Dysfunctional glia: contributors to neurodegenerative disorders
Marta Sidoryk-W?grzynowicz, Lidia Stru?yńska
Abstract Astrocytes are integral components of the central nervous system, where they are involved in numerous functions critical for neuronal development and functioning, including maintenance of blood-brain barrier, formation of synapses, supporting neurons with nutrients and trophic factors, and protecting them from injury. These roles are markedly affected in the course of chronic neurodegenerative disorders, often before the onset of the disease.In this review, we summarize the recent findings supporting the hypothesis that astrocytes play a fundamental role in the processes contributing to neurodegeneration. We focus on α-synucleinopathies and tauopathies as the most common neurodegenerative diseases. The mechanisms implicated in the development and progression of these disorders appear not to be exclusively neuronal, but are often related to the astrocytic-neuronal integrity and the response of astrocytes to the altered microglial function. A profound understanding of the multifaceted functions of astrocytes and identi fication of their communication pathways with neurons and microglia in health and in the disease is of critical signi ficance for the development of novel mechanism-based therapies against neurodegenerative disorders.
Key Words: astrocytes; microglia; neurodegeneration; neuroinflammation; synaptic dysfunction; synucleinopathies; tauopathies
Introduction
A growing body of evidence points to the crucial importance of astrocytes and their interplay with neurons in the brain function and dysfunction. Recent studies show that these interactions are even more complex, involving a contribution of the third factor, the microglial cells. In the first part of this article, we briefly overview the role of astrocytes and microglia in the functioning of the central nervous system(CNS). Then we focus on the contribution of the astrocytic dysfunction and defective astrocyte-neuron integrity for the pathogenesis of neurodegenerative disorders, mainly Parkinson’s disease (PD) and Alzheimer’s disease (AD) and other tauopathies. Finally, we discuss the involvement of impaired modulation of astrocytes by activated microglia in the mechanisms of neurodegeneration. More specifically,we are describing the metabolic, synaptic and inflammatory changes that affect microglia-astrocyte-neuron cross-talk in the course of neurodegeneration. We provide an overview of the novel insights into the key mechanisms of abnormal interactions among microglia, astrocytes and neurons that are involved in the neurodegenerative processes.
Database used in this review article to identify the most relevant papers included: https://www.ncbi.nlm.nih.gov/pubmed/.Keywords for searching: astrocytes, microglia, neurodegeneration,synaptic dysfunction, neuroinflammation, tau protein and α-synuclein. Majority dates of searching: 2013-2020.
Astrocyte-Neuron Interactions in the CentralNervous System
Astrocytes are considered as the most abundant glial cells in the CNS, where they make up 20-40% of glial and serve as the crucial regulators of the CNS in its development and functioning (Pekny and Pekna, 2014). Additionally,astrocytes may play a critical, either neuroprotective or neurotoxic function in virtually all the disorders of the CNS.Morphologically and functionally, heterogeneous populations of astrocytes are distinguished, including fibrous astrocytes,found mainly in the white matter, protoplasmic astrocytes present mostly in the grey matter, radial glia present in periventricular area during brain development, perivascular astrocytes, marginal astrocytes, Müller cells in the retina,Bergmann glia in the cerebellum, and pituicytes in the neuro-hypophysis (Verkhratsky et al., 2014). Physiologically,astrocytes provide structural and metabolic support to neurons, being involved in vascular homeostasis, fluid balance and regulation of ion concentrations in the brain(Volterra and Meldolesi, 2005). Perivascular astroglia are the part of neurovascular unit, where they maintain the proper functioning of the blood-brain barrier, i.e. by regulating fluid flow through the bidirectional water channel, aquaporin-4 (Bi et al., 2017). Also, astrocytes are enriched with the enzymes involved in glycolytic pathway and produce glycogen, as a source of lactate that can be transferred to neighboring neurons by monocarboxylate transporters (Magistretti and Allaman, 2018). This pathway, known as lactate shuttle, links astrocytic glycolysis with neuronal oxidative metabolism.
Metabolic interactions between neurons and astroglia are crucial for maintaining homeostasis of major neurotransmitters:glutamate (Glu) and γ-aminobutyric acid (GABA) (Bak et al.,2006). These interactions involve active translocation of two amino acids between neuron and astrocyte: glutamine(Gln) and Glu. In astrocytes, Gln is synthesized from Glu and ammonia, in a reaction mediated by astrocyte-speci fic enzyme,Gln synthetase (GS) (Martinez-Hernandez et al., 1977). Gln is transferred to neurons, where it is hydrolyzed to Glu by phosphate-activated glutaminase (PAG). Dependent on the neuron type, Glu can be included into neurotransmitter pool of Glu, or can be converted to GABA in the reaction mediated by Glu decarboxylase (Bak et al., 2006). Additionally, Glu can be converted to α-ketoglutaric acid by Glu dehydrogenase,to supplement tricarboxylic acid and neuronal energy production (Martinez-Hernandez et al., 1977). Although Glu can be produced by the carboxylation of pyruvate or the transamination of α-ketoglutaric acid, astrocyte-derived Gln is the main source of neurotransmitter pool of Glu (Hamberger et al., 1978). Following the release from synaptic terminals,Glu is taken up by neighboring astrocytes by Glu transporters and converted to Gln, what closes this circular astrocyticneuronal pathway, known as a Gln/Glu cycle (GGC) (Bak et al.,2006). This pathway involves cooperation of astrocytic and neuronal transmembrane transporters. Sodium-dependent Glu transporters, GLT1 (Glu transporter 1; EAAT2) and GLAST(glutamate-aspartate transporter; EAAT1) are localized perisynaptically on the astrocytic cell membrane (Takumi et al.,1997). Cellular translocation of Gln across the membranes of the CNS cells involves complex carrier systems that, apart from Gln, can transport some other amino acids. Individual members of these transporter families are characterized by defined cellular distribution and substrate specificity and different affinity to speci fic amino acids (Br?er, 2014). Among them, the sodium-dependent systems N, ASC, and A play major role in Gln transportation. The bi-directional transporters, belonging to the system N, sodium coupled neutral amino acid transporter SNAT3 and SNAT5 are specifically located in the astrocytes(Boulland et al., 2002). In addition, the outward translocation of Gln from astrocytes is also supported by the transporters representing the other systems - ASCT2 (alanine-serine-cysteine transporter 2, belonging to the system ASC) and LAT2 (L-type AA transporter 2, belonging to the sodium-independent system L)(Deitmer et al., 2003). The unidirectional System A transporter SNAT1 (alanine transporter 1) that mediates Gln uptake, is mostly responsible for Gln uptake by neurons (Chaudhry et al.,2002). Gln transporters are also present in brain capillaries.Endothelial cells of cerebral microvessels connected by tight junctions, forming the blood brain barrier are polarized into luminal (blood-facing) and abluminal (brain-facing) plasma membrane domains. The transporters belonging to the system N transfer Gln within the membrane vesicles enriched in the abluminal membranes of capillaries. The vesicles present in the luminal membranes transfer Gln by the sodium-independent transporter(s) that are yet poorly characterized (Br?er and Brookes, 2001).
In order to control neurotransmission properly, the neurons need to constantly replenish the neurotransmitters in the synaptic vesicles. These complex reactions involve surrounding glial cells that actively participate in this process by providing the precursors for neurotransmitter synthesis, recycling the transmitters and removing toxic metabolites. The majority of Gln release from astrocytes is mediated by an astrogliaspeci fic carrier, SNAT3, that is present in the close proximity to the synapse. The released Gln, is then taken up by a variety of transporter proteins localized on the nerve terminals and used for re-synthesis of Glu and GABA (Bak et al., 2006).
A recent evidence suggests that astrocytes, together with preand post-synaptic neuronal terminals, are a part of the so called tripartite synapse (Verkhratsky and Nedergaard, 2014). Such a localization of astrocytes, allows them to participate in the structural formation and functioning of the synapses (Allen and Lyons, 2018). It is well established that astrocytes constantly and actively release different neurotrophic factors including proteins, like chemokines or cytokines (e.g., glial cell linederived neurotrophic factor, nerve growth factor) as well as small metabolites (e.g., nucleosides and nucleotides) that support neuronal function and survival (Verkhratsky et al., 2016).
These factors can be delivered to the neurons either via exocytosis or can be contained in the astrocyte-released exosomes (Vanturini et al., 2019). Exocytosis is responsible for delivery of astrocyte-derived proteins, like extracellular matrix components, growth factors, chemokines and cytokines,when exosomes most often contain membrane proteins and RNA (Verkhratsky et al., 2016). These factors are essential for several processes, like maintaining neuronal health and extension of neurite outgrowths (Meyer-Franke et al., 1995).
Furthermore, neuronal cells are characterized by relatively low levels of endogenous antioxidants. Astroglia support neurons by supplying a major cellular antioxidant, a reduced form of glutathione (Mcbean, 2018).
Astrocytes in Diseased Brain
As mentioned above, the astrocytes, by providing essential support to neurons, play a pivotal role in the proper functioning of the healthy brain (Verkhratsky and Nedergaard,2014). Properly functioning astroglia are critical for antioxidative defense and neutralization of reactive oxygen and nitrogen species (ROS/RNS) in neurons. However, in the injury or disease, the CNS is often exposed to extensive oxidative and nitrosative stress. Overproduction of ROS/RNS challenges the antioxidative defense system, what induces astrocytic response, so called reactive astrogliosis, that involves a series of biochemical and morphological changes(Yates, 2015). Also cytokines (e.g., transforming growth factor), interleukin-6 (IL-6) or ciliary neurotrophic factor) may be involved in the activation of reactive astrogliosis via the STAT3 signaling pathway (Pekny and Pekna, 2014). This process is associated with upregulation of astrocyte-speci fic structural proteins (glial fibrillary acidic protein (GFAP) and vimentin)as well as functional abnormalities, including impaired expression and function of GGC-related transporters and enzymes. Astrocyte activation is also manifested by increased expression of potassium channels and by the disruption of antioxidant molecules (e.g., glutathione) homeostasis.Response of astrocytes to acute and chronic cellular stress and further progressive reactive astrogliosis can result in a release of toxic factors, directly supporting neuronal injury and dysfunction (Rizor et al., 2019).
Astrocytes and α-Synucleinopathies
Astrocytic failure appears to precede various forms of neurodegenerative pathologies. For example astrocytes are involved in the neuropathology of PD via augmentation of oxidative and nitrosative stress. The studies using PD transgenic (tg) animal models and post-mortem analysis of PD patient’s brains revealed astrocyte activation and ROS/RNS elevation as a substantial hallmark of the PD-associated pathology (Rizor et al., 2019).
An improper folding of disease-specific proteins, leading to neuronal damage, is the major feature of individual neurodegenerative disorders. α-Synuclein (αSYN) protein is present in the presynaptic terminals, and, under normal physiological conditions, plays a role in maintaining vesicular trafficking and SNARE complex formation. In the PD, αSYN undergoes incorrect folding, what results in its aggregation and formation of Lewy bodies (Lashuel et al., 2013). αSYN is the main component of the neuropathological lesions present also in some other disorders, collectively known as α-synucleinopathies, that include, apart from PD, also dementia with Lewy bodies, multiple system atrophy(Wegrzynowicz et al., 2019). Aggregation of αSYN is directly responsible for the disruption of dopaminergic and cholinergic neurotransmission and further cell death in PD. The exact mechanisms responsible for improper folding of αSYN remain to be clarified. Recent studies suggest that αSYN aggregates can spread between cells and tissues using a prion-like selfpropagation mechanism (Figure 1). It is well established that aggregated αSYN are present in the cytoplasm of astrocytes in human PD brain, what could suggest a role of these cells in the spreading of αSYN pathology. Indeed,in vitrostudy revealed that primary astrocytes can take up aggregated αSYN secreted from co-cultured SH-SY5Y human neuroblastoma cells The accumulation of pathological αSYN deposits in astroglia promotes, in turn, a secretion of chemokines and proin flammatory cytokines (e.g., IL-1, IL-6, TNFα; Song et al.,2009).
Astrocytes and Tau-Dependent Neurodegeneration
Under physiological conditions, tau protein is present in neurons as a soluble microtubule-associated protein, where it plays important functions in neurogenesis, cytoskeleton stabilization, axonal maintenance, and axonal transport(Spillantini and Goedert, 1998). Besides, tau may also interact with other cellular components, like cytoplasmic organelles,plasma membrane, actin cytoskeleton and nucleus (Nunez and Fischer, 1997). In the adult human brain, six tau isoforms are expressed, as a result of alternative splicing of exons 2, 3 and 10 of the tau gene,MAPT, linked with the 17q21-2 locus.Under pathological conditions, an excessive dissociation of tau from the microtubules leads to accumulation of unbound, misfolded tau in the cytosol, resulting in its hyperphosphorylation and aggregation. Misfolded and hyperphosphorylated tau aggregates into paired helical filaments what leads to the formation of more complex structures - neuro fibrillary tangles (NFTs). Abnormal assembly of tau protein is found in several human neurodegenerative diseases, known collectively as tauopathies. The presence of NFTs is a common feature of AD, frontotemporal dementia with parkinsonism linked to chromosome 17 (FTDP17) and several other disorders (Spillantini and Goedert, 1998).Identification of FTDP17T-related mutations in MAPT gene responsible for the disease pathogenesis through the misfolding and aggregation of mutated tau protein allowed to develop transgenic cellular and animal models of tau pathology (Spillantini and Goedert, 2013). The most common FTDP-17-related mutations (e.g., P301S or P301L),in transgenic mouse models, result in motor and cognitive dysfunctions correlated with age- and gene dose-dependent accumulation of NFTs (Lewis and Dickson, 2016). Although misfolded and dysfunctional tau was proven to be a primary factor in the development of tauopathies, the cellular mechanisms involved in tau-related neurodegeneration are still poorly understood. Astrocyte activation, reactive gliosis and dysfunction of glial cells are common for almost all human neurodegenerative disorders (Mohn and Koob,2015). In the tauopathies, both in the patients and inin vivomodels, astrogliosis is present in brain regions affected by the pathological process. Astrogliosis is found before neuronal loss, what may suggest a contribution of astrocytic pathology in the development of tau-related disorders. Direct evidence for astrocytic dysfunction was provided by the experiment,where transplantation of exogenous, neuron precursor cell-derived astrocytes resulted in significant reduction of neurodegenerative phenotypes in mice expressing P301S mutant tau under control of the neuronal Thy1.2 promoter.This indicates that endogenous astrocytes in the tauopathy are deprived of their neuroprotective function and/or may gain novel neurotoxic properties (Hampton et al., 2010).
A recent study with tg P301S mice expressing mutant human 4R/0N tau isoform revealed that transplantation of astrocytes prevents death of cortical neurons, suggesting that the endogenous astrocytes in tauopathy lose their neurosupportive properties, and instead intensify the neuropathological processes. A study usingin vitroco-culture systems demonstrated that primary cortical astrocytes or astrocyte-conditioned medium (ACM) from wild type mice have neuroprotective properties that are signi ficantly reduced in astrocytes or ACM from P301S mice (Sidoryk-Wegrzynowicz et al., 2019). Furthermore, ACM from tau mutant mice significantly decreased the expression of presynaptic and postsynaptic markers (synaptophysin and PSD95, respectively)in cortical neuronal cultures, whereas wild-type mouse ACM increased the expression of these proteins. This negative effect on neuronal viability and function was found for astrocytes derived from transgenic animals at the age when no tau pathology is yet observed in neuronsin vivo. This indicates that some pathological alterations precede tau aggregation in tauopathy, and that astroglia failure might be related to the manifestations of neuronal mutant tau toxicity at the early,pre-aggregation stage, before the onset of the disease. Such a loss of neurosupportive function was also confirmed for the astrocytes cultured from another established transgenic model of tauopathy, mice expressing human P301L 2N4R tau, speci fically in neurons, indicating that these findings can be generalized as being a common symptom of tau-related pathology. The same study also revealed that astrocytes in tau mutant mice exhibit altered phenotype compared to control animals already at early, presymptomatic stage. P301S mice were characterized by increased expression of astrocytic structural markers - GFAP and S100β in the cortex, con firming ongoing astrogliosis. In contrast, the cortical levels of astrocytic proteins involved in neuronal support, particularly related to the GGC (GS, GLAST, and GLT1) were decreased (Sidoryk-Wegrzynowicz et al., 2019). Similarly, signi ficant abnormalities in the expression of astrocytic proliferation markers and GGC components were found in the primary astrocyte cultures derived from P301S tau mice. These findings indicate that astrocytes from tau mutant mice, bothin vivoandin vitrogain a pathological phenotype beginning from an early postnatal stage what contributes to the tau-related neuropathology also in the adulthood (Sidoryk-Wegrzynowicz et al., 2019).
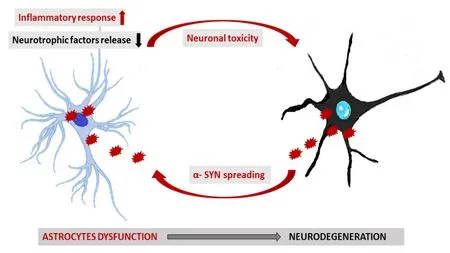
Figure 1|A scheme of proposed contribution of astrocytes to neurodegeneration in α-synucleinopathy.
Astrocytes and Tau Spreading
Under physiological conditions astrocytes do not express tau protein (Sidoryk-Wegrzynowicz et al., 2019). However, in some tauopathies, abnormal accumulation of tau protein is not only restricted to the neurons, but is also found in glial cells. While in AD, tau is aggregating in neurons, in the other tauopathies,including corticobasal degeneration (CBD) or progressive supranuclear palsy, tau protein is found in astrocytes and oligodendrocytes. A recent study shows that glial tau pathology can be reproduced in animal models of tauopathies by injection of brain homogenates derived from AD or FTLD-patients (Forrest et al., 2019). Most recently, pathological tau species, including astrocytic plaques or globular astroglial inclusions were found in several morphological types of pathological, tauopathy-related astrocytes, like: tufted,ramified, thorn-shaped, or granular astrocytes. Tufted astrocytes are characterized by symmetric tau inclusions in the proximal processes and are pathological hallmark lesions in progressive supranuclear palsy. Tau-immunopositive globular cytoplasmic deposits in proximal astrocytic processes are observed in the cortex in the globular glial tauopathy cases(Kovacs et al., 2017). Astrocytes with tau inclusions in distal processes are a distinguishing neuropathological hallmark of CBD (Forrest et al., 2019).
Growing body of evidence suggests that tau pathology spreads between neurons using a prion-like self-propagation mechanism, but also that the aggregated tau species are transferred from neurons to the other CNS cells (Yamada and Hamaguchi, 2018). A study revealed that cellular uptake of vesicle-bound or free tau may involve several cellular pathways(e.g., clathrin-dependent endocytosis, micropinocytosis, or direct membrane fusion). Interestingly, expression of the bridging integrator-1 gene that negatively regulates clathrindependent endocytosis, is oppositely correlated with tau pathology, suggesting that clathrin-dependent endocytosis contributes to tau translocation and pathology (Calafate et al., 2016). The other study revealed that tau inclusions are translocated to the between cells through heparan sulfate proteoglycans-dependent mechanism (Yamada and Hamaguchi,2018). More recently, the lysosomal pathway was suggested as a mechanism responsible for fibrillar tau internalization by the astrocytes (Martini-Stoica et al., 2018). Given that fibrillar and aggregated tau, is considered as a major histopathological hallmark of tauopathies, it is worth noting that the uptake of monomeric forms of the protein by astrocytes may be also involved in the toxicity and spreading of the disease (Falcon et al., 2017). Although the mechanism by which monomeric tau is taken up by the astrocytes is still unknown and deserves further investigation, the recent study suggests that it may be independent from HPSGs-mediated mechanism (Perea et al., 2019). Notably, recent studies confirmed tau spreading in the brains of AD patients, showing tau seeding between synaptically connected brain regions before the occurrence of advanced tau pathology (DeVos et al., 2018).
Neuroin flammation and Neurodegeneration
Microglia are derived from the macrophage cell lineage and account for about 12% of the cells in the CNS. These cells regulate several processes during both development and adulthood (Ransohoff and El Khoury, 2015). They are involved in a wide variety of functions, including removal of pathogens;phagocytosis of apoptotic cells and cellular debris; secretion of the growth factors, pro-inflammatory and anti-inflammatory signaling; as well as remodeling and elimination of synapses(Michell-Robinson et al., 2015). Microglia-derived factors are critical for the regulation of host response to in flammation -an important feature of regeneration and repair (Bolós et al.,2017). In a chronic condition, however, the prolonged state of in flammation is disruptive (Augusto-Oliveira et al., 2019).
It is well established that microglial dysfunction contributes to neurodegeneration (Bolós et al., 2017). Single-cell transcriptomic analyses in AD and other neurodegenerative diseases, like amyotrophic lateral sclerosis, revealed alterations in the expression of several genes involved in microglia functions as the disease progresses (Mathys et al., 2017). A hyperphosphorylated tau protein negatively affects neuronal function and promotes the pro-inflammatory activity of microglia, which, in turn, triggers AD pathology. A recent study revealed an aberrant exposure of phosphatidylserine on the outer surface of tau inclusion-positive neurons derived from P301S mice. Interestingly, co-culturing with microglial cells(BV2 cell line or primary microglia) led to the phagocytosis of these phosphatidylserine-exposing neurons through the mechanism dependent on the release of milk-fat-globule EGF-factor-8 and nitric oxide from the microglial cells. Furthermore,increased expression of milk-fat-globule EGF-factor-8 was found to be restricted to the tau inclusion-enriched areas of the brain in the transgenic P301S tau mice and different human tauopathies, that suggested a common mechanism of cell death in the tauopathies (Brelstaff et al., 2018).
More recently, an interest in the involvement of in flammationinduced reactive astrocytes in the neuronal pathology is increasing in the context of neurodegenerative diseases(Liddelow et al., 2017). A recent study has shown that abnormalities in the astrocytic functions contributing to the propagation of inflammation within the CNS are associated with the activation of microglia (Liddelow and Barres,2017). Microglia in pathological, neurodegeneration-related conditions activate astrocytes by secreting several proinflammatory mediators such as nitric oxide and cytokines(e.g., IL-1β, TNF-α, IL-6) what causes neuroinflammation.So called type-1 astrocytes (A1; nomenclature analogous to M1 state of microglial activation), once activated by microglia become neurotoxic and negatively affect neuronal functions by secreting proin flammatory cytokines: IL-α, TNF-α and C1q. Recent evidence suggests that the polarization of astrocytes to A1 phenotype results in a loss of their essential neuroprotective properties (Figure 2). For example,dysfunctional activation of astrocytes in a mouse models of AD impairs neuronal survival through activation of microglia(Sadick and Liddelow, 2019). Identi fications of the complement component 3 overexpressed specifically in A1 astrocytes allowed to identify a presence of active, neurotoxic astrocytes in different human neurodegenerative diseases including PD, AD, Huntington’s disease, amyotrophic lateral sclerosis or multiple sclerosis. These changes were observed in the speci fically affected brain regions in individual diseases (e.g.,caudate nucleus in HD, hippocampus and prefrontal cortex in AD, substantia nigra in PD, motor cortex in amyotrophic lateral sclerosis, and acute demyelinating lesions in multiple sclerosis). Anin vitrostudy, using retinal ganglion neurons cultured with A1 astrocytes, indicated that the neurons developed significantly less synapses compared to neurons cultured with control astrocytes suggesting that A1 astrocytes disassembled or failed to maintain the synapses. Furthermore,A1 astrocytes were found to release a soluble toxin that causes death of neurons and mature oligodendrocytes most likely via the induction of the apoptosis (Liddelow et al., 2017).
Given that glia is functioning as a key regulator of in flammation in the CNS (Clarke and Liddelow, 2017), modifications of these cells may help to prevent pro-inflammatory events.For example, minocycline, an antibiotic implicated in antiinflammatory response reduces the number of activated astrocytes in the cortex of transgenic mice expressing human tau, as identified by GFAP immunoreactivity and astrocytic morphological changes (Garwood et al., 2010). Minocycline administration to relatively young mice expressing human tau not only reduces astrogliosis but also decreases several pro-inflammatory mediators, what strongly correlated with the phosphorylation of tau at Ser396/404 in the cortex. In addition, minocycline treatment reduces the development of disease-associated aggregated tau species in the mouse model of human tauopathy (Noble et al., 2009). Altogether,identi fication of novel cytokine targets may help to establish novel therapeutic approach against AD and other tau-related neurodegenerative diseases.
Concluding Remarks
Growing body of evidence suggests heterogeneity of the processes related to the neuronal dysfunction and death observed in the neurodegenerative diseases. The major mechanism leading to neurodegeneration involves disruption of the astrocyte-neuron integrity. Recent studies additionally indicate the importance of microglia-astrocytic crosstalk at different stages of the diseases. The influence of microglia may shape the astrocytic response under neuropathological conditions. Understanding the mechanisms associated with astrocyte function/dysfunction may pave the way for the new, specific, glia-targeted therapeutic strategies against neurodegenerative disorders.
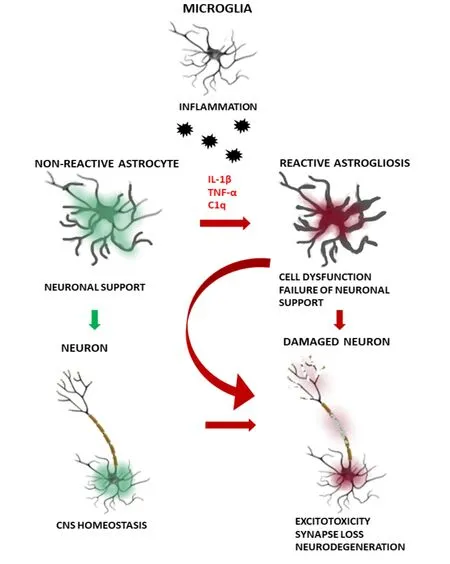
Figure 2|A scheme of the proposed impairment of astrocyte-neuronmicroglia interactions in the neurodegenerative diseases.
Author contributions:Manuscript conception, preparation of the manuscript draft: MSW; supervision of the manuscript, figure preparation and the manuscript design: LS. Both authors read and approved the final manuscript.
Con flicts of interest:The authors declare no con flicts of interest.
Financial support:This work was supported by statutory funds provided by the Polish Ministry of Science and Higher Education for Mossakowski Medical Research Centre Polish Academy of Sciences, Warsaw, Poland (9/2018, to LS).
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
 中國(guó)神經(jīng)再生研究(英文版)2021年2期
中國(guó)神經(jīng)再生研究(英文版)2021年2期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Role of apoptosis-inducing factor in perinatal hypoxic-ischemic brain injury
- In flammation/bioenergetics-associated neurodegenerative pathologies and concomitant diseases: a role of mitochondria targeted catalase and xanthophylls
- Potential therapeutic effects of polyphenols in Parkinson’s disease: in vivo and in vitro pre-clinical studies
- Possible implications of dysregulated nicotinic acetylcholine receptor diffusion and nanocluster formation in myasthenia gravis
- Hydrogel-based local drug delivery strategies for spinal cord repair
- Altered physiology of gastrointestinal vagal afferents following neurotrauma
