Low-dose lipopolysaccharide as an immune regulator for homeostasis maintenance in the central nervous system through transformation to neuroprotective microglia
Haruka Mizobuchi , Gen-Ichiro Soma
Abstract Microglia, which are tissue-resident macrophages in the brain, play a central role in the brain innate immunity and contribute to the maintenance of brain homeostasis.Lipopolysaccharide is a component of the outer membrane of gram-negative bacteria,and activates immune cells including microglia via Toll-like receptor 4 signaling.Lipopolysaccharide is generally known as an endotoxin, as administration of highdose lipopolysaccharide induces potent systemic inflammation. Also, it has long been recognized that lipopolysaccharide exacerbates neuroinflammation. In contrast, our study revealed that oral administration of lipopolysaccharide ameliorates Alzheimer’s disease pathology and suggested that neuroprotective microglia are involved in this phenomenon.Additionally, other recent studies have accumulated evidence demonstrating that controlled immune training with low-dose lipopolysaccharide prevents neuronal damage by transforming the microglia into a neuroprotective phenotype. Therefore,lipopolysaccharide may not a mere inflammatory inducer, but an immunomodulator that can lead to neuroprotective effects in the brain. In this review, we summarized current studies regarding neuroprotective microglia transformed by immune training with lipopolysaccharide. We state that microglia transformed by lipopolysaccharide preconditioning cannot simply be characterized by their general suppression of proinflammatory mediators and general promotion of anti-inflammatory mediators, but instead must be described by their complex profile comprising various molecules related to inflammatory regulation, phagocytosis, neuroprotection, anti-apoptosis, and antioxidation.In addition, microglial transformation seems to depend on the dose of lipopolysaccharide used during immune training. Immune training of neuroprotective microglia using lowdose lipopolysaccharide, especially through oral lipopolysaccharide administration, may represent an innovative prevention or treatment for neurological diseases; however more vigorous studies are still required to properly modulate these treatments.
Key Words: cognitive impairment; endotoxin; lipopolysaccharide; low-dose; microglia;neuroprotection; oral administration; preconditioning; tolerance; trained innate immunity
Introduction
Lipopolysaccharide (LPS) is a glycolipid present in the outer membrane of gram-negative bacteria, and induces innate inflammatory responses by binding to Toll-like receptor 4(TLR4). LPS has long been recognized as an endotoxin, as the first administration of a high dose of LPS induces potent systemic inflammation. Moreover, administration of high-dose LPS is generally used to generate animal models of Parkinson’s disease and encephalitis (Deng et al., 2020).
The first administration of high-dose LPS to Alzheimer’s disease model mice has been shown to exacerbate the accumulation of amyloid β (Aβ) and cognitive decline (Zhan et al., 2018). Among the most important immune cells involved in the background mechanism of LPS-induced neuropathology are microglia, which are brain tissue-resident macrophages that play a central role in brain innate immunity. Microglia are transformed by the first stimulation of high-dose LPS, changing into an inflammatory phenotype that promotes expression of proinflammatory mediators; these inflammatory microglia are thought to be involved in LPS-induced neuropathology (Ye et al., 2020). Thus, only pathological inflammation has been intensively studied in response to LPS.
However, it is important to also ask whether LPS can induce other responses than only pathological inflammation. Should LPS be completely excluded from our lives to promote health? Braun-Fahrl?nder et al. (2002) showed interesting epidemiological data stating that environmental LPS exposure was inversely associated with the incidence of childhood asthma incidence. These results suggested that continuous mild LPS exposure is required to control the immune response.Although few studies have been conducted in the brain, it has been reported that preconditioning with low-dose LPS leads to a neuroprotective effect. As such, we focused on oral administration of LPS, as continuous oral LPS administration has been shown to suppress cognitive decline and Aβ accumulation in an Alzheimer’s disease model (Kobayashi et al., 2018). In fact, in 1883, Mechnikov had already proposed the theory of “physiological inflammation,” which is a series of physiological immune reactions including phagocytosis andtissue repair leading to the maintenance of homeostasis and recovery from “pathological inflammation” (Tauber, 2003).Based on this concept, it is thought that preconditioning with low-dose LPS induces physiological immune responses rather than pathological inflammation and thus contributes to neuroprotection.
Several recent studies have begun to unveil the neuroprotective mechanism of LPS, in which preconditioning with low-dose LPS induces transformation of anti-inflammatory microglia instead of the inflammatory microglia induced by the first administration of high-dose LPS (Wendeln et al., 2018;Neher and Cunningham, 2019). We have also reported that repetitive treatment with low-dose LPSin vitro, which imitates oral administration of LPSin vivo, transforms microglia into a neuroprotective phenotype (Mizobuchi et al., 2020a). This transformation of neuroprotective microglia via preconditioning with low-dose LPS is an example of the concept of “trained innate immunity,” or “innate immune memory”. Trained innate immunity is the process by which changes in the subsequent reactivity of innate immune cells occur following repeated exposure to stimuli, either be an enhancement or a decrease (Netea et al., 2011; Boraschi and Italiani, 2018). In the past, an enhanced subsequent response was attributed to immune training, whereas a decreased subsequent response was attributed to immune tolerance after priming with the first exposure. However, it has been revealed that changes in immune response upon subsequent stimuli cannot be simply characterized by either training or tolerance, but must be instead characterized by complex reprogramming after which expression of some molecules is promoted while others are suppressed or unchanged.Therefore, we refer to immune training as changes in the immune response to subsequent stimuli, which can either be an enhancement or a decrease.
Immune training by preconditioning with low-dose LPS (LPS training) likely contributes to maintenance of brain homeostasis by transforming microglia into the neuroprotective phenotype; however, there are still few studies on microglial transformation by LPS preconditioning.In this review, we summarize previous studies on microglial transformation by LPS preconditioning to identify the gap between the current understanding and the as-yet unsolved problems. The data sources for this review were searched using the PubMed (last searched October 27, 2020). Data published within the last 5 years were prioritized, focusing on the novel and comprehensive reports. Consequently,this review takes the view that LPS training through oral administration may eventually be used as a prevention or an innovative therapy for neurological disorders.
Neuroprotective Effects of Immune Training with Lipopolysaccharide in vivo
Studies on neuroprotective effects induced byin vivoLPS training are summarized inAdditional Table 1. For example,LPS preconditioning prevented nerve damage after cerebral ischemia by modulating the inflammatory response (Tasaki et al., 1997; Rosenzweig et al., 2004, 2007; Furuya et al., 2005;Hickey et al., 2007, 2011; Lin et al., 2009; Marsh et al., 2009;Liang et al., 2011; Stevens et al., 2011; Vartanian et al., 2011;Halder et al., 2013; Wendeln et al., 2018; He et al., 2019;Hosseini et al., 2019). Similarly, LPS preconditioning provided neuroprotection against cerebral spine injury (Longhi et al.,2011; Chen et al., 2012, 2014; Li et al., 2013, 2014, 2016;Hayakawa et al., 2014; Turner et al., 2017). In an Alzheimer’s disease model, LPS preconditioning also protected or improves cognitive decline by suppressing Aβ deposition and tau phosphorylation (DiCarlo et al., 2001; Quinn et al., 2003; Herber et al., 2004, 2007; Go et al., 2016; Qin et al., 2016a; Kobayashi et al., 2018; Pourbadie et al., 2018;Thygesen et al., 2018; Wendeln et al., 2018; Jendresen et al.,2019). Neuroprotective effects by LPS preconditioning have additionally been reported in models of neuroinflammation(Ding and Li, 2008), epilepsy (Eslami et al., 2015; Amini et al., 2018; Ohgomori and Jinno, 2020), and surgery-induced cognitive impairment (Zhang et al., 2018).
Compared to the LD50 of LPS, which is 27 mg/kg (Li et al., 2018), or the LPS dose used in thein vivoto induce neuropathology, which is 5 mg/kg (Deng et al., 2020), the LPS dose used in immune training to successfully induce neuroprotective effects is very low, ranging from 0.2-0.9 mg/kg (Additional Table 1). Banks et al. (2015) showed that the degree of LPS-induced blood-brain barrier destruction depends on the dose of LPS. Erickson et al. (2018) also showed that different doses of LPS have different effects on the cytokine profile in both blood and brain. Therefore, it can be said that a low-dose is one of the requirements for inducing neuroprotection using LPS.
It has been suggested that LPS training modulates inflammatory responses by inducing suppression of proinflammatory mediators and by promoting antiinflammatory mediators in the brain. Although these mediators, such as cytokines, are not always classified as either pro- and anti-inflammatory, as their functions can change dynamically based on context, duration,and abundance, in this review, we applied the general classifications of proinflammatory and anti-inflammatory mediators to simplify comparison. In encephalitis, cerebral ischemia, cerebral spine injury, Alzheimer’s disease,epilepsy, and surgery-induced cognitive impairment models,low-dose LPS preconditioning suppressed expression of proinflammatory mediators induced by neuropathology, such as tumor necrosis factor alpha (TNF-α), interleukin 1 beta (IL-1β), IL-6, nitric oxide synthase 2 (NOS2), and nuclear factorkappa B in the brain (Ding and Li, 2008; Lin et al., 2009; Longhi et al., 2011; Vartanian et al., 2011; Eslami et al., 2015; Amini et al., 2018; Pourbadie et al., 2018; Zhang et al., 2018; Jendresen et al., 2019). In addition, LPS preconditioning prevented an excessive inflammatory response by suppressing microglial proliferation in response to neuronal damage in encephalitis,cerebral ischemia, cerebral spine injury, Alzheimer’s disease,and surgery-induced cognitive impairment models (Ding and Li, 2008; Lin et al., 2009; Halder et al., 2013; Hayakawa et al.,2014; Go et al., 2016; Zhang et al., 2018). On the other hand,low-dose LPS preconditioning promoted the expression of anti-inflammatory mediators such as arginase 1 (Arg1), IL-10,transforming growth factor beta 1, formyl peptide receptor 2, interferon beta, and nuclear factor erythroid 2-related factor 2 in the brain in cerebral ischemia, cerebral spine injury,Alzheimer’s disease, and epilepsy models (Longhi et al., 2011;Stevens et al., 2011; Vartanian et al., 2011; Li et al., 2013;Amini et al., 2018; Pourbadie et al., 2018).
In addition to promoting the expression of anti-inflammatory mediators, it was also reported that low-dose LPS preconditioning promoted the expression of B-cell lymphoma 2 via the the transcription factor forkhead box protein O1 in the brain, thus suppressing neuronal apoptosis in cerebral ischemia and cerebral spine injury models (Li et al., 2014; He et al., 2019). Furthermore, in cerebral spine injury model, LPS preconditioning promotes expression of antioxidant proteins such as heme oxygenase 1, NAD(P)H quinone oxidoreductase 1, and glutamate-cysteine ligase catalytic subunit in the brain (Li et al., 2016). Additionally, suppression of nuclear factor-kappa B and activation of nuclear factor erythroid 2-related factor 2, interferon regulatory factor 3 (IRF3), and IRF7 signaling pathways in the brain have been reported to induce expression of these mediators following by LPS preconditioning in cerebral ischemia and cerebral spine injury models (Marsh et al., 2009; Stevens et al., 2011; Vartanian et al., 2011; Li et al., 2013, 2014, 2016; Hayakawa et al., 2014).Interestingly, in a cerebral ischemia model, low-dose LPS preconditioning suppressed expression of the proinflammatory mediator TNF-α, but complete deletion of TNF-α prevented the neuroprotection induced by low-dose LPS preconditioning (Tasaki et al., 1997; Rosenzweig et al.,2007). This indicates that low concentrations of TNF-α can be neuroprotective and important for maintaining homeostasis,whereas high concentrations of TNF-α can be harmful, as they induce potent inflammatory signaling cascade. In other words,the correlation between TNF-α dose and neuroprotective effect is considered to shows a bell-shaped curve. Therefore, it is suggested that low concentrations of TNF-α induced by lowdose LPS preconditioning are required for neuroprotection.The neuroprotective mechanism behind LPS training is therefore more complex than just simply via suppressing proinflammatory mediators.
In summary, LPS training contributes to neuroprotection by inducing anti-inflammatory, anti-apoptotic, and antioxidant effects. Low-dose and pre-stimulation (immune training) are important conditions to induce LPS-mediated neuroprotective effects by LPS. LPS training also has the advantage of being more sensitive than other TLR agonists, and may be expected to have a more comprehensive effect than treatment with a single cytokine. The expression of some typical cytokines induced by low-dose LPS preconditioning show similar tendencies, even if the administration routes are different,such as oral administration, peritoneal administration,and intravenous administration. However, the detailed cytokine profile is likely to vary depending on the route of administration, and further characterization and comparative studies will be needed in the future.
Oral administration of LPS can be advantageous over other routes of administration, as it can have fewer side effects than intraperitoneal administration or intravenous administration.However, most studies that have reported LPS preconditioning used intraperitoneal injection of LPS. Even at a low-dose,LPS preconditioning by intraperitoneal injection induces side effects such as transient systemic inflammation with mild symptoms of illness and temporary weight loss (Wendeln et al., 2018). As humans are generally more sensitive to LPS than mice (Fink, 2014; Sandiego et al., 2015), LPS injection that induces serious side effects is not practical for therapeutic application.
We therefore proposed a method of immune training by oral administration of LPS, and demonstrated that continuous oral administration of LPS reduced Aβ accumulation and improved cognitive decline without causing side effects in an Alzheimer’s disease mouse model (Kobayashi et al.,2018). Importantly, the safety of oral LPS administration was ensured by previous studies, in which animals given LPS orally did not exhibit systemic inflammation, mild illness,or temporary weight loss (Taniguchi et al., 2009; Inagawa et al., 2011; Phipps et al., 2020). Since LPS is abundantly present in foods and herbal medicines commonly consumed by humans (Taniguchi et al., 2009; Tamura et al., 2015;Inagawa et al., 2016), it is certain that humans commonly ingest LPS in their daily lives. Additionally, we have also demonstrated in humans studies that oral administration of LPS improves hyperglycemia, hyperlipidemia (Nakata et al., 2011), reduced bone density (Nakata et al., 2014), and blood flow (Nakata et al., 2017). We also confirmed that oral administration of LPS does not have side effects of systemic inflammation by measuring biomarkers such as white blood cell count, red blood cell count, aspartate aminotransferase,alanine aminotransferase, creatinine, C-reactive protein, and immunoglobulin A in human peripheral blood samples (Nakata et al., 2017). Therefore, simple immune training through LPS oral administration may be expected as a revolutionary solution to neurological disorders.
Phenotype of Neuroprotective Microglia Transformed by Lipopolysaccharide Training
Microglia have drawn attention as key players in the neuroprotective mechanism of LPS training (Neher and Cunningham, 2019). Studies on microglial transformation by LPS training are summarized inAdditional Table 2. It has been reported that microglia transformed by LPS training (LPStrained microglia) exhibit an anti-inflammatory phenotypein vivo. For example, Chen et al. (2012) reported that in a cerebral ischemia model, low-dose LPS preconditioning promoted the expression of anti-inflammatory mediators such as chitinase-like 3, suppressor of cytokine signaling 3,IL-4 receptor, alpha (IL-4RA), CD163, IL-1 receptor antagonist(IL-1RA), and mannose receptor c-type 1 (Mrc1, CD206) in the microglia. Similarly, Hayakawa et al. (2014) reported in a cerebral ischemia model that low-dose LPS preconditioning promoted the expression of anti-inflammatory mediators such as Arg1, Mrc1, and IRF3 in the microglia.
Wendeln et al. (2018) showed that microglial transformation induced by LPS training is important in improving neuronal dysfunction in Alzheimer’s disease and cerebral ischemia.They revealed that low-dose LPS preconditioning induced epigenetic reprogramming in microglia, and activated the Rap1 signal and phagocytosis-related signals while suppressing hypoxia inducible factor 1 signal and glycolysis(Wendeln et al., 2018). Importantly, they also verified that microglial immune memory persists for at least 6 months.A recent study by Ohgomori and Jinno (2020) reported that phagocytosis-related gene expression and IL-1β suppression in the microglia are involved in improvement of epilepsy symptoms through LPS training. Consistent with their reports,our previous study also suggested that oral administration of LPS enhances Aβ phagocytosis mediated via microglia(Kobayashi et al., 2018). These studies indicate that microglia transformed by LPS training contribute to neuroprotection by promoting phagocytosis of Aβ and other undesirable brain aggregates. Although it has been reported that excessive activation of inflammatory microglia led to phagocytosis of normal nerve cells (Yanuck, 2019), quality and/or quantity of microglia phagocytosis may depending on LPS dose and route of administration. These results indicate that LPS trainingin vivoprevents neuronal dysfunction by inducing microglial transformation to a neuroprotective phenotype with both anti-inflammatory and phagocytic activity.
Consistent with thein vivostudies, low-dose LPS preconditioningin vitrosuppresses proinflammatory mediators and promotes anti-inflammatory mediators in the microglia. Commonly, it has been observed that low-dose LPS preconditioning suppressed the expression of TNF-α, IL-1β,and IL-6 while promoting the expression of Arg1 and IL-10 in the microglia (Ajmone-Cat et al., 2003, 2013, 2016; Cacci et al., 2008; Schaafsma et al., 2015; Chu et al., 2016; Liu et al.,2016; Qin et al., 2016b; Sun et al., 2018; Lajqi et al., 2019;Twayana et al., 2019; Mizobuchi et al., 2020a). Furthermore,Qin et al. (2016b) analyzed additional molecules afterin vitroLPS preconditioning (10 ng/mL), and characterized LPS-trained microglia in terms of their suppressed proinflammatory mediators TNF-α, IL-β, IL-6, prostaglandin-endoperoxide synthase, C-X-C Motif Chemokine Ligand (CXCL) 9, CXCL10,CXCL11, and CD54, as well as enhanced anti-inflammatory mediators Arg1, Fizz 1, IL-4, IL-10, C-C motif chemokine (CCL)2, CCL17, CCL22, CD206, and heme oxygenase 1. Yousefi et al. (2019) also reported that LPS preconditioning (1 μg/mL)prior to Aβ stimulationin vitropromoted the expression of anti-inflammatory mediators such as TIR-domain-containing adapter-inducing interferon-β, IRF3, and interferon beta.
On the other hand, LPS preconditioning also promoted the expression of prostaglandin E2, despite its classical proinflammatory roles (Ajmone-Cat et al., 2003; Cacci et al., 2008; Antonietta Ajmone-Cat et al., 2013). The regulation of NOS2, another proinflammatory mediator, by LPS preconditioning is controversial, as there are reports of both promotion and suppression (Ajmone-Cat et al., 2003,2016; Cacci et al., 2008; Antonietta Ajmone-Cat et al., 2013;Schaafsma et al., 2015; Mizobuchi et al., 2020a). These reports suggest that not all proinflammatory mediators are suppressed in LPS-trained microglia.
In addition to inflammatory modulation, it was reported that LPS preconditioning promoted expression of cellular inhibitor of apoptosis protein in microglia (Twayana et al., 2019).Microglia phagocytic ability seems to be generally promoted by LPS preconditioning, although there is one report suggests suppressed phagocytosis as a result of LPS preconditioning(Antonietta Ajmone-Cat et al., 2013; Schaafsma et al., 2015;Twayana et al., 2019; Mizobuchi et al., 2020a).
Importantly, it has been demonstrated using anin vitroneuron-microglia co-culture system that LPS-trained microglia restore neuronal function that is disrupted by the primary stimulation with LPS (Cacci et al., 2008; Chu et al.,2016). Therefore, the above-mentioned characteristics of LPS-trained microglia are thought to be deeply involved in neuroprotective effects. Since low-dose LPS does not readily cross the blood brain barrier (Banks and Robinson, 2010),it is suggested that the microglial transformation is induced through activation of cerebrovascular endothelial cells,ependymal and choroid plexus cells at the brain ventricles,and circulating monocytes/macrophages (Tang et al., 2017;Haruwaka et al., 2019; Liu et al., 2019). For example, IL-1 is a typical cytokine induced by LPS, and Liu et al. (2019) reported that LPS-induced IL-1 stimulated IL-1 receptors of endothelial cells at cerebral ventricles to induce microglial activation.In addition, Tarr et al. (2012) reported that c-fos, which is induced downstream of IL-1 (Schiller et al., 2006), is activated in the brain in a LPS dose-dependent manner. As it has been suggested that physiological IL-1 stimulation is necessary for the physiological regulation of memory processing (Labrousse et al., 2009), LPS-induced IL-1 in endothelial cells at cerebral ventricle may play an important role in microglia-mediated neuroprotection.
However, the characterization of LPS-trained neuroprotective microglia has been limited to analysis of several representative molecules, and their true characteristics may not yet have been fully explored. Moreover, although LPS doses used for immune trainingin vitrovary considerably, it has recently been shown that different LPS doses induce distinct transformation of microglia (Lajqi et al., 2019; Mizobuchi et al., 2020a). Hence, further characterization of LPS-trained neuroprotective microglia is needed in the future.
Dynamic Transformation of Lipopolysaccharide-Trained Microglia Modulated by Varying Lipopolysaccharide Doses
We have established anin vitroLPS training model imitating continuous oral administration of LPS (Mizobuchi et al.,2020a). This model is a repetitive stimulation system using 1 ng/mL LPS, which is even lower than previously reported doses. Training with 100 ng/mL LPS showed microglia toxicity as in previous reports (Harada et al., 2011; Kim and Li, 2013),whereas training with 1 ng/mL LPS did not exhibit cytotoxicity(Mizobuchi et al., 2020b).
Using this model, we demonstrated that microglia transformed by LPS preconditioning at 1 ng/mL were characterized by high phagocytic activity and high expression of proinflammatory (NOS2, CCL1, IL-12B, and CD86), antiinflammatory (IL-10, Arg1, IL-13 receptor subunit alpha 2 (IL-13RA2), and Mrc1), and neuroprotective molecules(neurotrophin 4/5 (NT4/5), CCL7, and gastric inhibitory polypeptide receptor (GIPR)) (Mizobuchi et al., 2020a). These results indicate that LPS trained microglia have the potential to regulate inflammation, neuroprotection, and phagocytic clearance. As CCL7 is involved in neuronal differentiation(Edman et al., 2008), and NT4/5 and GIPR prevent neuronal damage through their antioxidant and antiapoptotic effects(Seino et al., 2010; Faivre et al., 2011; Meirelles et al., 2017;Spielman et al., 2017), it is suggested that such a mechanism may also be involved in the neuroprotective effects of LPStrained microglia. In addition, our results indicated that LPStrained microglia could not be characterized in terms of their general suppression of proinflammatory mediators or general promotion of anti-inflammatory mediators; instead,LPS-trained microglia exhibit complex molecular expression profiles of both pro- and anti-inflammatory mediators.Hence, it is assumed that microglia transformed by oral administration of LPSin vivoalso exhibit similarly complex molecular expression profiles.
Furthermore, microglia transformed by low-dose LPS (1 ng/mL) showed characteristics distinct from microglia trained with high dose LPS (100 ng/mL) (Mizobuchi et al.,2020a). On the other hand, Lajqi et al. (2019) reported a Schwartzman-like reaction by examining the effect of ultralow-dose LPS preconditioning. They observed that highdose LPS preconditioning at 100 ng/mL suppressed TNF-α and IL-6 secretion by microglia, while ultra-low-dose LPS preconditioning at 1 fg/mL enhanced TNF-α and IL-6 secretion from the microglia via phosphoinositide 3-kinase (PI3K)-mediated signaling. In other words, the difference in response to secondary LPS stimulation (proinflammatory or antiinflammatory response) depended on the LPS dose. These results indicated that the molecular expression profiles of microglia do not simply change proportionally to the LPS doses, and that microglia exhibit diverse transformation depending on the administered LPS dose. It is important to understand the diversity of microglial transformations in order to determine the appropriate LPS dosing conditions to induce neuroprotective microglia transformation.
Although it has been reported that the reactivity of peripheral monocytes and the adaptive immune system differed depending on LPS dose (Morris and Li, 2012; Zakeri and Russo, 2018), little attention has been paid to the difference in microglial transformation by LPS dose. As microglia are derived from the yolk sac and monocytes are derived from the bone marrow, results from LPS-trained monocytes cannot be simply applied to LPS-trained microglia. Therefore, in order to characterize LPS-trained neuroprotective microglia transformed by oral administration of LPS, conditions regarding LPS dosing are issues that need more thorough examination.
Signal Transduction Mechanisms That Induce Lipopolysaccharide-Trained Neuroprotective Microglia
Several studies on signal transduction mechanisms that induce transformation to a LPS-trained neuroprotective microglial phenotype have been performed, but consensus has not yet been reached as the number of reports is small.
For example, Schaafsma et al. (2015) showed that RelB was required for the suppression of TNF-α and IL-1β by epigenetic reprogramming in microglia trained with LPS at 100 ng/mL.Liu et al. (2016) revealed that in microglia trained with LPS at 100 ng/mL, Smad4 was required for IL-6 suppression and IL-10 promotion via the inactivation of nuclear factor-kappa B signaling. Qin et al. (2016b) reported that LPS preconditioning at 10 ng/mL promoted phosphorylation of Smad2 (p-Smad2),Smad3, as well as expression of Kruppel-like factor 4, and peroxisome proliferator-activated receptor γ in microglia.Ajmone-Cat et al. (2003) reported that expression of IκBα and phosphorylation of cAMP response element binding protein were promoted in microglia trained with LPS at 10 ng/mL.Moreover, they also showed using a hippocampal slice culture system that inhibition of glycogen synthase kinase 3 beta may be involved in the transformation to neuroprotective microglia (Ajmone-Cat et al., 2016). Sun et al. (2018) reported that suppressor of cytokine signaling 1 was involved in TNF-α suppression in microglia trained with LPS at 10 ng/mL.Interestingly, Chu et al. (2016) revealed that the colony stimulating factor 1 from neurons and astroglia also played an important role in the transformation to neuroprotective microglia by LPS preconditioning at 15 ng/mL.
Thus, diverse background signals may be involved in the transformation of neuroprotective microglia upon LPS training. In other words, the signal transduction that induces transformation to LPS-trained neuroprotective microglia cannot be explained by a single factor, and multiple factors are likely involved in this process and may act in a complex manner. Furthermore LPS-trained microglia may transform into more diverse phenotypein vivodepending on changes in microenvironmental factors, including LPS dose and factors released by surrounding cells.
Unsolved Issues for Elucidating Neuroprotective Mechanisms by Lipopolysaccharide-Trained Microglia
Our understanding of neuroprotective microglia transformed by LPS training is still in its infancy. Further studies are required to characterize these LPS-trained neuroprotective microglia, and to elucidate the mechanisms by which they repair neurons or protect against neurological disorders.Studies comparing the effects of low- and high-dose LPS under microglial depletion may provide clues to elucidate the mechanisms by which LPS training impart beneficial effects.Additionally, it is necessary to consider the conditions for treatment with LPS that can induce neuroprotective microglia without side effects. Our studies indicate that immune training by oral administration of LPS is particularly desirable,but dose, timing, and duration should be further investigated.In addition, it has been reported that there are strain and sex differences in the response to LPS in rodents (Erickson et al., 2018; Kadkhodaee et al., 2020); This suggests that there may be sex differences in the effects of LPS training even in humans, although further research is needed to confirm this.Moreover, the mechanism by which LPS preconditioning induces neuroprotective microglia is unclear. Wendeln et al.(2018) suggested that peripheral IL-10 may be involved in neuroprotection induced by LPS training. In this context, it was reported that either orally administered LPS or enterobacterial LPS regulated physiological hepatic gluconeogenesis by activating adipose tissue macrophages to restore decreased peripheral IL-10 induced by a high-fat diet (Kobayashi et al.,2018; Toda et al., 2020). Therefore, IL-10 may be involved in the mechanism of transformation to neuroprotective microglia following the oral administration of LPS.
On the other hand, the possibility that LPS directly activates microglia cannot be excluded. Toda et al. (2020) reported that LPS concentration in the blood increased transiently after feeding, even in a healthy state. It remains unclear whether LPS directly activates microglia or indirectly activates microglia via other cells and humoral factors. Solving these issues may lead to the potential treatment of neuronal dysfunction by LPS training, especially via oral administration of LPS.
Conclusion
As shown inFigure 1, LPS training through oral administration,prevents neuronal dysfunction by inducing transformation of microglia to a neuroprotective phenotype. LPS-trained microglia cannot be simply characterized by their general suppression of proinflammatory mediators or general promotion of anti-inflammatory mediators; instead, they must be characterized according to complex profiles comprising various molecules related to inflammatory regulation, phagocytosis, neuroprotection, anti-apoptosis, and antioxidation. In addition, LPS-trained microglia exhibit diverse transformation depending on LPS doses.
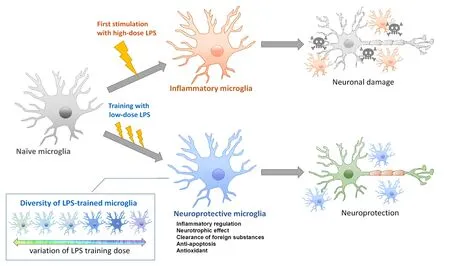
Figure 1|Model of neuroprotective microglia transformed by training with low-dose LPS.Initial stimulation with high-dose LPS induces transformation to inflammatory microglia, thus exacerbating neuronal damage. In contrast, training with low-dose LPS prevents or repairs neuronal damage. LPS-trained microglia cannot be simply characterized by their general suppression of proinflammatory mediators or general promotion of anti-inflammatory mediators; instead, LPS-trained microglia exhibit complex molecular expression profiles related to inflammatory regulation,phagocytosis, neuroprotection, anti-apoptosis,and antioxidation. Moreover, LPS-trained microglia are diversely transformed into diverse phenotypes dependent on the administered LPS dose. LPS:Lipopolysaccharide.
Molecular-targeted drugs, which are the current primary treatment for neurological diseases, have limited therapeutic effects. Controlling transformation of neuroprotective microglia by LPS training with a focus on the oral administration of LPS may serve as an innovative treatment or prevention method for various neurological disorders that are currently considered as intractable diseases.
Author contributions:HM and GIS participated in study concept,definition of intellectual content, and manuscript editing and review.HM participated in study design, literature search, data acquisition, and manuscript preparation. GIS was a guarantor. Both authors approved the final version of the paper.
Conflicts of interest:HM and GS are employed by the Control of Innate Immunity, Technology Research Association. GS are employed also by Macrophi Inc. This does not affect our adherence to journal policies.
Financial support:This work was funded by Control of Innate Immunity Technology Research Association; a grant of Cross-ministerial Strategic Innovation Promotion Program, SIP-No. 14533073 (to GIS) from the Council for Science from Technology and Innovation (CSTI) in Cabinet Office of Japanese Government and the National Agriculture and Food Research Organization (NARO). CSTI and NARO had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Aric F. Logsdon, University of Washington, USA;Claudia Espinosa-Garcia, Emory University, USA; Wensheng Lin, University of Minnesota Twin Cities, USA.
Additional files:
Additional Table 1: Neuroprotective effects induced by in vivo immune training with LPS.
Additional Table 2: Phenotypes of neuroprotective microglia transformed by LPS training.
Additional file 1: Open peer review reports 1 and 2.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Axonal regeneration and sprouting as a potential therapeutic target for nervous system disorders
- The role of gap junctions in cell death and neuromodulation in the retina
- Don’t know what you got till it’s gone: microglial depletion and neurodegeneration
- Protein post-translational modifications after spinal cord injury
- Axonal mRNA localization and local translation in neurodegenerative diseases
- Alzheimer’s disease: a tale of two diseases?