Concepts,processing,and recent developments in encapsulating essential oils
Qirui Tian,Weiqing Zhou,Qiong Cai,Guanghui Ma,*,Guoping Lian,4,*
1 Department of Chemical and Process Engineering,University of Surrey,Guildford GU2 7XH,UK
2 State Key Laboratory of Biochemical Engineering,Institute of Process Engineering,Chinese Academy of Sciences,Beijing 100190,China
3 University of Chinese Academy of Sciences,School of Chemical Sciences,Beijing 100049,China
4 Unilever Research Colworth,Colworth Park,Sharnbrook,Bedfordshire MK44 1LQ,UK
ABSTRACT By the virtue of their olfactory,physicochemical,and biological characteristics,essential oils (EOs) have drawn wide attention as additives in daily chemicals like perfume or personal care products.Nevertheless,they are physicochemically unstable and susceptible to degradation or loss.Microencapsulation offers a feasible strategy to stabilize and prolong release of EO.This review summarizes the recognized benefits and functional properties of various preparation and characterization methods,wherein innovative fabrication strategies and their formation mechanisms are especially emphasized.Progress in combining detecting/measuring technologies with kinetic modelling are discussed,to give an integral approach of controlling the dynamic release of encapsulated EOs.Moreover,new development trends of EOs capsules are also highlighted.
Keywords:Essential oils Encapsulation Sustained release
1.Introduction
Essential oils (EOs) are liquids of complex biosynthesized compounds characterized by strong odors or aromas [1–5].As traditional materials with a long history,EOs have been widely used in fast moving consumer goods as additives to provide pleasant feeling and deliver pleasant olfactory and gustatory sensations[6].For instance,flavor enhancement for food,drink and medicine,long-lasting fragrance release from textile,personal care,and home care [7,8].Moreover,EOs could also provide therapeutic functions in delivering emotional,sensorial and health benefits in well-being and higher standards of living [9,10].Nevertheless,composed of unstable and susceptible volatile compounds,EOs will undergo continuous deterioration as exposed to oxygen,light,moisture,and heat[11,12].These changes have negative effects on product quality like inducing off-flavors that impairs sensorial properties,or generating free radicals that breaks stability [13].Also,constant EOs degradation and loss will shorten the shelf life and reduce the overall quality of the products [11,14].
Various encapsulation approaches have been applied to retain stability and functional properties of EOs [15,16].Encapsulation is defined as a method in which tiny particles or droplets are surrounded by a coating wall,or embedded in a homogeneous or heterogeneous matrix [17].The matrix wall isolates the active compound as a functional barrier from the surrounding environment to avoid chemical and physical reactions to prolong their stability and bioavailability [18].Emulsion-based encapsulation allows the compartmentalization of oily active compounds in oil droplets and the aqueous phase favoring process of wall-forming[19,20].For instance,coacervation at the exterior of the emulsion droplets is widely developed,enabling trials of different filming polymers[21]and complex structures[22].Inorganic capsules like silica particles [23,24]and graphene oxide-silica hybrid [25]have also been reported with enhanced mechanical stability.Besides,the emulsion structure is also regulated to separate the compartments containing the compounds to encapsulate and the locus of wall-polymerization,oil-in-water-in-oil (O/W/O) double emulsion[26–28]is a feasible approach in regionalizing multi-functional designs.Despite these improvements on functionality and applicability,however,the wider utilization of current EOs capsules still appear to confined by the limited retention of volatile components.It is desirable to pursue new developments to further advance from current scientific levels for novel composite materials [2].This review will focus on concluding and analyzing various procedures employed for EOs encapsulation,the recent researches with innovative developments are highlighted.Improvement of characterization methodology for such structures and release kinetics of the core materials are also summarized based on current studies.
2.EO Encapsulation Strategies
Encapsulation,a process for entrapping active and high value materials in small particles,has been widely used in cosmetics and food industry to protect oils against oxidation and evaporation,achieve sustained release,promote easier handling during manufacturing and storage,and prolong the shelf life of odor-added products[29,30].Most reported EO fixation methods can be classified into encapsulation and absorption.Physical methods are generally productive and widely utilized in industrial processes [31],while structural stability of the obtained capsule is limited.Chemical processes,by contrast,achieves better stability in protecting EOs and offers long-lasting releasing potential,while most of them require complicated production processes with high environmental impact [32].Representative techniques of encapsulation continue to emerge and relevant technologies for encapsulating EOs are as shown in Fig.1.
2.1.Physical encapsulation
2.1.1.Spray drying
Spray drying is a typical physical procedure involving the atomization of emulsions sprayed into a drying chamber at a relatively high temperature,followed by subsequent water evaporation [33,34].To make sure the sudden evaporating,the inlet air temperature can be as high as 200 °C [35].Spray drying has been widely utilized for microencapsulation of EOs as its output of good quality and easy handling powder [36].
Carbohydrates and proteins are most widely used wall materials in the process of spray drying[37,38].Apart from hydrophilicity and biocompatibility,carbohydrates and proteins generally have low viscosity in water,good dispersion property during emulsification,and good film forming property.A comparison of the encapsulating properties of typical carbohydrates and proteins used for encapsulates are listed in Table 1.Chitosan (CS),for instance,is a feasible biomaterial as capsule material [39].Spray dried CS microparticles are characterized by highly spheroid shape and high specific surface area,as well as size controllability.The solubility of CS can be raised by introducing acid solution[40]or modifying carboxylation extend on the CS wall [41],and the reported encapsulating efficiency could over 90% as calculated by Tzhayiket al.[40].However,CS possesses functional selectivity for several EO types with specific structures,Kumaret al.[42]proposed it not suitable for squalene as both the efficiency ((26 ± 0.577)% and the anti-oxidation capability was not satisfactory,directly impacted by the molecular interactions between the wall and the core agents.
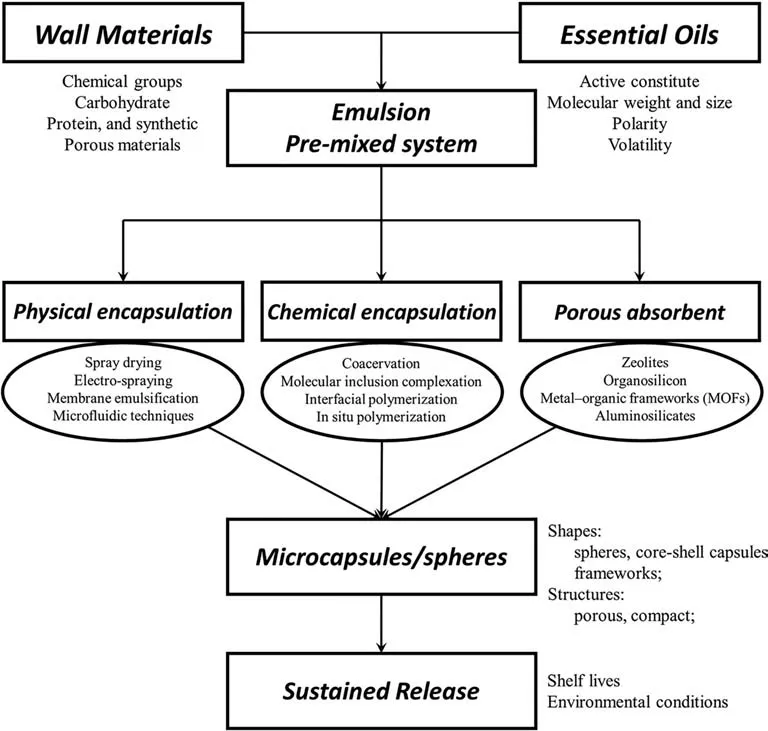
Fig.1.Encapsulation or fixation processes for EOs.Parameters listed outside the boxes are key factors affecting encapsulated product and its characteristics.
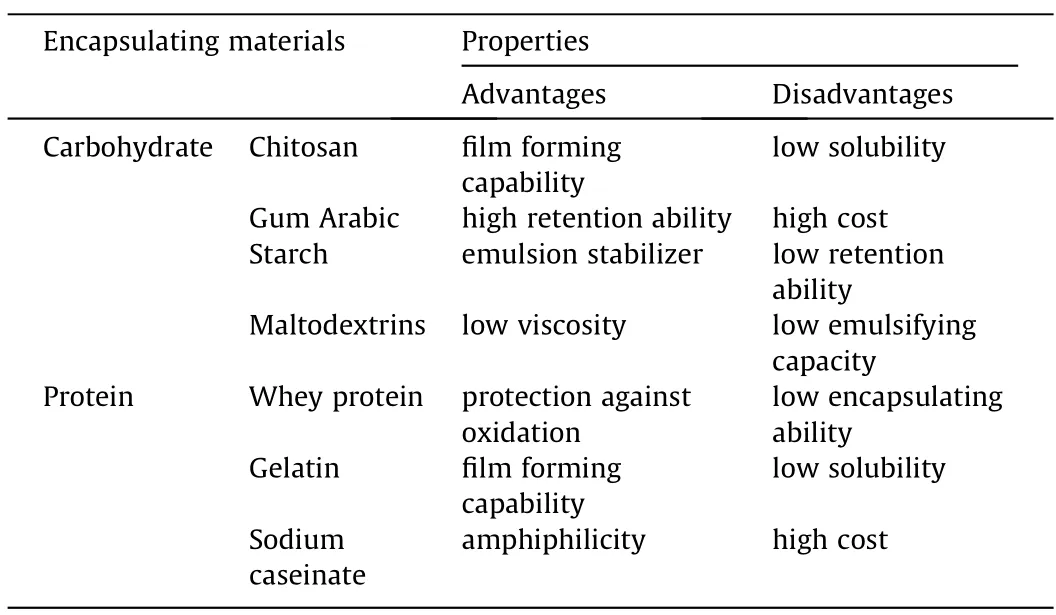
Table 1 Typical wall materials used for spray dried encapsulates
The above-mentioned limitation of CS revealed a wide-existing weakness for wall-forming biopolymers,that single-component capsule will lead to inevitably functional defections.To overcome the weakness,coupled carbohydrate and proteins have been studied to achieve better encapsulating capability.Protein and carbohydrate are polyelectrolytes exhibiting opposite electric potentials.At a controlled pH,accordingly,the complexation between protein and carbohydrate can be strengthened due to electrostatic interactions,and further enhance the stability of the capsules.For instance,maltodextrin (MD) is a soluble and low viscous wall material with good protection of oxidation to core oils [36],but has poor emulsification and film-forming properties [43].Complexation between MD and Soy protein isolate (SPI),whey protein(WP),and sodium caseinate(NaCas)were proved to have encapsulation efficiency as much as 89.8%[43].Likewise,Shamaeiel al.[44]proved that incorporating Tween 80 into the wall material formula of skim milk powder (SMP) resulted in the highest encapsulation efficiency of 91.01%.
Although spray drying are widely applied and easily scaled up,they are still limited by the high temperature,wherein many heatsensitive EOs can be easily lost or deteriorated[17].Besides,some core materials may also stick to the surface of spray dried capsules,which can cause oxidation,leaking,and possible contamination of the encapsulated materials [45].
2.1.2.Microfluidic techniques
Apart from wall materials,the capsule morphology also plays key role in releasing performance [46].Oil droplets are spherical dispersoid of o/w emulsions where smaller droplets constantly shrink and larges ones enlarge due to diffusion.Under this circumstance,the oil droplets are preferred to be as uniform as possible to avoid Ostwald ripening or subsequent demulsification.Several methods are utilized to obtain uniform emulsion system,for instance,electro-spraying [47],microfluidic techniques [48],and membrane emulsification [49].
Microfluidic techniques are getting increasingly focused in comparison to conventional processes [50–52].By pumping dispersing phase through a microarray of controlled size and geometry into the continuous phase,monodispersed emulsion is produced and the size distribution is normally less than 1% [48].Microfluidic devices also involve mild generating environment with low interchange of energy,making it preferable in preventing denaturation of sensitive bioactive compounds [51,53].Early researches related to microfluidic EOs mainly focus on the emulsion compositional and stability optimization [54–56].Leeet al.firstly introduced a capsule-forming strategy outside the microfluid-emulsified droplets by photocurable oil as shell material[57].Fragrance-in-water(F/W)emulsion was initially prepared by bulk emulsification,and surrounded by photocurable oil phase(trimethylolpropane ethoxylate triacrylate,ETPTA) at a tapered cylindrical injection.As injected into the cylindrical collection capillary,EO was directly flow-focused by the outer aqueous continuousphaseintoisolateddroplets.UV-triggered photopolymerization of ETPTA was subsequently applied to solidify the hydrogel capsule as depicted in Fig.2.The release assessment of the hydrogel network showed that (49 ± 6)% of α-pinene remained in the microcapsule after 5 days.Kawakatsuet al.also developed a feasible encapsulating strategy by crosslinking the pectin molecules dissolved in the outer phase of a W/O/W emulsion obtained through microfluidics by introducing calcium ions[58].
Newly developed encapsulating methods like microfluidics are feasible in obtaining high encapsulating efficiency and sustained release performance due to their exquisite structures and regulable polar surroundings.However,complex processes of these techniques also limit the production efficiency of the capsules,an emulsion in a microfluidic device is made by precisely fabricating one drop at a time[50],weakened its relative commercial application related to the scale up for larger throughput [51].
2.2.Chemical encapsulation
2.2.1.Molecular inclusion complexation
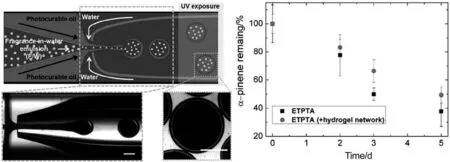
Fig.2.(A) Schematic illustration of the glass capillary microfluidic device for preparing polymer microcapsules encapsulating a preformed F/W emulsion.Bottom optical microscope image shows the generation of triple emulsion drops containing multiple internal fragrance drops.Upon UV irradiation,the photocurable oil in the middle phase polymerizes to form a polymeric shell.Scale bar represents 200 μm.(B) Graph showing the percentage of α-pinene remaining with time upon removal of the dispersion media.Reprinted from Ref.[57](Copyright 2016,American Chemical Society).
Molecular inclusion complexation refers to the fabrication technique for special structured host–guest complexation [59].Cyclodextrins(CDs)are representative oligosaccharides with compatible cavities for molecule retentions [33,60].Although the encapsulating efficiency is limited by the low solubility of CDs,studies have proved their capability in increasing the stability of volatile molecules,converting the EOs from liquid to powder form by forming inclusion complexations and further prolong their release [61].This process is influenced by both steric hindrance and supramolecular interaction [62–64].
2.2.2.Interfacial polymerization
Interfacial polymerization,also known as interfacial polycondensation,is another well researched strategy that forms encapsulating layer through crosslinking and solidifying of the reagent monomers dissolved in two immiscible phases [65].Polymerization occurs at the interface of the droplets and form capsules with active compounds entrapped by the polymer walls [66]as illustrated in Fig.3.
In practice,interfacial polymerized EOs capsules have a diameter between 3 and 800 μm and contain 10%–90%core[67].According to different types of monomers,reported wall structures of microcapsules can be classified as polyamide [68–72],polyurea[73],polyurethane [74],polyester [67],and thiol-acrylate [75].Interfacial polymerization manifests advantage in their rapid reaction speed and mild reaction course compared to other encapsulating process [75].“clickable”wall-forming procedure with high reaction efficiency and rate can be achieved in generating stable microcapsules with long EOs retention ability.One disadvantage of the technique is that the formation of a thin interfacial polymeric layer between the reagents can hinder further reaction between the separated phases,thus capsules with low mechanical integrity may then be produced [66].Additionally,abundant ions attached on the shell may offer a tunable hydration state of the capsule influenced by the pH environment,so that their permeability under specific solution system would be weakened [65].
2.2.3.In situ polymerization
In situ polymerization is similar to interfacial polymerization in their mechanism of encapsulation with no reactant of capsule formation is dispersed in core oils [11].During polymerization,the reactions between monomers or oligomers occur in the continuous phase,leading to higher mechanical integrity of the capsule [3].After emulsification,controlled deposition of the wall-forming polymers take place at the o/w interface by a change in pH,temperature,or solvent quality [66].
In encapsulating EOs,wall materials are generally aminoplast resins,such as melamine–formaldehyde(MF)[76],urea–formaldehyde(UF)[77],or third-monomer-modified system like methanolmodified MF(MMF)[78]or polyethylene glycol modified MF(PMF)[79].Amongst those,MF are extensively adopted in preparing EOs capsules.Due to the strong reactivity of formaldehyde with the trifunctional melamine monomer,the resulted wall structure consists of a densely packed matrix with a high crosslinking density[16],possessing the capsules of high thermal-and chemicalstability [80].Early researches focused mainly on factors affecting the characteristics of MF resin capsules containing EOs[76,81–84].Demonstrated that MF-encapsulating efficiency is affected by stirring time,stirring rate,emulsifier type,pH,emulsifier concentration,and the viscosity of the core material.Many other researches also designed new strategies to strengthen the MF droplet attachment by building covalent bonds within.For instance,high molecular copolymers like as poly (acrylamide-acrylic acid sodium) [85]and styrene-maleic anhydride polymer [78,79]were used as emulsifiers to stabilize oil droplets and provide driving forces for capturing MF pre-condensates to the oil droplets’surfaces.
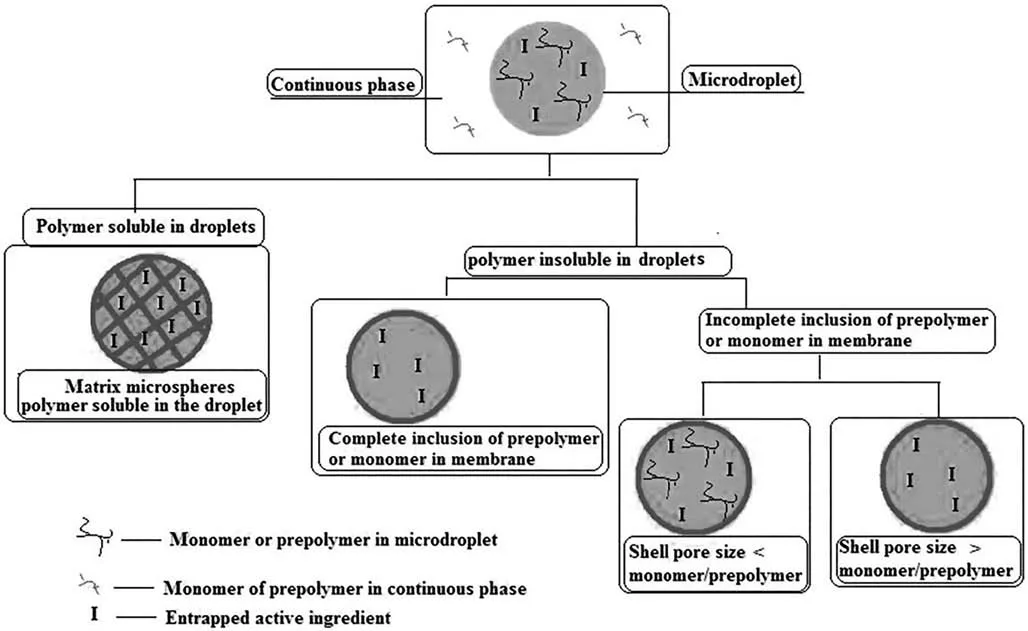
Fig.3.Scheme of microcapsule formation by interfacial polycondensation.Reprinted from Ref.[65](Copyright 2011,Taylor &Francis).
Nevertheless,MF system is not workable for EOs of high active unsaturated bonds on the structure such as clove oil,Litsea cubeba oil,and lemongrass oil,because the existing active bonds or radicals in the shell would possibly change the physical and chemical properties of the core oils.Heet al.[86]combined MF in situ polymerization with pre-solvent evaporation of a polyacrylate(PAA)as an inner intermediate bridging layer.This special conformation not only stabilizes the droplets from agglomeration,but also provides electrostatic attractions to MF pre-condensates with(Fig.4).Williamset al.incorporated MF resin at the outer layer of the Magnafloc/Laponite stabilized Pickering emulsion of sunflower oil [85].Magnafloc,an amine-based polyelectrolyte,offers active sites that can further combine MF pre-condensates and lead to chemical grafting via intermediate layer.
By introducing a composite structure,the intermediate layer will separate the chemically active MF shell from the inner core to improve its chemical stability.However,the mechanical feature of MF resin still limits its utilization in EOs encapsulation as a consequence of stiff and brittle shell wall of most aminoplast-type capsules [16].Release process of the of active compounds is controlled by mechanical force on the MF shell,which causes an outburst,instead of sustained release,of the inner EOs[87].Guoet al.designed a polyethylene glycol-modified MF resin with branched structure to raise the flexibility of the wall molecules,and obtained MF capsules could prompt the impact strength and surface hardness as dopant materials [79].In the recent decade,researches diversified the aminoplast capsule morphologies to achieve better controllable processes.Fuet al.developed microcapsules with nano-scaled pores (from 5 to 200 nm) on the surface formed by the self-assembly template of nonionic surfactant micelles [88],and polyoxyethylene (10) nonyphenyl ether (NP-10) was selected as the surfactant and pore-foaming agent[89].This study revealed that raising temperature facilitated smaller capsule sizes and larger pore sizes throughout the capsule walls.Fig.5A illustrates the formation mechanism of external pores,and Fig.5B exhibited SEM images of obtained porous microcapsules.Porous microcapsules display a large specific surface area and adjustable permeability,proving potential applications for the sustained release of core materials.
In general,aminoplast resins obtained through in situ polymerization is a widely applicative method in encapsulating specific types of EOs.Nevertheless,deficiencies in their toughness and chemical instability limit its further applications.Moreover,in regards to biodegradability,it should be mentioned that MF microcapsules will not be biodegraded until an extensive amount of time[16].In the ecofriendly context of modern manufacturing industry,establishing sustainable chemistry considerations based on traditional materials remains to be further investigated.
2.2.4.Simple coacervation
With coacervation,a colloidal system is separated into two liquid phases by forming coacervating shells as interfaces[32].Coacervation is generally classified into simple and complex coacervation [11].Simple coacervation involves only one polymer that forms the capsules,and the solvated oil droplets are gradually wrapped by the desolvated polymer in the presence of dehydrating agents or raising temperature.
Film forming in simple coacervation uses partially-hydrophilic natural/synthetic polymers [90]or surfactants [91]that form colloids in solution,and the encapsulating process is driven by“water deficit”phenomenon in the presence of a competing hydrophilic substance [92].The size of coacervated beads is influenced by the initial droplet size,and the strength depended on the concentration of the dehydrating agent.Both natural and synthetic polymers can be chosen as wall materials.Gelatin is a commonly used coacervation material derived from collagen in skins,bones,and connective tissues of animals [93],owing to its biocompatibility,biodegradability,nontoxicity,and low cost [94].Apart from protein,carbohydrate also offers alternative selection as film polymers.For example,hydroxypropyl methylcellulose (HPMC) is a carbohydrate of good water solubility and emulsifying-ability[95].Notably,respective characteristics of natural polymers as wall materials has been concluded as Table 1.
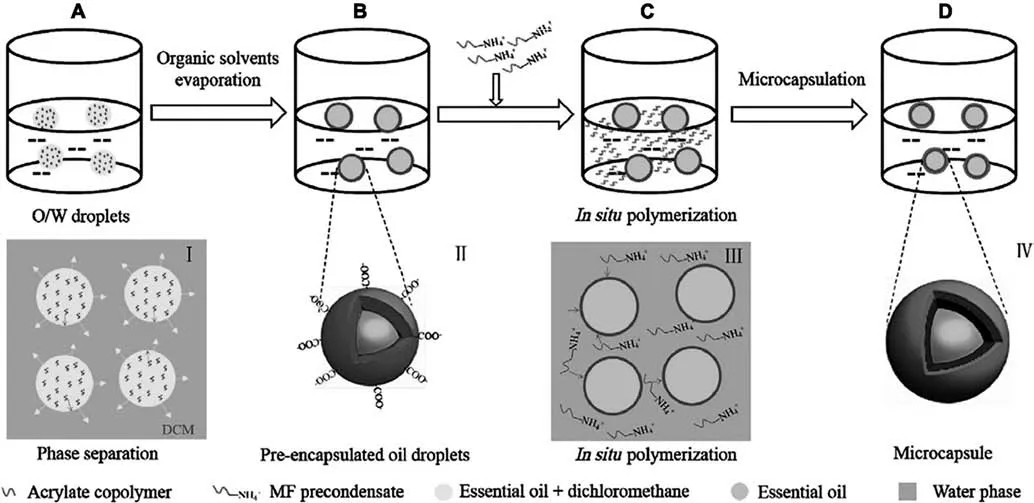
Fig.4.Sketched mechanism of the fabrication process of microcapsules containing essential oil by combining the solvent evaporation process and in situ polymerization.Reprinted from Ref.[86](Copyright 2019,Elsevier Ltd).
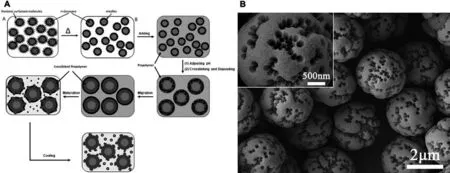
Fig.5.(A)Schematic illustration of the encapsulation process of long chain normal alkanes with NP-10 micelles as template of the MF resin surface pores.(B)SEM images of porous microcapsules prepared by in situ polymerization via self-assembly of NP-10 micelles as template within the range of Tcp.The inset is the high-magnification image.Reprinted from Ref.[88](Copyright 2011,Elsevier Ltd).
Synthetic polymers are also reported as coacervate agent.Poly(vinyl alcohol) (PVA) solution exhibits a lower critical solution temperature (LCST) separating into two phases upon increasing the temperature of the aqueous solution.Bachtsiet al.[96]published a study on the oil permeability through cross-linked PVA wall and its dependence on the preparation conditions(e.g.degree of desolvation,degree of cross-linking of the membrane).The crosslinking ratio and surfactant concentration are found to be key factors controlling the releasing rate.Leimannet al.[97]and Sahlan [98]further proved the feasibility in encapsulating various EO models and dependent variables in taking PVA as wall polymers.
Simple coacervation offers important advantages with costsaving and flexible operations.However,this method has shortcomings of size control and sustained release availability.Also,encapsulations of EOs by coacervation is likely to induce evaporation of volatiles,dissolution of active compound into the processing solvent and oxidation of product,as core materials sometimes also cling to the exterior of capsules.
2.2.5.Complex coacervation
Complex coacervation is another encapsulating approach by changing the phase-separating mechanism into the mutual neutralization of opposite-charged polymers,usually proteins and polysaccharides [1,99].Microcapsules produced by complex coacervation have a typical core–shell structure with two main conformations:mononuclear and multinuclear microcapsules.The major driving factor for complex coacervation is electrostatic interaction where charged macromolecules present in the reaction medium [92,100].Both conformation changes and intramolecular interactions have important acts on the process due to entropy variations induced by van der Waals intermolecular force,as showed in Fig.6A[15,100,101].Complex coacervation is widely utilized in foods [11],daily chemicals [16],pharmaceuticals [19],and agricultural applications [60].The technology is simple,low cost,scalable,and reproducible,yielding high encapsulation efficiency with very high payload.
For complex coacervation,interactions between participating polymer chains are key factors influencing the structure assembly and the stability of coacervates and entrapped materials [92].To optimize the manufacturing process and obtain microcapsules with uniform size distribution and encapsulating stability,critical component such as temperature,ionic strength,relative polymer mixing ratio,molecular weight,total concentration,and charge densities need to be controlled during initiation,continuation,and termination of the coacervation process [101].The ionization degree of the polyions is also fundamental parameter.Polyelectrolytes (macromolecules) carry a relatively large number of functional groups that are either charged or neutralized according to the solution pH [3].With the co-existence of amino groups (protein) and carboxyl groups (polysaccharide),the pH variation will give two scenarios:(1) pH lower than the isoelectric point of protein,the proteins carry positive charge;(2)pH is slightly above pKaof carboxyl group,polysaccharides carry negative charge.A schematic phase diagram was presented in combination by Kaibaraet al.[102]and Mattisonet al.[103].The authors firstly researched the impact of the charging state on phase separation,as shown in Fig.6B.By lowering the pH blow 7,soluble complexes are formed at pHc,a critical acidity to induce intra-polymeric association.Between pHφ1and pHφ2,coacervation-activated phase separation happens as there are approximately equal amounts of positive and negative charges.Further lowering pH below pHφ2generates more cations than anions,thus the coacervation diminishes.Additionally,the diagram has also revealed that adding strong electrolyte could narrow the two-phase region,an inevitable consequence of electrostatic screening [32,104,105].
2.2.5.1.Traditional coacervation applied in EOs encapsulation.Consistent with above-mentioned theoretical observations are the wide range of reported materials for successful encapsulation of different EOs by coacervation with better stability and controllability [3,106].Amongst those,complex coacervation endows abundant protein/polysaccharides pairing tragedies taking pH and stirring rate as control variables to match different product quality requirements as listed in Table 2.Complex coacervation shows advantages in obtaining controllable capsule size (range less than 10 μm),and preferable encapsulating efficiency (>80%).
However,use of complex coacervation has encountered main drawbacks for process scale up in daily chemicals industries,generally concluded as:
(1) Sustained release.Fabric softeners/conditioners,liquid laundry detergents,and powdered laundry products often come with encapsulated fragrances with specific release profiles,preferably long-lasting releasing of fragrances for weeks after their last impregnation wash [16].Coacervates shells are far more permeable to small molecules due to their porous hydrogel-like structure and interfacial morphology[125,139],leading to an upper limit duration to reach long lasting release.
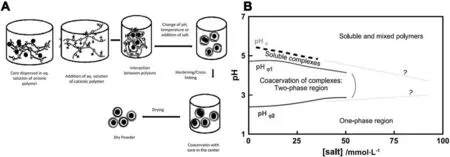
Fig.6.(A) Schematic representation of complex coacervation for encapsulation of EOs;(B) State diagram as a function of pH and salt concentration for phase separation.Reprinted from Ref.[92](Copyright 2019,Elsevier Ltd) and Ref.[32](Copyright 2004,Elsevier Ltd),respectively.

Table 2 Summary of EO encapsulation by chemical methods
(continued on next page)
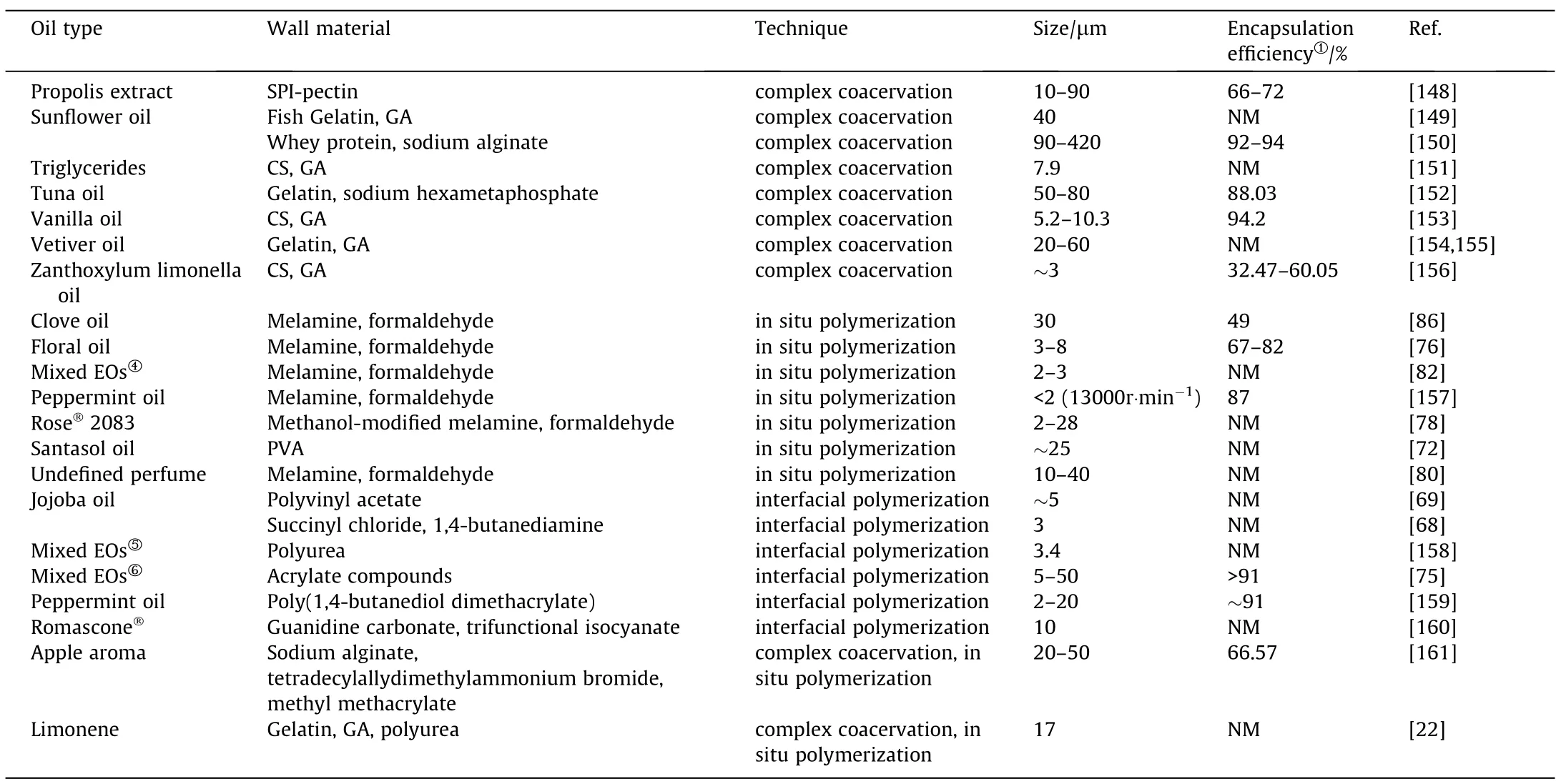
Table 2 (continued)
(2) Enhanced stability of formulations in the presence of light,heat,and oxidizing conditions,or transform liquid oil compound into a free-flowing powder.
(3) Size reproducibility of the commonly used stirring method is not conducive to stabilize size distribution and not reproducible for scaling up emulsion production [149].
Consequently,though complex coacervation has proved its feasibility in encapsulating EOs,harsh storage conditions of the capsules and requirement for sustained release have significantly limited the selection of viable wall materials and chemistries for industrial applications.
2.2.5.2.Combination of complex coacervation and other techniques.By constructing complex shells or introducing sizecontrol techniques into coacervation,several newly proposed developments have offered potential to optimize capsule production and achieving long-lasting release.In this chapter we emphatically introduce some illuminating works in developing complex coacervation.
Dardelleet al.[22]synthesized a double-layered core–shell limonene microcapsule with strong composite barrier combining synthetic polyurea with the outstanding adhesive properties of complex coacervates of gelatin and GA.The polyurea membrane is designed to be synthesized in situ from the interface between the coacervate and the oil core,by previously mixing monomer precursors into the inner oil cores and the outer water phase.(Fig.7).Further tests have proved the outstanding encapsulating stability for the microcapsules over limonene oil against surfactant-driven leakage.UV–vis spectrum with capsules immersed by SDS solution testified the relatively low releasing rate for the composite capsules.Similar double-layered strategy constructed through in situ polymerization based on coacervate templatehasbeenreportedbySongetal.[161]Tetradecylallydimethylammonium bromide (TADAB) and sodium alginate (SA),a pair of oppositely charged molecule has been utilized as coacervate wall material,in which TADAB contains unsaturated one C=C bond that could be further initiated to copolymerize with methyl methacrylate (MMA) to manufacture another polymer shell composited with complex coacervate wall.The revised coacervation capsules exert enhanced capacity in EOs retention,only 10.8% aroma released out from the aroma microcapsules after 100 hours and the encapsulation efficiency of aroma was about 66.57%.
In addition to improving mechanical and morphological properties of capsule shells,the design of microcapsules with tailor-made features in terms of size reproducibility has drawn growing attention.A challenge to develop standardized protocols and to improve the quality of encapsulated products.From an engineering perspective,it is important to ensure the maximum lot-to-lot size consistency.Membrane emulsification is a newly proposed technique that allows the production of uniform particles of controlled size by producing droplets of one liquid phase in a second immiscible liquid phase through a membrane with determined sizes[49,162–166].Piacentiniet al.[149]firstly developed a membrane-emulsification-assisted complex coacervation.Oil droplets with a controllable median size of 40–240 μm and a particle span as low as 0.46 were generated using a micro engineered membrane with a pore size of 10 μm,in which the mixtures of fish Gelatin and GA were used as a continuous phase.As given in Fig.8,very narrowly dispersed droplets are formed as soon as the oil was pressed into the continuous phase through the membrane pores,and very low amounts of anionic emulsifier was needed to obtain small droplets.Unfortunately,EOs releasing tests were not developed in the report,and related researches are still scarce so far.Also,further utilization of membrane emulsification in EOs encapsulation rarely reported.
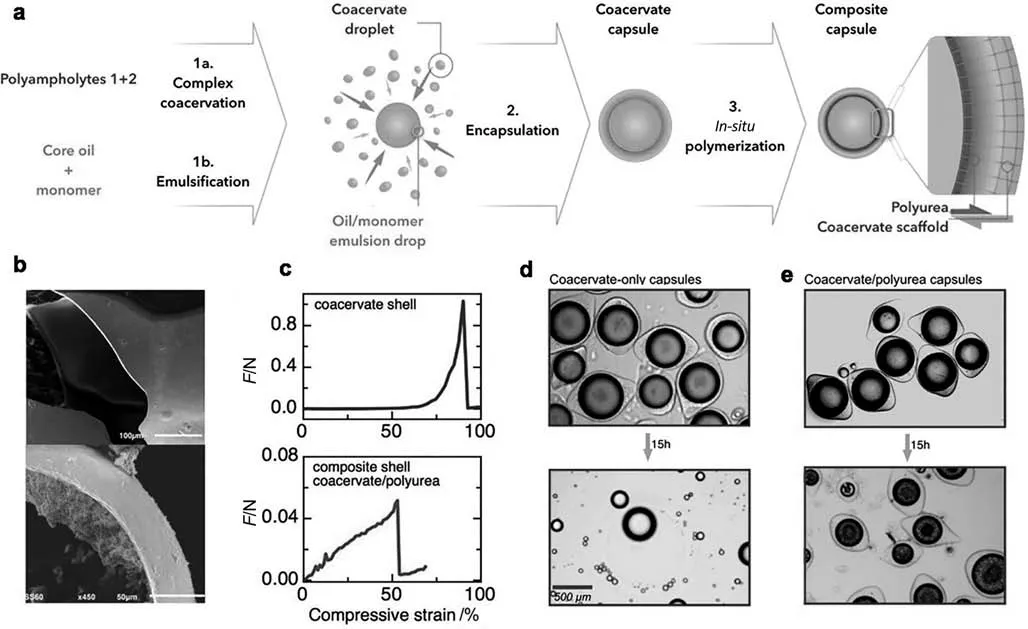
Fig.7.(a) Formation of composite core/shell capsules polyurea/coacervate multilayer membranes.Stability against surfactant-driven leakage for encapsulated model volatiles:side-by side comparison of (a) SEM micrographs of coacervate-only (top)and composite polyurea/coacervate core/shell capsule shells.(c) Mechanical behavior of single capsules measured in compression mode standard coacervate-only capsules,and stability against surfactant-driven leakage for encapsulated model volatiles:side-byside comparison of (d) standard coacervate-only capsules and (e) composite coacervate/polyurea capsules upon exposure to a surfactant solution.Reprinted from Ref.[22](Copyright 2017,John Wiley &Sons Inc.).
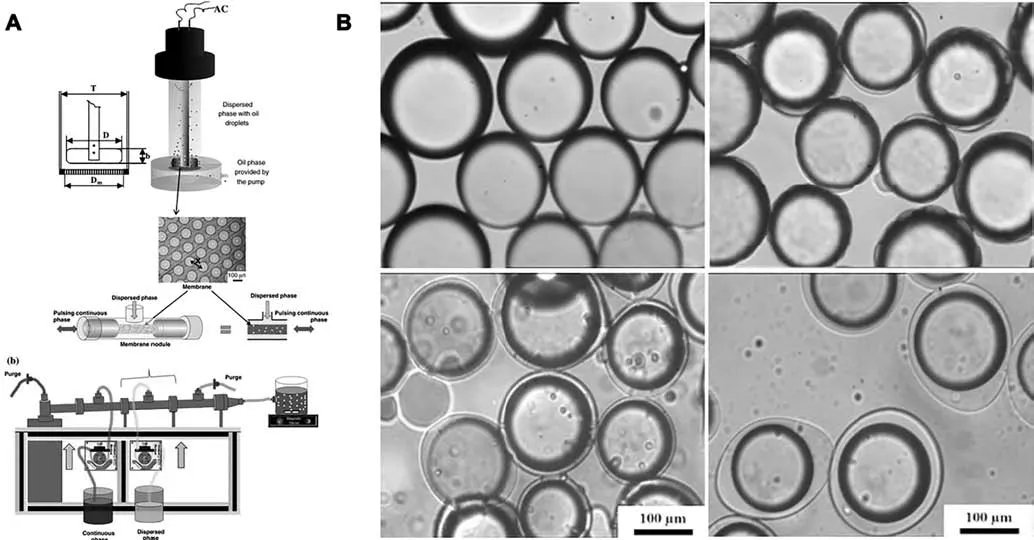
Fig.8.(A) A schematic representation of the membrane emulsification process;(B) Microscope images of microcapsules crosslinked with glutaraldehyde and washed with hot water at different ratio between gelatin and GA.Reprinted from Ref.[149](Copyright 2013,Elsevier Ltd).
The above results demonstrate that new types of complex coacervation are valid in meeting more critical requirements.Traditional coacervation technique has proved wide feasibility and high productivity in constructing EOs capsules,while its releasing performance are not satisfying limited by weak emulsion stability and capsule strength.Introducing double-layered structure or mono-sized oil template endows prolonged stability and release sustainability of the EOs capsules.Moreover,combination techniques based on complex coacervation also broadened the scope of the EOs availability as new functional materials.
2.3.Porous absorbent
More recently,porous materials have attracted new interests as absorbent for EOs storage and separation due to specific structural advantages[167–169].Zeolites and zeolite-like materials with aluminosilicates channels and pores are good matrices as EOs carriers,wherein natural zeolites represented non-specific absorbance over various kinds of EOs at higher temperature(100°C),and synthetic molecular sieve exhibited better absorbing efficiency [170].More specifically,lab-synthesized zeolite X products with different average particle diameters (20 μm and 4 μm) were tested in encapsulating/releasing EO molecules [171].Desorption tests indicated larger crystals to have lower desorption rate constants,revealing a volume-dependent kinetic releasing model of the zeolite frameworks.Also,mesoporous silica nanocapsules for EOs encapsulation were obtained via interfacial sol–gel solidification of the EOs/silica emulsions [172].
Metal–organic frameworks (MOFs) are broadly studied porous materials composed of metals and organic ligands with permanent porosity and designable hydrophilicity [173].Three-dimensional network of MOFs offers an ideal and flexible spatial array in hosting and sustained release of EOs simply by soaking [61,174].Rutgers recyclable porous materials (RPMs) were firstly investigated in encapsulating EOs [175].Releasing profiles under dispersed state represented a clear lowered desorption speed for both hydrophilic and hydrophobic models,and the EOs protecting percentage over 1.5 h could reach 100%.Lashkariet al.investigated the feasibility of MOFs as delivery systems for encapsulation and sustained release of volatile allyl isothiocyanate (AITC) molecules [176].Upon releasing,water vapor of high concentration was found to be an effective trigger to release AITC molecules from MOFs,with an underlying mechanism of the structural transformation from a porous to a nonporous structure at high humidity [177].Studies also improved the release duration of polar ester EOs by incorporating hydroxyl pendants onto MOFs,and further achieved the designability of the hydrophilicity extent of the specific capsule structure to meet the requirement of on-demand release [178].
In addition,studies have revealed EOs absorption and delivery with longevity of porous clay materials [179,180],and the releasing kinetic represented pH-responsiveness that increased releasing velocity was observed as pH decreased,probably influenced by the electric potential variation of the porous structure.Likewise,organosilicon was also studied as EOs carriers,and EOsconjugated organosilicon could be faster hydrolyzed with acidic catalysis upon releasing [181].In general,development in porous sorption has enlightened new aspects in applying natural or synthetic inorganic materials as EOs carriers.Mostly,absorbing strategies offer efficient encapsulating approach by simply soaking[171]or blending[172],save the process of complicated pre-mixing and wall forming.Besides,porous skeletons of high strength and thermal stability could meet the needs of harsh environments with considered economically cheap in manufacturing [182].However,deficiency of bio-compatibility might limit the feasibility of inorganic structures in skin-contactable chemicals like lotions or toothpastes.Also,diffusion kinetic within porosity skeletons is a typical concentration-gradient-controlled process,that prolonged release in the open system is hard to achieve [179].
3.EOs Measurement Methods
Releasing kinetic measurement is the main evaluation procedure in testing EOs capsule performance.EOs release is a continuous and time-relevant process of different classes of target analytes,thus dynamic experimental methods are mostly reported to characterize their sustainability.Considering different applicability for each of these methods,it is essential to give a classification and comparison as guidance for future work.In this chapter,we sorted related reports as gaseous phasic-,liquid phasic-,and thermogravimetric-techniques,wherein respective characteristics are analyzed.
3.1.Gaseous phasic measurement
Because of their inherent physicochemical properties,EOs can be easily analyzed by gas chromatography (GC),and one can conclude from the researches that methods based on GC coupled to mass spectrometry(MS)is the main approach for the identification and quantification of these compounds [183].
Headspace-GC (HS-GC) is a sampling technique applied to the determination of volatiles in the gaseous phase in equilibrium with the EOs matrix [184].Typical devices of HS-GC are generally classified as static or dynamic.Static HS-GC refers to a process that the equilibrium phases of the volatile active is hermetically sealed in the vial,in the dynamic HS-GC,the targeted sample is obtained by capturing the volatiles in a gaseous effluent passed through or over the matrix on to a suitable trapping system [185].
The effective parameters in static HS-GC analysis are temperature,sample volume,and incubation time[186,187].Lianet al.utilized static HS-GC to verify the amount and types of oil in different samples[188].All generated headspace was injected into the nonpolar GC column at 200°C,followed by the calculation of the retention times and peak areas by software.Samakradhamrongthaiet al.calculated the releasing profile and rate by multiple sampling at specific time intervals [142].The releasing profile showed clear head-dependent “ON/OFF”performance along with the temperature variation.For EOs-CD inclusion capsules,static HS-GC could also be applied to calculate the complexation stability by release monitoring [189,190].
Dynamic HS-GC techniques use a flow of inert gas for continuous extraction of volatile compounds from a sample[186].An inert gas is purged through the sample chamber as long as all or most volatile compounds extracted from the sample and swept to one or two analytical traps that adsorb the volatiles [191,192].Compared to static HS-GC,the trapping stage of the analysis features can enhance the detection precision up to ng/g level,and types of sorbents can be selected in trapping stage to achieve EOs absorbance of a wide range [193].Kimet al.utilized dynamic HS-GC in evaluating release rate of different EOs of specific capsule structure[194].The same procedure was applied by Günayet al.to verify the EOs retention performance of a peptides-bonded capsule [160].
For complicated EOs systems,electronic nose (e-nose) device shows its advantage in characterizing complicated components[195].They usually consist of cross-reactive sensing arrays and analytical algorithms that produce signatures upon recognition of specific EO molecules.Applying e-nose in EOs release kinetics are seldomly reported.Rodriguezet al.qualitatively examined the time dependent pattern of the EOs 5 days onward since encapsulation to measure the releasing profile,and improved discrimination was obtained using raw data and supervised analysis methods [196].
Apart from chromatographic devices,weighing method is also reported to measure the EOs release amount in open air[40],offers a direct but rough comparison between different testing systems.Dardelleet al.also developed a human sensory panel tests to quantitate intuitive feelings over the EOs release,and four standards were set to define the perceived olfactive intensity [22].This method could effectively distinguish the user feelings in commodities of with essence.
3.2.Liquid phasic measurement
Analytical techniques like liquid chromatography (LC) and UV–visible spectrophotometer are feasible approaches for EOs releasing experiments conducted in liquid phases without change of EOs state.High performance liquid chromatography (HPLC) is widely adopted on measuring the active concentration after rinse and diffusion for certain period[197,198].A representative utilization can be referred from Gumíet al.[199,200],who periodically sampled the bulk solution that was hermetically stored until analysis to monitor the vanillin release from polysulfone microcapsules Likewise,UV–vis spectrometry is employed for the liquidphasic release of the EOs[201].Deviet al.rinsed a known quantity of microcapsules of EOs by certain volume of Tween 80 solution,followed by removal of aliquot sample of known volume at appropriate time intervals to conduct UV–vis spectrometry tests [202].Similar methods were also implemented in measuring the release rate of vanillin [21],limonene [22],Lavender oil [132],osmanthus flower fragrance [203],Rosmarinic acid [41],and thyme essential oi [204].Notably,Dardelleet al.modified traditional UV–vis spectrometry by designing an in situ UV–vis spectrometry of capsules immersed in the surfactant solution directly inside glass cells[22].A layer of porous membrane was fixed in the glass cell to separate immersed capsules from testing area of light path,as shown in Fig.9.A continuous release concentration curve of EO was obtained as controlled weigh of capsules placed onto the membrane and soaked into SDS solution.This method avoided the experimental error induced by multiple sampling.
3.3.Thermogravimetric analysis (TGA)
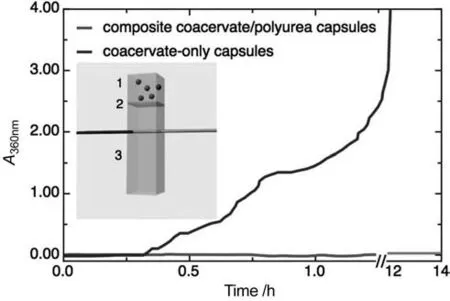
Fig.9.Loss of encapsulated compounds from the capsules:absorbance in the surfactant phase in contact with capsules measured by UV–vis spectrometry(inset:schematic of the custom sample cell used;(1) capsules in SDS solution;(2) porous membrane;(3) measuring compartment with SDS solution).Reprinted from Ref.[22](Copyright 2017,John Wiley &Sons Inc.).
TGA are widely applied to determine the thermal stability of the wall materials[205]and the total amount of the loaded oil[178]in the aspect of in EOs encapsulation.Some reports also extended this strategy to measure the release kinetics of the encapsulated EOs by monitoring the weight loss at constant temperature [14,158,206].In these studies,TGA was conducted as a feasible observation method for the capsules with relatively long release endurance.
4.Sustained Release of the EOs
Regardless of the methodologies and applications,sustained release is a dominating function of the EOs microcapsules.In the UK,EOs labelled products are normally suggested to have a shelf life over 1 year[207].Herein we should clarify the term“sustained release”as a specific process that controlled merely by the diffusional progress of the encapsulated EOs.Any releasing actions triggeredbyexternalstimuli,bothmechanicallyand physicochemically,are not concluded in the kinetic discussion.A typical diffusion progress of polymer matrices may be through the free volume within polymers chains or pores preexisting in the polymer [208,209].For inorganic capsules,the diffusion is dependent on the porosity of the capsule [210].
4.1.Steady state release
Steady state release,or zero-order release,is a convenient process model in dynamic mathematics [211].Steady state release is for special cases that outflow rate is continuous and constant per unit of time in the capsule system,as concluded by Eq.(1) [212].The validity of the model is proved for several types of modified release powders,as in matrix particles with low soluble EOs,coated forms,osmotic systems [213].

whereMtandM∞r(nóng)efer to the amounts of EOs released at timestand infinity,respectively,so theMt/M∞stands for the cumulative release at timet.
The permeation of the active in the microcapsule depends on both kinetic and thermodynamic parameters in the barrier [210].The difference of the active solubility between the core and polymer shell is defined by partition coefficient,K,as calculated by Eq.(2) [214].CAandCBare the equilibrium concentration of the active in phase A and B,respectively.The total flux (J) is decided by the oil concentration (C) in core and total resistance (R) of the permeation pathway (Eq.(3)),whereRis an inductive parameter integrating the thickness δ,the apparent diffusion coefficientD,and the partition coefficientKbetween the core and shell,as defined in Eq.(4).
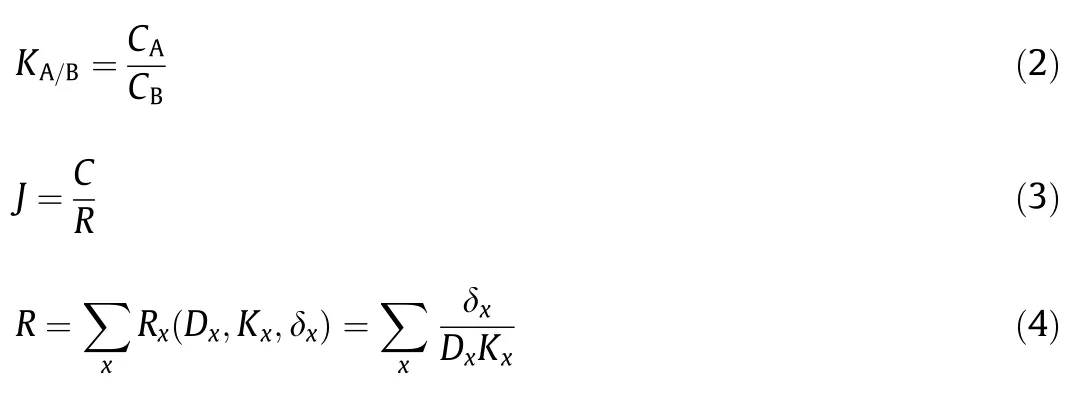
Dis an apparent diffusion coefficient influenced by the inner structural porosity and the tortuosity[215].Also,the conformation of the polymer chain and relaxation of the segments,which inflect the viscosity of the wall,will also exert impacts on the diffusing kinetics of the active molecules [210].The diffusion coefficient of a spherical molecule through a homogeneous fluid is given by the Stokes–Einstein equation (Eq.(5)) [216].Here,kis Boltzmann constant,Tis the absolute temperature,ηSis the viscosity of the shell,andris the effective radius of the EOs molecule.Low diffusion coefficient will be obtained at high shell viscosity and effective radius of the bioactive.To sum up,sustained release of EOs at steady state can be prolonged by(1)decreasing the partition coefficient of the core oils;(2) increasing the shell thickness;(3)increasing the polymer viscosity;and (4) increasing the effective radius of the encapsulated molecules.

Steady state release model is feasible in describing erosioninduced release or early stage of the capsule releasing process,when the concentration of EOs in the core varies slowly with time(pseudo-steady state).Also,some incompact and porose capsule structure can fit the zero-order kinetic.Peppaset al.proved that,continuous steady state release is possible to achieve as the polymer crosslinking ratio was controlled at a very low level(less than 1%)[217].Nevertheless,availability of steady state release model is limited to exceptional cases.
4.2.Non-steady state release
4.2.1.Empirical models
The overall mathematical theories are based on real phenomena,such as diffusion,dissolution,swelling,erosion,precipitation,and degradation [218,219].Empirically,the releasing kinetics can statistically facilitate the understanding of release pattern by equations with different orders of reaction.Table 3 lists several commonly used models in core oil transporting,together with their most befitting situations.Notably,the Weibull model is generally adopted in dissolution/release process based on an empirical equation[225].This empirical relationship has not theoretically related specific kinetic fundament with single parameters of the intrinsic dissolution mechanism,however,has proved its utility in certain practical problems [226].
4.2.2.Diffusion controlled models
For a monolithic system in which the active agent is equally dispersed throughout the polymeric matrix,unsteady-state diffusion and its time correlation in a one-dimensional matrix(thus the edge effects can be neglected) can be described by Fick’s second law of diffusion [227].In general EOs releasing cases,where diffusion coefficient is concentration-dependent,a revised diffusive expression is given as Eq.(6),where ?C/?xis the concentration gradient.

Derived from the revised Fick’s law,an analytical diffusion model of monolithic spherical devices was reported by Crank,given as Eq.(7),wherem(t)is the fractional part of the release part at timetof total amount of the EOs (mTOT) [211].
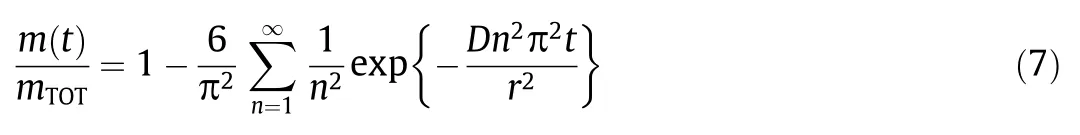
This approximation is based on three assumptions:(1) the absence of significant changes in the polymer matrix during the release,(2) perfect sink conditions during the release,and (3) the release of EOs is mostly controlled by diffusion through the polymer matrix.Exact agreement was proved by Danet al.between predictions of Eq.(7) and simulation data for homogeneous particles [228].Notably,subsequent researches have also solved the Fick’s diffusion equation for various distributions of the active substance in the microspheres of more realistic situations[13,209,229].
As similar with monolithic devices,core–shell capsules follow diffusional transport of the releasing reservoir.According to Crank[211],the fractional part of the released amount can be expressed as a function of timet,effective diffusion coefficientDin the coating,and coating thickness δ(Eq.(8)).This equation shows that the release rate of an EO component decreases as the shell permeability decreases,i.e.,its diffusion coefficient in the shell decreases or the thickness of the shell increases.
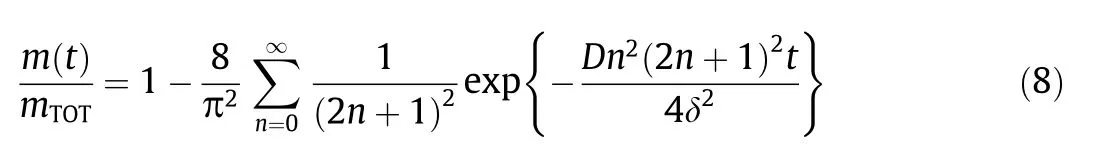
5.Conclusions and Perspective
This review highlights the preparation and characterization methodologies of the EOs microcapsules.The relative advances in designing and constructing capsules by different approaches have promoted a better understanding of EOs’utilization in daily chemicals.We report examples of various encapsulation according to kinds of capsule structures,including (1) physical processes like spay drying and microfluidic devices;(2) chemical processes like inclusion complexation,interfacial polymerization,in situ polymerization,and coacervation,where innovative fabrication strategies and their formation mechanisms are emphasized;and(3)porous absorbance like novel MOFs that gained fresh attraction in the recent decade.A conclusive table is given as Table 4 for respective features.Common experimental means to trace and measure EOs molecules are also summarized to provide references for releasing tests of different phase states.Moreover,we have reported a simple review of releasing kinetic models feasible for types of capsule structures.
Critical assessment should be notified as EOs encapsulation combines considerations of technical and economic factors,together with efficiency,safety,and the pleasing appeal of the essences [230].One of the bigger challenges involves the compromised balance between long-term stability and the releasing controllability of various packing structures.Most reviewed researches focus on obtaining promoted longevity of the EOs capsule with different wall structures.However,in view of the complex composition of EO products,it is also challenging to quantify the influence of partition coefficient and molecular dimension forspecific bioactive.As such,for glassy biopolymer excipients,moisture renders them shortcomings like plasticizing or swelling in practical utilization conditions[231].Such shortcomings are manifested in the limited confinement of odor products after unsealed.Moreover,with the expansion of capsule forming materials,the assessment regarding to biocompatibility and toxicity recognitions for novel structures like MOFs has not been accomplished to date[173].It is also necessary to establish such safety testing criterions to prompt the further productization of novel carriers.
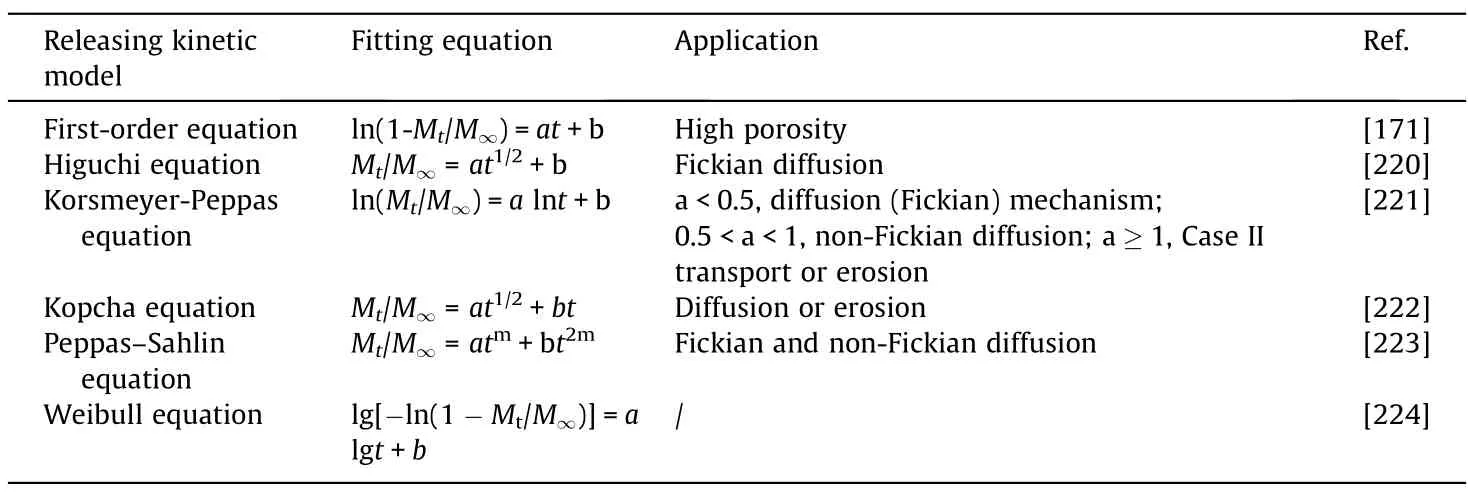
Table 3 Release kinetics models
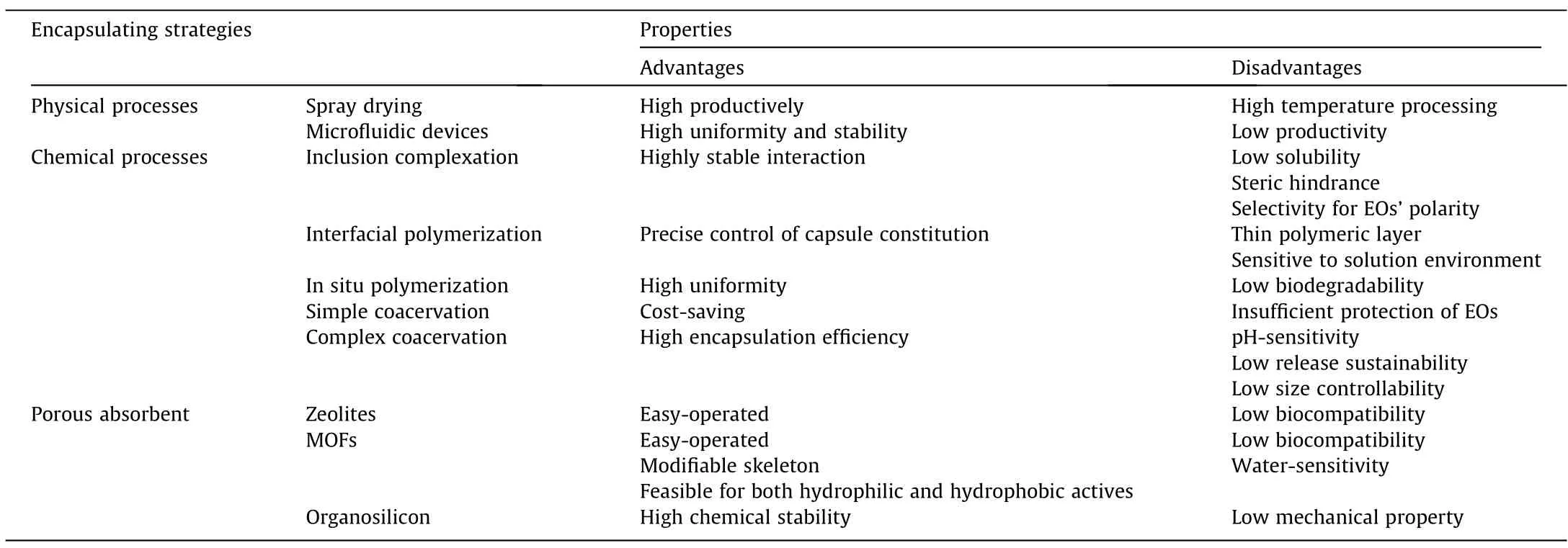
Table 4 An overview of the features for different encapsulation strategies
When looking at the overall development scope of the EOs encapsulation,one can conclude a trend towards complexity and hybridizations as a perspective in fabricating new materials in a context of sophisticated and diversiform product requirements.Future encapsulating strategies should focus on further functional optimization.(1)Biodegradability.As green chemistry becoming a growing consensus,biodegradable materials have drawn concerns to diminish the impact on environment caused by traditional plastics.Biopolymers-based coacervation offers better eco-friendliness compared to MF and solution polymerized capsules,that represents wider application prospect.(2) Functional designability.Chemically and morphologically composite shells with higher designability will be one of the future directions to achieve better controlled,even specifically triggered,release of the EOs.Deepened combination of polymeric-inorganic materials can offer higher processability with improved mechanical stability,synergistic construction of multiple material types is hopeful to broaden the performance boundaries of current encapsulation techniques.(3) Better applicability.EOs capsules are expected to be applied in more scenarios,including but not limited to antimicrobial edible films,“off-flavor”masking films,large-scale manufacturing technologies,or biomimetic adhesion and release,these perspectives need further exploration on the material and structural characteristics.In short,microencapsulation of EOs in carrier matrices is of great importance in the food,textile,and care product industries.It can provide protection against degradation,prevent the loss of volatile ingredients,and achieve sustained release for desired shelf life.Reviewing relevant processes offer a referenced toolbox for broadening its versatility and providing guidance to future rationales.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by the Strategic Priority Research Program of the Chinese Academy of Sciences (Grant No.XDA16020405),National Natural Science Foundation of China(Nos.21821005,81772417,and 21902160).Also,we would like to acknowledge the financial support provided by the joint PhD program with the University of Surrey (UK),Unilever (UK),and Institute of Process Engineering (China).
 Chinese Journal of Chemical Engineering2021年2期
Chinese Journal of Chemical Engineering2021年2期
- Chinese Journal of Chemical Engineering的其它文章
- Molecular design,synthesis strategies and recent advances of hydrogels for wound dressing applications
- Monoclonal antibody-based cancer therapies
- Recent advances in systemic and local delivery of ginsenosides using nanoparticles and nanofibers
- State of arts on the bio-synthesis of noble metal nanoparticles and their biological application
- Engineering organoid microfluidic system for biomedical and health engineering:A review
- The potential of ionic liquids in biopharmaceutical engineering
