L, 24:00 11.90 nmol/L; insulinlike growth factor-1 (IGF-1) < 25.00 ng/mL (115.00-358.00); free triiodothyronine 2.1500 pmol/L (2.6300-5.7000); free thyroxine 8.5700 pmol/L (9.0100-19.0500); thyroid stimulating hormone (TSH) 0.3372 mIU/L (0.3500–4.9400); follicle-stimulating hormone 0.56 mIU/mL; luteinizing hormone 0.14 mIU/mL; serum estradiol < 73.40 pmol/L; progesterone < 0.64 nmol/L; immunoglobulin A 1.25 g/L (0.70-3.80); immunoglobulin G 12.58 g/L (7.00-17.00); immunoglobulin M 2.73 g/L (0.60-2.50); γ globulin 26.1% (9.8-19.8); normal α and β globulin, C3 0.38 g/L (0.60-1.50); and C4 0.10 g/L (0.12-0.36). The related tests of hepatitis, autoimmune liver disease and rheumatic antibodies were negative. The serum ceruloplasmin was normal.

Table 1 Gene/chromosome mutations related to pituitary stalk interruption syndrome
On the basis of her history and the examination results, we considered that the liver cirrhosis was caused by a hormone deficiency. The patient was finally diagnosed with PSIS and received hormone replacement therapy.
NAFLD is a disease spectrum, including nonalcoholic fatty liver (NAFL), nonalcoholic steatohepatitis (NASH), cirrhosis and related complications[54]. The prevalence of global NAFLD is estimated to be 24%[55]. Many factors such as genetic background, insulin resistance, hormones secreted by adipose tissue and intestinal microbiota play certain roles in the pathogenesis of NAFLD[56,57]. Significantly, many reports have pointed out that hypopituitarism is related to liver changes[58,59]. A Japanese study reported that the prevalence of NAFLD in hypopituitary patients with GH deficiency was higher (77%) compared with controls[60].
The progression of NAFLD is generally slower than that of other liver diseases. The progression from nonalcoholic fatty liver or NASH to cirrhosis or liver cancer generally takes 57 years and 28 years, respectively, and only 2.5% of NASH patients progress from NASH to cirrhosis or liver cancer[61,62]. Gonzalez Rozaset al[63]reported a patient with liver cirrhosis due to hypopituitarism. They believed that NAFLD in patients with hypopituitarism may develop rapidly into cirrhosis. Yanget al[64]reported that hypopituitarism could cause NAFLD and decompensated cirrhosis, and the average time from liver dysfunction to decompensated cirrhosis was only 6.9 yrs. Therefore, liver changes in patients with hypopituitarism are of concern.
The characteristics of cholestasis caused by hypopituitarism are giant cell formation of hepatocytes with dysplasia of the bile duct and no or only a small amount of inflammatory cell infiltration. However, the giant cell formation of hepatocytes could be reversed after hormone therapy[65]. This suggests that early recognition of liver changes in patients with hypopituitarism could be reversed. In the current literature reports, growth hormone, thyroid hormone, gonadal hormones and prolactin are related to NAFLD. The mechanisms of hormone deficiency causing NAFLD are shown in Figure 4.
GH
There have been many reports on the relationship between GH and the liver. Xuet al[66]reported that the prevalence of NAFLD increased when GH decreased. A decrease of GH level could independently predict the occurrence of NAFLD in male patients[67]. Moreover, a study reported that GH resistance can promote the development of liver cirrhosis[68], and GH replacement therapy can significantly improve the NASH in an adult patient with GH deficiency[69]. Therefore, GH deficiency in an adult may be a risk factor for hepatic steatosis and NASH[70].
It has been found that GH deficiency prevents the activation of signal transducer and activator of transcription-5 (STAT5), which leads to an increase of liver lipid uptake and an increase of phosphorylation of STAT1 and STAT3[71]. The activation of STAT1 and STAT3 can promote the development of NAFLD[72], and an increase of STAT1 would cause a decrease of the liver’s regeneration ability[73].
Oxidative stress plays an important role in the pathogenesis of NAFLD[74], and GH replacement therapy could reduce oxidative stress in the liver and serum in the GH deficiency patient with NASH[69]. In addition, Sesmiloet al[75]carried out a study on men with growth hormone deficiency and found that supplementing them with GH could reduce the levels of serum interleukin-6 and C-reactive protein, which may suggest that growth hormone can regulate the inflammatory response. The proinflammatory factor interleukin-6 can promote hepatic insulin resistance[74].
IGF-1 deficiency caused by GH deficiency may also play an important role in the development of NAFLD. The effects of IGF-1 and GH supplementation on hepatic steatosis and fibrosis in GH deficient rats were similar[76]. GH regulated the synthesis and secretion of IGF-1 through the GHR-STAT-5B signaling pathway[77,78]. The main site of IGF-1 synthesis and secretion was the liver[79]. IGF-1 was greatly reduced after knockout of the GH receptor in the mouse liver[80]. An imbalance of the GH/IGF-1 axis will cause NAFLD[81]. In addition, IGF-1 can improve mitochondrial function and reduce oxidative stress[82,83]. As a result, a decrease of IGF-1 may increase oxidative stress. The levels of GH and IGF-1 in NAFLD patients decreased[84], and IGF-1 levels further decreased with the development of NAFLD[85-87]. This may be one of the reasons for the rapid progression of liver lesions in patients with hypopituitarism.
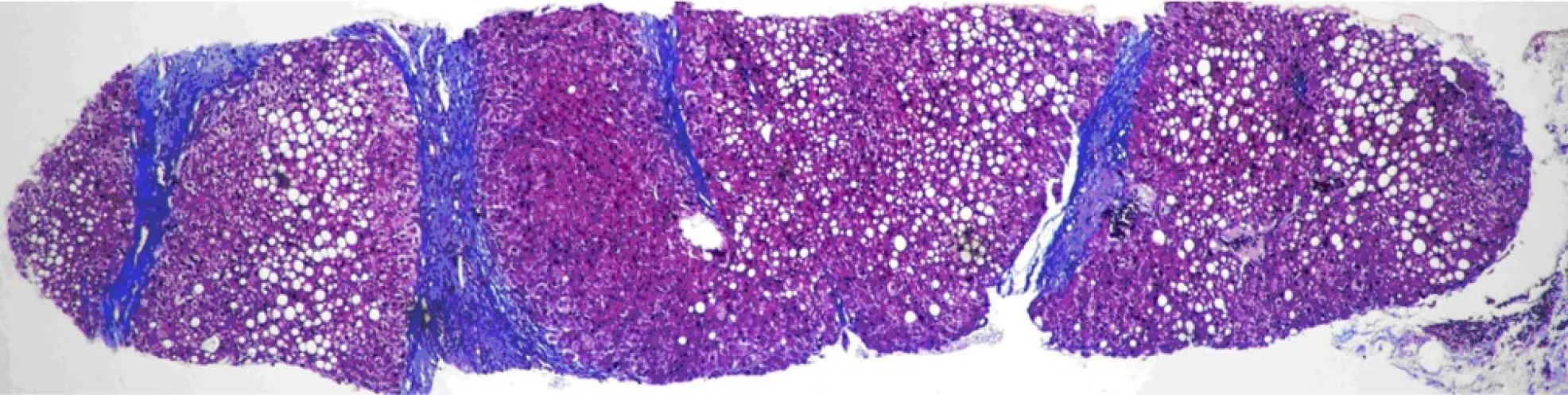
Figure 1 The liver tissue is divided into nodules by fibrous septa of different widths. Most of the hepatocytes in the nodules appear as bullous steatosis.
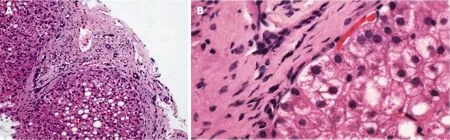
Figure 2 Edema of hepatocytes around the fibrous septum, a few of which showed balloon-like changes, and Mallory body can be seen. A: Hematoxylin-eosin staining (HE), 200 ×; B: HE, 400 ×.
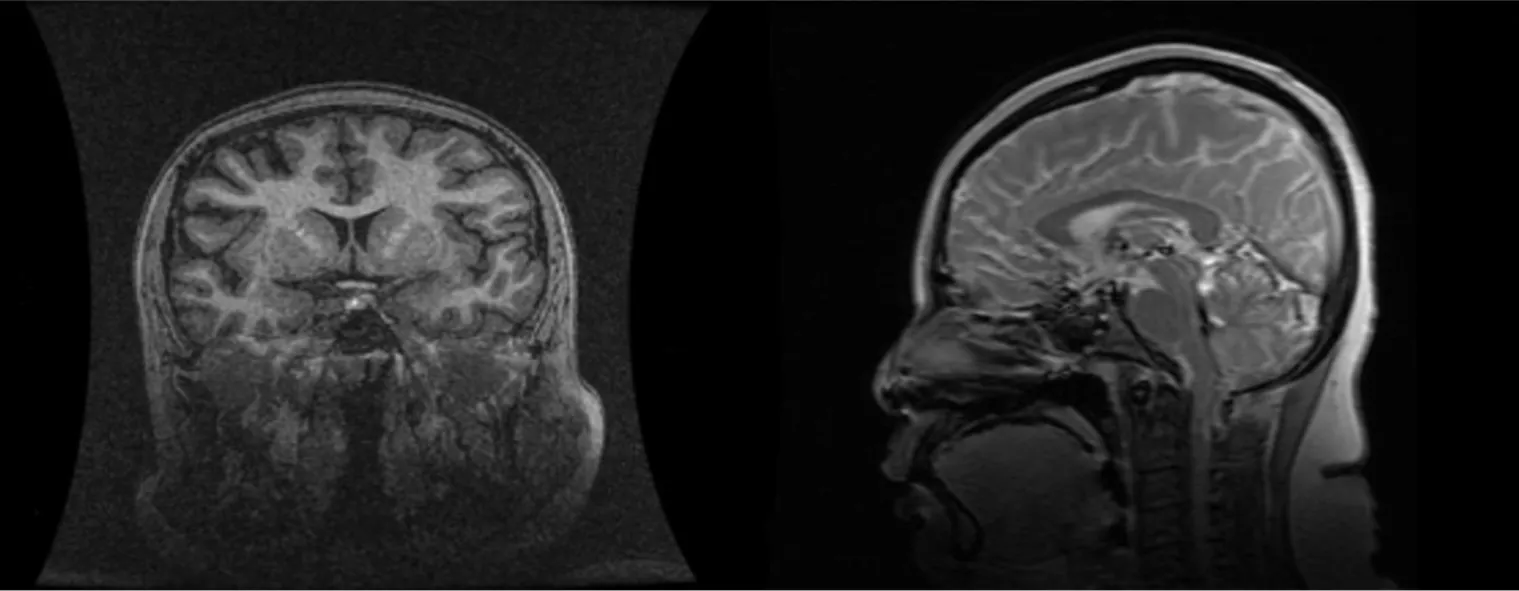
Figure 3 Cranial magnetic resonance: abnormal pituitary.
However, another study found that GH deficiency had no significant effect on the liver. Meienberget al[88]did not find any difference in liver fat content or the prevalence of liver steatosis between patients with GH deficiency and healthy controls after matching for age, sex, BMI and ethnicity. Moreover, GH replacement therapy had no effect on liver fat in patients with GH deficiency. We speculate that the possible reasons are as follows: First, the sample size of the study was relatively small, and thus the conclusion was biased; second, the treatment time of the study was 6 mo, which may not be long enough; and third, the dosage of growth hormone was not high enough.
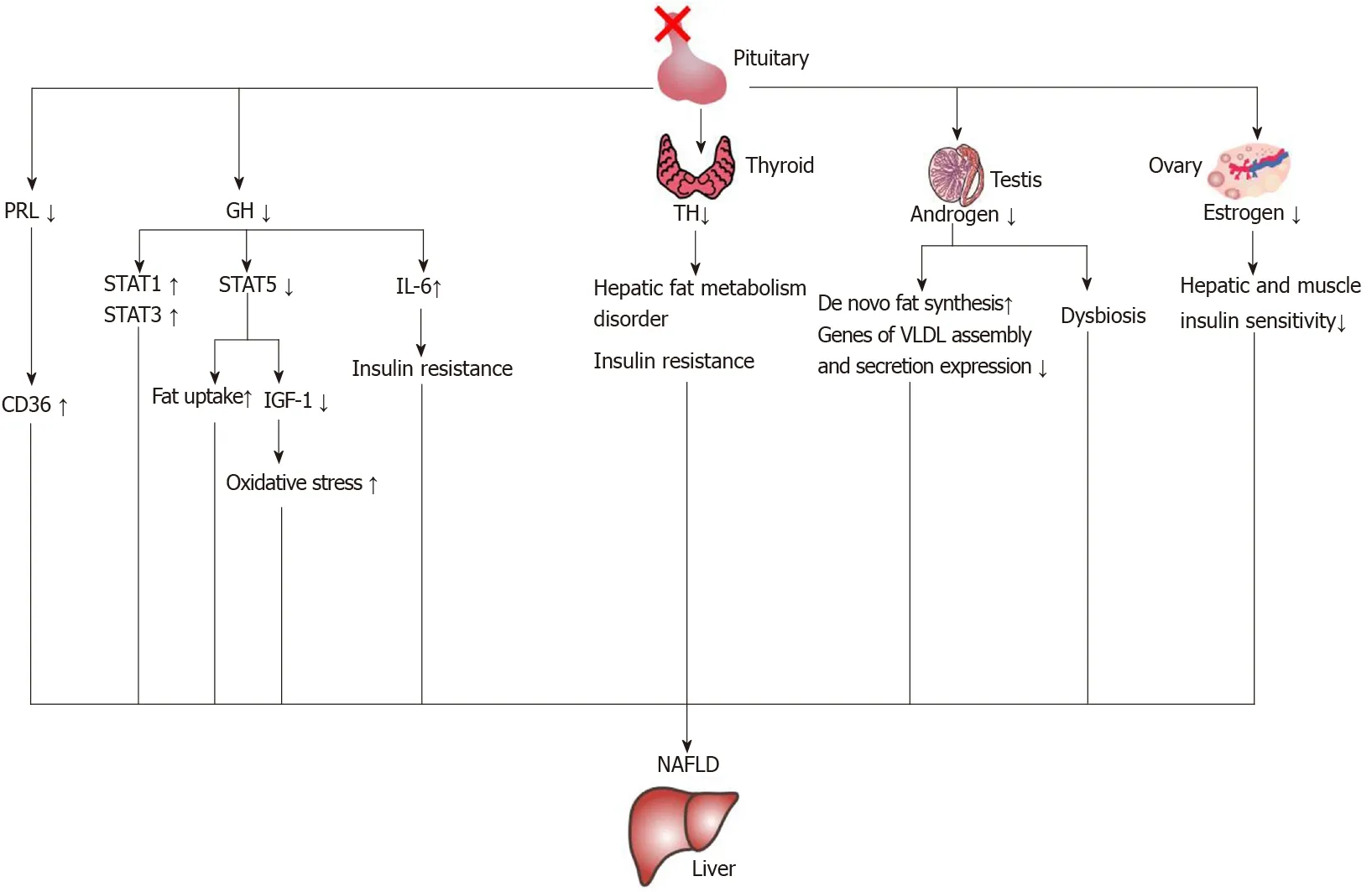
Figure 4 The mechanisms of nonalcoholic fatty liver disease induced by hormone deficiency. IGF-1: Insulin-like growth factor-1; IL-6: Interleukin-6; NAFLD: Nonalcoholic fatty liver disease; PRL: Prolactin; GH: Growth hormone; STAT: Signal transducer and activator of transcription; TH: Thyroid hormone.
Thyroid hormone
It has been reported that the prevalence of central hypothyroidism in patients with PSIS is 79.8%, but one study found that only 5.6% of PSIS patients with hypothyroidism have low TSH levels. Therefore, the biological activity of TSH in patients with PSIS may be decreased[89]. Thyroid dysfunction might play an important role in the development of NAFLD[90]. Demiret al[91]carried out a histological study and found that rats with hypothyroidism had mild liver steatosis suggesting that hypothyroidism could cause NAFLD. Two systematic reviews found that patients with hypothyroidism had a higher risk of NAFLD[92,93].
Bothin vitroandin vivoexperiments confirmed that thyroid hormone can promote liver fat transformation and prevent hepatic steatosis through degradation of lipid droplets induced by hepatic autophagy, also known as lipophagy[94-96]In addition, in patients with hypothyroidism, low-density lipoprotein increased and liver triglyceride deposition increased[97]. Therefore, hypothyroidism can cause a liver fat metabolism disorder, which can lead to NAFLD. Hypothyroidism may also promote insulin resistance[98], which also plays a role in promoting the occurrence and development of NAFLD.
However, some studies have come to the opposite conclusions. One study reported that hypothyroidism was not associated with the occurrence of NAFLD[99]. However, there are some deficiencies in that study. The NAFLD was not confirmed by histology, only by ultrasound diagnosis, and the sample group was relatively young people[99]. Jaruvongvanichet al[100]carried out a meta-analysis and found no correlation between hypothyroidism and NAFLD, which may be related to multiple factors. First, the sample size of some included studies were small. Second, some studies did not use liver biopsies to diagnose NAFLD, just ultrasound for diagnosis. Therefore, early mild NAFLD may not have been correctly diagnosed. Third, the severity of hypothyroidism in the included population may not have been evenly distributed. In addition, an experiment conducted in mice fed a high-fat diet found that after thyroidectomyinduced hypothyroidism, the level of glucagon like peptide-1 (GLP-1) increased in mice, relieving hepatic steatosis[101]. A relationship between GLP-1 concentration and subclinical hypothyroidism was also found in humans. The serum GLP-1 concentration in patients with subclinical hypothyroidism increased[102]. The relationship between hypothyroidism and NAFLD needs to be further studied on a larger scale.
Gonadal hormones
Estrogen plays an important role in liver lipid metabolism[103]. Studies have found that the prevalence of metabolic syndrome and NAFLD in postmenopausal women increased[104-106]. Animal experiments also found that ovariectomized mice could develop liver steatosis[107]. Estrogen can improve the liver and muscle insulin sensitivity of ovariectomized mice[108]and reduce liver fat deposition[109]. Another gonadal hormone, testosterone, is also associated with NAFLD, and a decrease of testosterone level indicates an increased risk of NAFLD[110,111]. Animal experiments showed that androgen receptor knockout mice were prone to develop insulin resistance and hepatic steatosis[112]. Testosterone could influence de novo fat synthesis through regulating expression of enzymes related to fatty acid synthesis[113]. It has also been found that in a high-fat fed mouse model of orchiectomy, a decrease in testosterone would cause changes in gene expression related to liver synthesis and secretion of very low-density lipoprotein, such as decreases in mRNA expression of microsomal triglyceride transporter, lipin-1, PGC-1α,etc., resulting in hepatic steatosis[114].
Androgen deficiency has an impact on the intestinal microbiota, such as an increase in the ratio ofFirmicutestoBacteroidesand an increase ofLactobacillusspecies in the cecum[115]. A high fat diet could induce NAFLD through increasing the ratio ofFirmicutes/Bacteroidetes, while reducing this ratio could relieve the inflammation of NAFLD[116]. The intestinal microbiota ofFirmicutesandBacteroidescan regulate insulin resistance by regulating the secretion of GLP-1. A decrease ofFirmicutesandBacteroidescould cause an increase of taurocholic acid, and an increase of taurocholic acid could promote the secretion of GLP-1 and improve insulin resistance[117]. Hypogonadism might influence the liver through the intestinal microbiota. The mechanisms require further study.
Prolactin
Zhanget al[118]found that a low level of prolactin (PRL) is a risk factor for the occurrence and development of NAFLD, and PRL can improve liver steatosis through PRL receptor mediated inhibition of fatty acid translocase/CD36. An increase of CD36 is associated with insulin resistance and hepatic steatosis[119]. It has been found that knockout of the PRL receptor in the mouse liver can increase the accumulation of triglycerides in the liver[120]. Therefore, a change of PRL may play a role in the development of NAFLD in patients with PSIS.
In conclusion, each hormone deficiency alone can cause liver metabolism changes and NAFLD, and NAFLD caused by multiple hormone deficiencies may be more serious and develop more rapidly.
TREATMENT
The main treatment of PSIS is hormone replacement therapy. For PSIS, early diagnosis and monitoring are very important. It has been found that the shorter the baseline height, the better the response to GH treatment[20]. A retrospective analysis of 75 patients with PSIS of Han ethnicity in China found that GH supplement therapy was beneficial for adults[121]. Therefore, for PSIS hormone replacement therapy should be started as soon as possible, whether it is diagnosed in infants or adults. In view of the hypogonadism of PSIS patients, a study in China reported that after micropump pulse infusion of gonadorelin treatment for 12 wk, symptoms caused by androgen deficiency improved, and gonadal hormone levels increased[122].
CONCLUSION
PSIS can cause changes in pituitary hormones, and the changes in pituitary hormones can not only cause abnormal growth and development but also cause metabolic changes in the human body and lead to NAFLD. Moreover, NAFLD caused by pituitary hormone deficiency develops rapidly. For patients with a late onset, liver lesions may already exist at the time of onset. Therefore, in the clinical management of PSIS, besides the need for early identification of PSIS and timely hormone replacement therapy and monitoring of relevant hormone levels, routine assessments of the liver condition are necessary. At present, the etiology of PSIS is still unclear. Genes and adverse events during pregnancy and the perinatal period may be involved in its pathogenesis. The related genes of PSIS should be screened in the neonatal stage, and the possibility of PSIS should be kept in mind. Abnormalities of the pituitary gland should be excluded if any of these conditions, such as an abnormal birth position especially breech delivery, hypoxia, dystocia, recurrent hypoglycemia and/or prolonged jaundice, occur in the neonate. It should be noted that regardless of the age of the patient, it is necessary for patients with PSIS to actively take hormone replacement therapy.
 World Journal of Gastroenterology2020年44期
World Journal of Gastroenterology2020年44期
- World Journal of Gastroenterology的其它文章
- Prognostic value of changes in serum carcinoembryonic antigen levels for preoperative chemoradiotherapy response in locally advanced rectal cancer
- Hepatocellular carcinoma with tumor thrombus in bile duct: A proposal of new classification according to resectability of primary lesion
- Fatty liver is an independent risk factor for gallbladder polyps
- Artificial intelligence based real-time microcirculation analysis system for laparoscopic colorectal surgery
- Emerging use of artificial intelligence in inflammatory bowel disease
- Endoscopic pancreaticobiliary drainage with overlength stents to prevent delayed perforation after endoscopic papillectomy: A pilot study
