Study on the Anti-inflammatory and Repairing Effects of Cannabis Sativa Leaf Extract by Epikutis Model Substitution
Guangzhou Zoom-i Cosmetic Co.,Ltd.,China
Wan Jie
New Era Health Industry(Group)Co.,Ltd.,China
Abstract To explore the anti-inflammatory effect of Cannabis sativa leaf extracts and their repair to damaged skin,a 3D epidermal model (Epikutis?) was used.During the experiment,after epithelial cell surface was pretreated with SLS for 24 h,the anti-inflammatory effect and repair effect of Cannabis sativa leaf extract were observed by testing tissue viability and the representative pro-inflammatory factors such as IL-1α,TNF-α and IL-8.The tissue viability was detected by the MTT test.The results showed that the Cannabis sativa leaf extract effectively inhibited the secretion of inflammatory factors IL-1α,TNF-α and IL-8,and the tissue viability of the skin model was significantly improved after using the sample.The Cannabis sativa leaf extract has a repairing effect on skin barrier damage by inhibiting skin inflammatory reaction.
Key words Epikutis model; Cannabis sativa; Cannabis sativa leaf extract; inflammatory factors; cosmetics
Cannabis sativa,known as hemp,kenaf,flax,mountain seedlings and wild hemp,is annual herbaceous plant of Moraceae.Modern pharmacological studies have shown that Cannabis sativa has antibacterial and anti-inflammatory biological activities.[1,2]
Industrial cannabis is a Cannabis species that has been optimized for breeding to reduce its THC content,which is typically less than 0.3%.[3,4]The detoxified flower and leaf extract of industrial cannabis is commonly known as detoxified CBD full spectrum oil in China,in which THC is not higher than 0.3%.More than 100 kinds of natural cannabinoids have been found in the leaves,flowers and stems of industrial cannabis,and most of the active substances have good antibacterial and antiinflammatory effects.[5,6].
According to the research results of Hungarian scientist Attila Oláh et al.in 2014,cannabidiol(CBD),the main ingredient of cannabinoid leaf extract,has the effects of inhibiting sebum secretion of sebaceous gland cells,anti-proliferation and antiinflammation.[7]According to the research conducted by Nalli Y et al.in 2019,the active ingredients such as cannabidiol(CBD) and α-bisabolol in the cannabis leaf extract can be added into cosmetics to have antiinflammatory and antibacterial effects.[8]
The 2013 EU Regulation EC 1223/2009 on New Cosmetics applies the 3D skin model to cosmetic safety evaluation.In addition,thein vitrostimulation test,especially the application of 3D skin model,has been adopted in the cosmetics industry in China.[9,10]In this paper,the inhibitory and repair effects of cannabis leaf extract on inflammation were preliminarily explored and detected by the 3D epidermis model (Epikutis?),which provided an experimental basis for the application of industrial hemp in cosmetics.
1 Experiment
1.1 Main reagents
Keratinocyte (BatchNo.: p190826,GenerationNo.:P7),Guangdong Boxi Biotechnology Co.,Ltd.;Maintenance medium,homemade;Epikutis? artificial skin model,homemade,batch number: MS200501;WY-14643(PPAR activator); Isopropyl alcohol (IPA);Sodium lauryl sulfate (SLS); 3-[4,5-Dimenylthiazol-2-yl]-2,5-diphenyl tetrazole bromide (MTT);dimethyl sulfoxide (DMSO);
Phosphate buffered saline (PBS),purchased from Sigma; Cannabis sativa leaf extract,Yunnan Hempson biotechnology Co.,Ltd.
The test samples used in this experiment were derived from industrial cannabis varieties planted on high mountain slopes with an altitude of 1,500~2,500 meters in Yunnan Province.The full spectrum oil of cannabis leaves extracted by the supercritical CO2extraction process contained 62.3% of CBD,0.18%of THC,and 0.95% of α-bisabolol.The sample to be tested was a pale yellow liquid,and it was stored at room temperature in the dark.
1.2 Preparation of maintenance medium
TU culture medium: MCDB153 powder culture medium was used as the main component,and growth factors 1-7 that correspondingly regulate keratinocytes were weighed,prepared in the corresponding order,filtered and subpackaged for use.
TA culture medium: MCDB153 powder culture medium was used as the main ingredient,and the corresponding key factors 1-14 that can regulate the balance of keratinization proliferation and differentiation were weighed and prepared in the corresponding order,followed by filtration and subpackaging.
1.3 Construction of EpiKutis artificial skin model
Separating and purifying keratinocytes from human tissues,culturing for standby after passage to a certain generation; Inoculate that obtained keratinocyte in a culture chamber according to a specific ratio,and adding a self-research TU culture medium for culture under the solution for 2 days; The medium was switched to TA culture medium suitable for the proliferation and differentiation of epidermal cells,and after 7 days of culture in the gas liquid surface,the model was prepared and used for the detection experiment.
1.4 Preparation of working solution of positive control group (WY14643 activator)
Weigh 10 mg WY14643 powder and dissolve in 1 mL DMSO to prepare 30 mmol/L WY14643 mother liquor; Add 8.3 μL of WY14643 mother liquor to 5 mL of model medium to prepare a 50 μmol/L working solution for later use.
1.5 Sample Preparation
In the anti-inflammatory test,the concentration of test samples greatly affected the experimental results,and the test concentration was mainly determined based on the safe concentration in the cytotoxicity test.Under normal circumstances,the amplification factor should be appropriately adjusted based on the safe concentration,and 10 times amplification factor should be selected.If concentrations greater than or less than the safe concentration were selected for the anti-inflammatory test,the anti-inflammatory effects would be affected,which is not representative.At the same time,the specific to the corresponding experimental results will be different.
According to the experimental design in Table 1,test article working solutions with different concentrations were prepared,and two repeated wells were set in each group.The keratinocytes were inoculated into the 24-well plates according to the inoculation density of 1×104cells/well.After the inoculation operation,the 24-well plates was placed in a CO2incubator (37℃,5% CO2,and 95%RH) for incubation overnight,and the drug was administered when the cell plating rate in the 24-well plates reached 50%.After the dosing procedure,96-well plates were incubated in a CO2incubator (37 ℃,5%CO2,RH 95%) for 24 h.After incubation,the cell morphology was observed under a microscope and photographed (Figure 1).
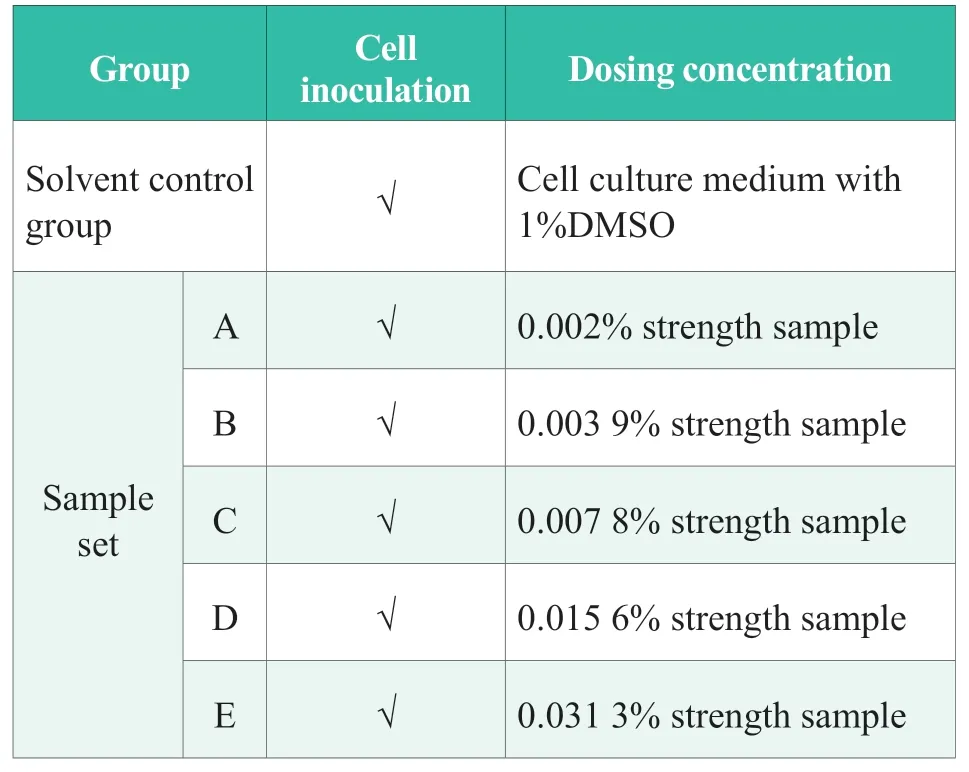
Table 1.Experimental design
As shown in Figure 1,the morphological test results show that,when the concentration of sample is less than 0.007 8%,the cell morphology of the sample has no significant difference compared with the solvent control group.When the concentration is higher than this,the difference between the cell morphology of the sample and the solvent control group is very significant,and the number of cells is significantly reduced.Within the concentration range of 0.002%~0.031 3%,the number of cells tended to decrease and the cytotoxicity of samples increased with the increase of sample concentration.Therefore,according to the selection basis of the content of the anti-inflammatory test sample,the concentration of the anti-inflammatory test sample could be set to 0.023 4%.
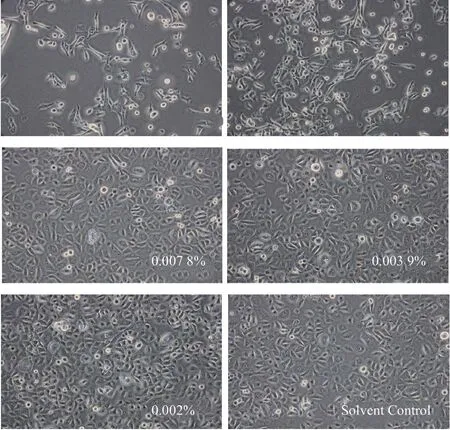
Figure 1.Morphological test results of sample
Absorb 500 μL of prepared sample mother solution (0.046 8%) and add to 500 μL 0.4% SLS solution,and mix well for standby.
1.6 Experimental process
According to the design of Table 2,the 3D epidermal skin model was transferred to a 6-well plate (with 0.9 mL of model culture medium added in advance) and the test group number was marked on the 6-well plate.25 μL prepared working solutions of different groups were added on the surface of the model.SLS solution with 0.2% concentration was added on the surface of NC and PC groups,and the working solution with corresponding concentration was added in the sample group.The samples were uniformly distributed on the surface of the model and incubated in a CO2incubator (37 ℃,5%CO2,RH 95%) for 24 h.After incubation,the residual test substance on the surface of the model was washed with sterile PBS solution,and the residual liquid in and out of the model was gently wiped with a sterile cotton swab.
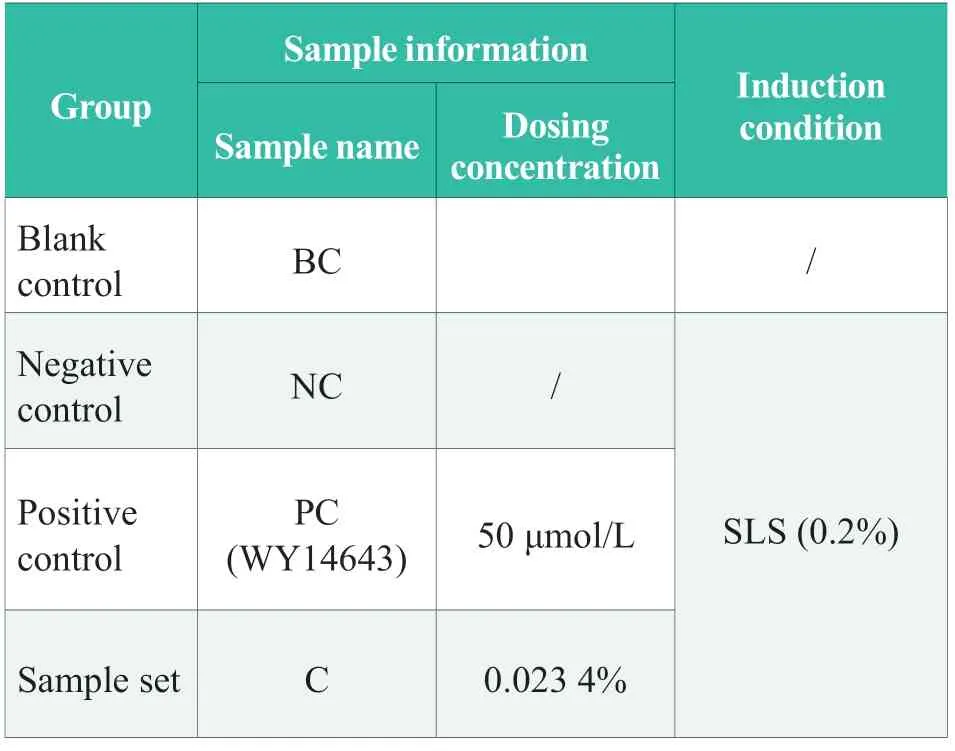
Table 2.Experimental design
1.6.1 Detection of inflammatory factors
After incubation for 24 h,the supernatant of the 3D skin model cultures was collected into EP tubes and the samples for ELISA were frozen in a refrigerator at -80 ℃.Detection analysis was performed according to the ELISA kit operating instructions.
1.6.2 Tissue viability test
According to the experimental grouping,the models used for tissue viability test were washed,and 24-well plates (I) were prepared according to the number of models and marked accordingly.Then,0.3mL of MTT working solution (1 mg/mL) was added to each well in sequence.The 24-well plate with the model was placed in an incubator (37 ℃,5% CO2,and RH 95%) for 3 h ± 5 min.
After incubation,the outer surface of the model was washed with a wash bottle filled with sterile PBS solution,and the residual liquid outside the model was gently wiped with a sterile cotton swab and transferred to a new 24-well plate (II) and marked accordingly.Then 2 mL isopropanol was added into the model,and the 24-well plate was sealed with a sealing membrane and oscillated on a plate shaker for 2 h.
The 200 μL pipette tip was used to pierce the model,and the isopropyl alcohol extract was allowed to flow out of the model into 24-well plate (II).Discard the pierced mold and blow the 2-propanol extract 3 times in each well to mix well.
After uniform mixing,two parts of 200 μL isopropyl alcohol extract from each well were added into the corresponding wells of 96-well plate,and marked.2-propanol was used as the zero adjustment well.The absorbance was read by a microplate reader at 570 nm.
The calculation formula of relative tissue viability of each experimental group was as follows:Relative tissue viability =(Aadministration well -Azero adjustment well) /(Acontrol well -Azero adjustment well) ×100%
Data analysis was performed using SPSS17.0 statistical software.The experiment was repeated three times,and the data results were expressed as mean±standard deviation (mean s).Analysis of variance was used to compare the differences among multiple groups of data,and T-test statistical analysis was used to compare the differences between two groups.All the statistical analyses were 2-tailed,andP<0.05 indicated significant difference,andP<0.01 indicated extremely significant difference.
2 Results and discussion
2.1 Tissue viability test results
The results of tissue viability test are shown in Figure 2.in the figure indicatesp<0.01 compared with BC,* * indicatesp<0.01 compared with NC.Compared with the blank control group (BC),the tissue viability of the NC group was significantly decreased (p< 0.01),indicating that the SLS stimulation condition was effective in this experiment.Compared with the NC group,the tissue viability of the PC(WY14643)group was significantly increased (p< 0.01),indicating that the positive control test was effective.Compared with the NC group,the tissue viability of the sample(0.023 4%) was significantly increased (p< 0.01),and it was able to significantly repair the tissue damage.
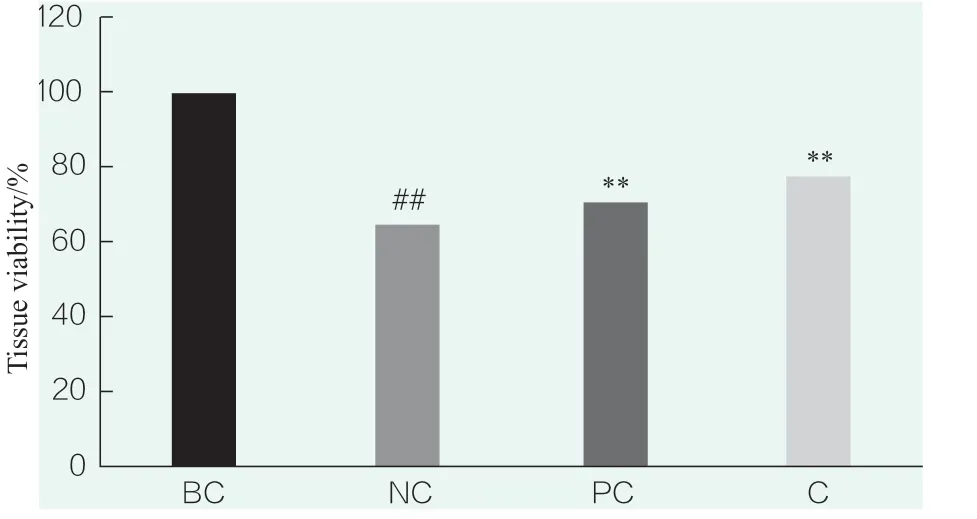
Figure 2.Tissue viability test results
2.2 Detection results of inflammatory factors
2.2.1 IL-1α detection
The results of IL-1α detection are shown in Figure 3.In the figure,# # indicatesp<0.01 compared with BC,* * indicatesp<0.01 compared with NC,* indicatesp<0.05 compared with NC.Compared with the blank control group (BC),the secretion of IL-1α in the NC group was significantly increased(p<0.01),indicating that SLS stimulation could significantly increase the secretion of IL-1α,and that the SLS stimulation conditions were effective in this experiment.Compared with NC group,PC(WY14643)group could significantly inhibit the secretion of IL-1α(p<0.01),indicating that the positive control test was effective.The sample (0.023 4%) could significantly inhibit the secretion of IL-1α (p<0.05).
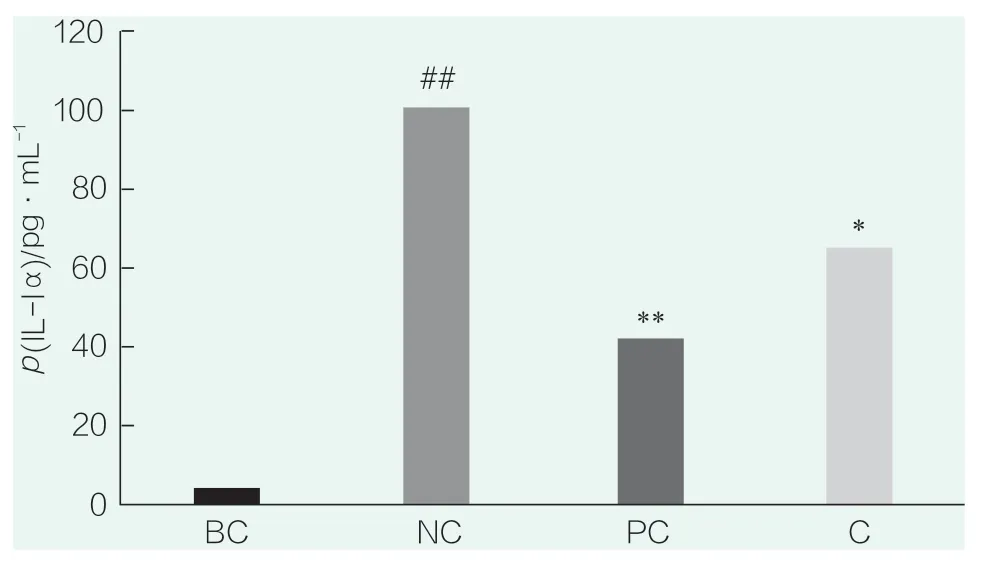
Figure 3.IL-1α test results
2.2.2 IL-8 detection
The results of IL-8 detection are shown in Figure 4.# # in the figure indicatesp<0.01 compared with BC,* * indicatesp<0.01 compared with NC.Compared with the blank control group (BC),the secretion of IL-8 in the NC group was significantly increased(p<0.01),indicating that SLS stimulation could significantly increase the secretion of IL-8,and that the SLS stimulation conditions were effective in this experiment.Compared with NC group,PC(WY14643)group could significantly inhibit the secretion of IL-8(p<0.01),indicating that the positive control test was effective.The sample (0.023 4%) could significantly inhibit the secretion of IL-8 (p<0.01).
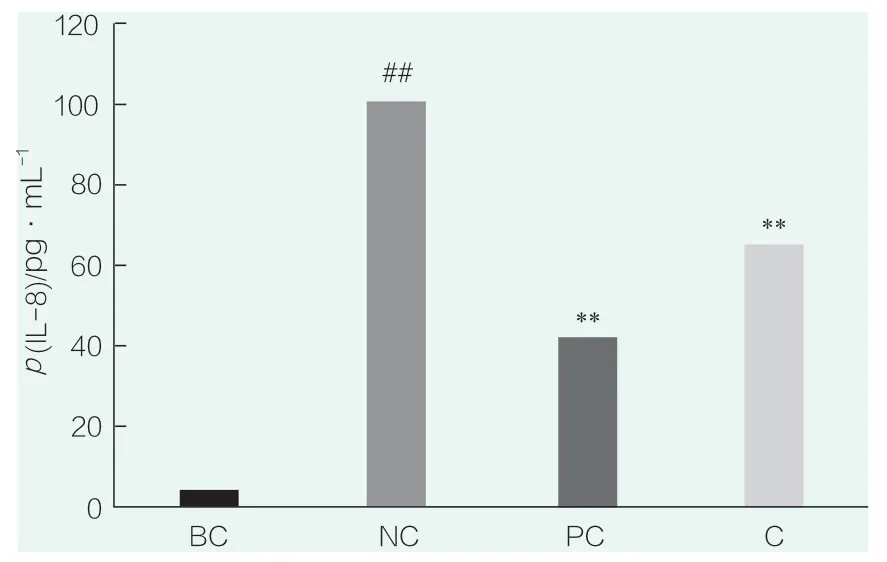
Figure 4.IL-8 test results
2.2.3 TNF-α detection
The results of TNF-α detection are shown in Figure 5.# # in the figure representsp<0.01 compared with BC,* * representsp<0.01 compared with NC,* representsp<0.05 compared with NC.Compared with the blank control group (BC),the secretion level of TNF-α in the NC group was significantly increased (p<0.05),indicating that SLS stimulation could significantly increase the secretion of IL-8,and indicating that the SLS stimulation conditions were effective in this experiment.Compared with the NC group,PC(WY14643) group significantly inhibited the secretion of TNF-α (p<0.01),and compared with the NC group,samples (0.023 4%) significantly inhibited the secretion of TNF-α (p<0.05).
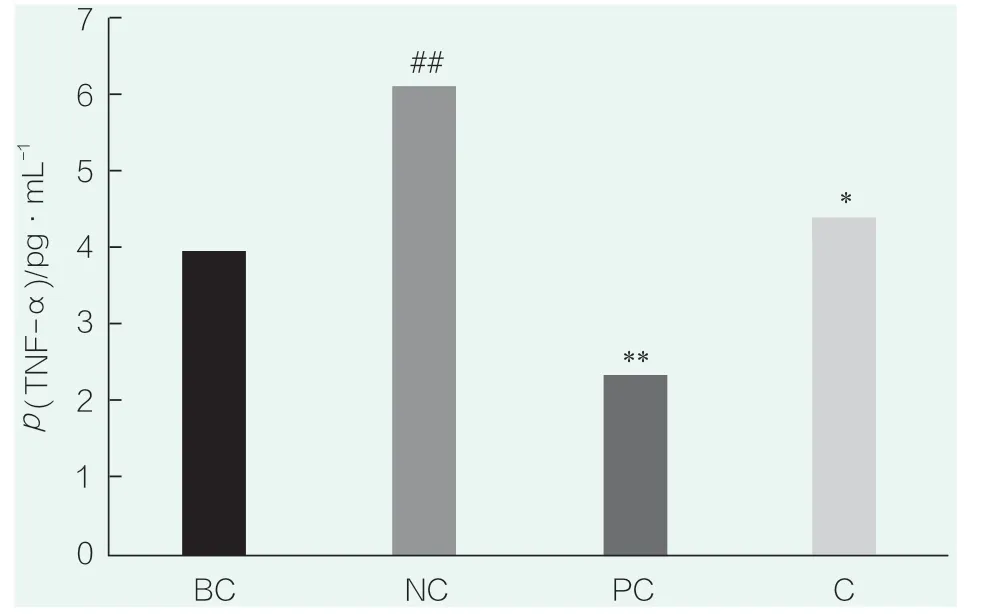
Figure 5.TNF-α test results
3 Conclusion
The research and test results showed that the cannabis leaf extract could inhibit TNF-α-induced NF-kB transcription,reduce the release of IL-8 and IL-1α,and significantly enhance the activity of skin model tissue after the addition of the appropriate concentration of cannabis leaf extract,indicating that at the concentration of 0.023 4% of cannabis leaf extract ,the efficacy of repairing barrier damage was achieved by repairing cell damage and inhibiting skin inflammatory reaction.

 China Detergent & Cosmetics2021年1期
China Detergent & Cosmetics2021年1期
- China Detergent & Cosmetics的其它文章
- Specification on cosmetics registration record ready to be operated
- Risk Analysis and Quality Control of Cosmetic Raw Materials
- The Application of Modified Oil Ethoxylates in Laundry Beads Formulation
- Efficacy Evaluation of a Compound Plant Extract Used in Makeup Base
- Laboratory Study on the Whitening Ability of A New Type of Toothpaste Against the Tobacco Stains on Teeth
- Research and Application of Nanofiber Technology in Mask Products
