In flammation/bioenergetics-associated neurodegenerative pathologies and concomitant diseases: a role of mitochondria targeted catalase and xanthophylls
Natalia V. PozdnyakovaVasily V. Vorobyov
Abstract Various inflammatory stimuli are able to modify or even “re-program” the mitochondrial metabolism that results in generation of reactive oxygen species. In noncommunicable chronic diseases such as atherosclerosis and other cardiovascular pathologies, type 2 diabetes and metabolic syndrome, these modifications become systemic and are characterized by chronic inflammation and, in particular, “neuroinflammation” in the central nervous system. The processes associated with chronic inflammation are frequently grouped into “vicious circles” which are able to stimulate each other constantly amplifying the pathological events. These circles are evidently observed in Alzheimer’s disease, atherosclerosis, type 2 diabetes, metabolic syndrome and, possibly,other associated pathologies. Furthermore, chronic inflammation in peripheral tissues is frequently concomitant to Alzheimer’s disease. This is supposedly associated with some common genetic polymorphisms, for example, Apolipoprotein-E ε4 allele carriers with Alzheimer’s disease can also develop atherosclerosis. Notably, in the transgenic mice expressing the recombinant mitochondria targeted catalase, that removes hydrogen peroxide from mitochondria, demonstrates the significant pathology amelioration and health improvements. In addition, the beneficial effects of some natural products from the xanthophyll family, astaxanthin and fucoxanthin, which are able to target the reactive oxygen species at cellular or mitochondrial membranes, have been demonstrated in both animal and human studies. We propose that the normalization of mitochondrial functions could play a key role in the treatment of neurodegenerative disorders and other noncommunicable diseases associated with chronic inflammation in ageing. Furthermore,some prospective drugs based on mitochondria targeted catalase or xanthophylls could be used as an effective treatment of these pathologies, especially at early stages of their development.
Key Words: algae xanthophylls; Alzheimer’s disease; atherosclerosis; depression; type 2 diabetes; metabolic syndrome; mitochondria-targeted catalase; noncommunicable chronic diseases; stress
Introduction
Noncommunicable chronic diseases (NCDs), associated with cardiovascular, metabolic, neurological, diabetic,rheumatologic, and neurodegenerative pathologies, began to be fundamental medical problems of the 21stcentury(Bousquet et al., 2011). A leading role of the cardiovascular diseases (CVDs) in mortality rate in the human population seems to arise from insufficient measures to prevent CVDs through the healthy lifestyle, underestimation and poor assessment of risk factors for early diagnosis during asymptomatic phase of CVDs (Cohn et al., 2003). Furthermore,most ageing people with CVDs suffer from several diseases,which are frequently etiologically related. The risk factors for NCDs development are age, genetic predisposition, high arterial blood pressure, dyslipidemia, insulin resistance,overweight, smoking, poor nutrition, low level of physical activity, excessive amount of alcohol consumption. Thus,NCDs are caused by complex interactions between genetic,nutritional, stressful and environmental factors affecting people throughout their lives. The key role in NCDs development is chronic inflammation (frequently termed as“l(fā)ow grade chronic inflammation”) with systemic damage to either the whole organism or speci fic organs/tissues.
Inflammatory processes are mediated by various immune cells, in particular, by differentially activated M1 and M2 macrophages triggering either pro-in flammatory or resolution processes, respectively (Mescher, 2017; Oishi and Manabe,2018). In chronic inflammation, M1/M2 balance is biased towards the prevalence of M1 macrophages targeting the potential “threat” that instead of foreign microorganisms or antigens now becomes its own “host” cells and/or proteins (Liu et al., 2014; Parisi et al., 2018). Usually, this is accompanied by in filtration of activated macrophages into in flamed internal organs. In the atherosclerosis, the macrophages infiltrate the walls of large blood vessels and capture the oxidized low-density lipoproteins which results in their subsequent transformation into “foamy cells” (Fadini and Ciciliot, 2014;Tabas and Lichtman, 2017). In diabetes of both types (1 and 2),the immune cells attack insulin-producing pancreatic beta-cells(McDevitt, 2005; Burrack et al., 2017; Zhou et al., 2018; de Candia et al., 2019). In metabolic syndrome, the macrophages infiltrate the visceral fat and can attack adipocytes (Lumeng et al., 2007; McNelis and Olefsky, 2014; Ieronymaki et al.,2019). In chronic alcoholism and non-alcoholic fatty liver disease, the macrophages attack the hepatocytes triggering the fibrosis (Ju and Mandrekar, 2015; Cha et al., 2018). In the inflammatory bowel disease, macrophages destroy the intestine (Na et al., 2019). One of the hallmarks of Alzheimer’s disease (AD) is the extracellular beta-amyloid (Aβ) in the brain, which is able to activate the microglia (Klegeris et al.,1994) that triggers the “vicious circle” of neuroin flammation(Cai et al., 2014). Autoimmune reactions in the central nervous system (CNS) target the variety of own proteins of the organism: for example, myelin (in autoimmune demyelinating polyneuropathies and multiple sclerosis), synaptic proteins of the extracellular matrix leucine-rich-glioma-inactivated 1 and contactin-associated protein 2 (in autoimmune encephalitis),glutamate decarboxylase 2 and pro-insulin (in diabetes) and others. The autoimmune reactions are characterized by high concentration of antibodies against the host proteins in the blood that in turn can activate myeloid cells and thereby establish the pro-inflammatory milieu negating the immune tolerance mechanisms (Suurmond and Diamond,2015). The peripheral macrophages can also infiltrate the brain as a consequence of ischemic stroke, epileptic seizure,autoimmune reaction and bacterial infection (Cox et al., 2013;Raj et al., 2015; Lopes Pinheiro et al., 2016; Varvel et al., 2016;Rajan et al., 2019). Hence, the peripheral macrophages in conjunction with the brain resident microglia can orchestrate the processes of neuroinflammation in the brain (Fischer and Reichmann, 2001; Sevenich, 2018). In particular,ex vivolabelled peripheral macrophages have been shown to cross the blood-brain barrier (BBB) and attack Aβ plaques together with residential microglia in AD animal models (Gate et al.,2010; Lebson et al., 2010).
Search Strategy and Selection Criteria
Literature research was performed using PubMed, Google and Google Scholar databases using the following combination of keywords: “Alzheimer’s disease” OR “neurodegenerative diseases” OR “atherosclerosis” OR “type 2 diabetes” OR“metabolic syndrome” AND “inflammation” OR “chronic in flammation” OR “concomitant diseases” OR “mitochondrial functions” AND “mitochondria-targeted catalase” OR“xanthophylls” OR “algae xanthophylls” OR “oxidative stress”O(jiān)R “photosynthesis and oxidative stress” until February 2020. The studies identified were further screened using the following inclusion criteria: studies in animals, humans,algae and sometimes plants (i), articles and studies written in English (ii) that had available abstracts and/or full texts (iii).
Mitochondrial Dysfunction and Metabolism
The chronic inflammation in NCDs is characterized by mitochondrial dysfunctions. Disturbances in mitochondria functioning are usually manifested with the oxidative stress(OS) which is expressed in the reactive oxygen species (ROS)accumulation in affected cells. OS is a characteristic of chronic inflammatory processes and observed in liver diseases(Satapati et al., 2015, 2016; Mansouri et al., 2018), metabolic syndrome (Bhatti et al., 2017), atherosclerosis (Madamanchi and Runge, 2007; Peng et al., 2019), and type 2 diabetes(De Felice and Ferreira, 2014). Genetic abnormalities in mitochondria were demonstrated in the in flammatory bowel disease (Wang et al., 2013; van Tilburg et al., 2014) and mitochondrial dysfunctions were revealed in AD (Moreira et al., 2010; Garcia-Escudero et al., 2013; De Felice and Ferreira,2014; Swerdlow, 2018).
The studies of mechanisms of intracellular inflammatory processes indicate that the mitochondrial “dysfunction” is a natural reaction caused by the infections or trauma/stress.OS has been directly demonstrated to be a component of the immune response. In particular, the immune cells have been shown to accumulate the hydrogen peroxide that is“programmed” by impaired function of mitochondrial complex I and III (Chandel et al., 2000; Zmijewski et al., 2008, 2009).OS is also characterized by the reduction of enzymatic activity of pyruvate decarboxylase and alpha-keto-dehydrogenase that is associated with impairments in the Krebs cycle functioning (van Horssen et al., 2019) and decreased glutathione peroxidase activity resulting in hydrogen peroxide accumulation (Soto et al., 2014). The in flammation-associated alterations are essential for ROS generation and activation of the inflammasome complex (Tschopp, 2011). They are accompanied by energy deficit in the cells and in turn by greater mitochondrial dysfunctions that inevitably “push” the cells into a vicious in flammatory circle (Lopez-Armada et al.,2013; van Horssen et al., 2019).
Interestingly, the high-fat diet, so-called “the calorie bomb”, is known to attenuate the glucose intake from the extracellular medium and increase both the lipid metabolism and its oxidation. This provokes the development of insulin resistance and can generate the conditions for the pathological lipid oxidation. This in turn provokes the mitochondrial dysfunction through the oxidation and degradation of mitochondrial membranes (Hernandez-Aguilera et al.,2013; Lopaschuk, 2016). In AD, the neuroinflammation is accompanied by the endocytosis of Aβ with its subsequent entry into the mitochondria. Highly toxic Aβ(1-42), which is more hydrophobic than the “normal” Aβ(1-40), enters into membranes and catalyzes the oxidation of unsaturated lipids (Butterfield et al., 2013). The bioenergetics profile disturbances (insulin signaling impairment, abnormal mitochondrial metabolism, metabolic biases towards glycolysis) are observed in fibroblasts from AD patients(Sonntag et al., 2017), and inhibition of both basic metabolism and glycolysis in astrocytes) may play substantial role in Aβ accumulation (Fu et al., 2015).
Residential Microglia/Macrophages and Chronic In flammation
Another important aspect of chronic inflammation is the activation of residential macrophages which are not derived from bone marrow monocytes. In particular, residential brain microglia is well known to be formed from erythro-myeloid fetal bladder precursors (Lenz and Nelson, 2018; Thion et al., 2018) and Kupffer cells, which are specialized hepatic macrophages originated from the embryonic yolk sac (Lobritto,2017) and they are capable for mitosis (Crofton et al., 1978).Functionally, Kupffer cells can also be in different states: proin flammatory M1 and resolving anti-in flammatory M2. There are several subtypes of M2 cells whose correct regulation may play a key role in inflammatory outcomes and thus the inflammation treatment (Dixon et al., 2013). The induced prevalence of M2 over M1 macrophages could promote the pro-inflammatory state downregulation. M1/M2 balance is dependent on genetics and environmental factors and de fines the pathogenesis development. Even in intracerebral pathologies (in particular, AD), when the brain is protected by the BBB from external toxicity, M1/M2 ratio in microglia could be dependent on dietary and traumatic external factors(Katsumoto et al., 2018).
In AD animal models the enhanced levels of both proinflammatory M1 microglia and alternatively activated M2 microglia are observed (Wang et al., 2015b). M2 microglia provides important mechanisms for neuroprotection:it secretes interleukin (IL)-10, which suppresses the inflammation and insulin-like growth factor 1, which has essential neurotrophic function (Gray et al., 2020). At early stages of neurodegeneration, the neuroprotective M2 microglia prevails, however over time, M1 microglia activity is increased that stimulates inflammatory processes.This in turn increases beta-secretase activity that initiates Aβ production (Sastre et al., 2008). Thus, the vicious circle begins: the inflammation triggers Aβ generation,Aβ interacts with microglia that amplifies the production of tumor necrosis factor (TNF)-α, IL-1β, IL-6, and IL-18 in favor of the inflammation. This circle is accompanied by hyperphosphorylation of tau protein, tau protein paired helical filament formation, that disrupts the axonal transport system in neurons, and, finally, the neuronal death (von Bernhardi et al., 2010). The initiation of the “vicious circle”can be associated with two features of capillary blood vessels:a) their endothelial cell ability to release pro-inflammatory cytokines, provoked by Aβ (Grammas and Ovase, 2001), and b)in filtration of the peripheral proin flammatory signals into the brain due to increased endothelial permeability observed in in flammation (Perry et al., 2007; Holmes et al., 2009; Kamer,2010). Proin flammatory cytokines can pass through disturbed BBB, whereas chronic inflammation in turn increases its permeability even more (Varatharaj and Galea, 2017).Thus, peripheral macrophages infiltrate into the brain and residential M1 microglia orchestrates further development of inflammatory reactions and neurodegeneration in AD(Sevenich, 2018). This explains why AD could be associated with prolonged chronic in flammation in the peripheral organs linked with such concomitant diseases as atherosclerosis,diabetes, metabolic syndrome (MS), and chronic infections.
Apolipoprotein-E ε4 Associated Genetic Problem
Genetic predisposition, stress, inappropriate lifestyle and age-related pathological processes have been shown to be the main factors initiating and supporting the development of chronic inflammation in NCDs (Hernandez-Aguilera et al.,2013). The particular interest is in common genetics among different diseases. One typical example is polymorphisms in the APOE gene encodingApolipoprotein-E ε4(APOE4)allele which is associated with the worst variant APOE as a cholesterol carrier. Human beings with APOE4 allele have significantly enhanced risk of AD development (25-65% of the “l(fā)ate onset” of AD at age of above 65 years, https://www.ncbi.nlm.nih.gov/books/NBK1161/). The same APOE4 allele increases the risk of the atherosclerosis by 4-14 times that depends on the presence of one or two copies of the allele(Mahley, 2016). The APOE knock-out mice are very sensitive to the atherosclerosis-provoking factors making this mouse line as a very usable genetic model of atherosclerosis and CVDs (Lo Sasso et al., 2016). Atherosclerosis and CVDs are dominant in the mortality list from the “developed” countries.In Europe, according to the World Health Organization, APOE4 carriers are in the cohort of patients associated with 40-75%of deaths from CVDs (Abondio et al., 2019).
Thus, the genetic polymorphisms which are considered as pathogenic in conjunction with chronic inflammation can provide the basis for the malfunction of organs/tissues and consequently their degeneration or failure. CVDs,atherosclerosis, arterial hypertension, obesity, type 2 diabetes,inflammatory diseases of the liver and intestines are factors associated with common mechanisms of pathogenesis based on the chronic in flammation processes.
Alzheimer’s Disease and Concomitant Diseases
Atherosclerosis and chronic in flammation
Atherosclerosis is probably the most important concomitant disease related to AD. Dyslipidemia results in accumulation of atherosclerotic plaques in the walls of large blood vessels that sooner or later may result in endothelial rupture and generation of the blood clots (Hess and Grant, 2011)raising the risk of heart attacks and/or strokes. Lipid driven immune-metabolic responses are associated with OS and subendothelial infiltration of oxidized lipoproteins followed by the migration of activated macrophages (Gistera and Ketelhuth, 2018). Activated macrophages absorb the oxidized lipoproteins followed by their transformation into the “foam cells” and atherosclerotic plaque formation. Dyslipidemia is triggered in the liver, one of its main functions is to control the cholesterol release into the bloodstream via very low density lipoproteins. Very low density lipoproteins deliver the cholesterol to organs and tissues where it is transformed to low density lipoproteins (LDL) for subsequent return into the liver. This process is controlled by the subtilisinkexin type 9 proprotein convertase (PCSK9) which allocates low density lipoprotein receptors (LDLR) from cell surface for the endocytosis in hepatocytes (Lusis et al., 2004).PCSK9 binds to LDLR resulting in LDLR endocytosis and degradation. Large amounts of serum PCSK9 prevent LDL from LDLR absorbance that provokes LDL accumulation in the blood consequently creating favorable advantageous conditions for their infiltration into the large vessel walls.Thus, impaired cholesterol recirculation in the blood and OS evoked by in flammatory processes potentiate synergically the atherosclerotic plaques formation. Interestingly, PCSK9 gene expression, which is regulated by the nuclear transcription factor 1 in the hepatocytes, is blocked by insulin (Glerup et al., 2017). On the other hand, the insulin signal was demonstrated to be blocked by the inflammation (McNelis and Olefsky, 2014). Thus, the chronic inflammation seems to be accompanied by high levels of PCSK9, whereas, the LDL recirculation in the liver is deactivated because of LDLR endocytosis and degradation. The modern treatments for atherosclerosis include therapeutic antibodies against PCSK9(e.g., Evolocumab or Alirocumab) which inactivate PCSK9 and consequently attenuate the pathological process (McDonagh et al., 2016). However these antibodies do not necessarily stop the inflammation. One of “traditional” approaches oriented on prevention of LDL accumulation in the blood via the inhibition of cholesterol synthesis in the liver by statins(Tian et al., 2012). However, it results in lots of side effects including pancreatitis (Jones et al., 2015), that can seriously affect patient life quality.
In some studies, PCSK9 is indeed considered as an indicator of inflammation because it induces the pro-inflammatory responses in macrophages (Ricci et al., 2018). It also promotes both Toll-like receptor (TLR) 4 expression and TLR4/NF-kappa B genetic pathway activation (Tang et al., 2017). Upregulation of TLR2 and TLR4 expression is consistent with increased serum lipids (Zhu et al., 2015). Local overexpression of TLR2/TLR4 in the vessel walls can induce the atherosclerotic plaque formation (Shinohara et al., 2007). Moreover, significant local PCSK9 expression increase was demonstrated in the endothelial cells inside of the atherosclerotic plaques (Tang et al., 2017). Thus, in chronic in flammation, the loop of TLR2/TLR4 and PCSK9 activation/expression can create the vicious circle that leads to permanent LDLR degradation on the hepatocytes surface and persistent increase of LDL level in the blood. Interestingly, people with impaired PCSK9 functions demonstrate lower levels of both LDL cholesterol and risk to develop the coronary heart disease (Kent et al., 2017).Unfortunately, they could be more susceptible to infectious diseases and sepsis (Mitchell et al., 2019).
Cholesterol recirculation via LDLR is not only the mechanism of cholesterol reverse transport to the liver. Another mechanism is associated with high density lipoproteins(HDLs), that are dedicated for uptake by hepatocytes.However, in atherosclerosis, HDL synthesis is depressed and its secretion is driven by two ATP-binding cassette transporter subfamilies: A1 (ABCA1) and G1 (ABCG1). The expressions of ABCA1 and ABCG1 have been revealed to be inhibited by the in flammation (Soumian et al., 2005; Yvan-Charvet et al., 2010)meaning that additional mechanism of excessive accumulation of cholesterol in the blood is possible.
As mentioned above, the most frequent treatment of atherosclerosis is associated with the use of statins that,unfortunately, produces many side effects (Bellosta and Corsini, 2018; Taylor and Thompson, 2018; Toth et al., 2018).More advanced approaches are based on the treatments with the use of PCSK9 specific inhibitors, Evolocumab or Alirocumab (McDonagh et al., 2016). These artificial antibodies are effective, however, they are expensive. Below,some potentially effective alternatives will be discussed.
Type 2 diabetes mellitus
Type 2 diabetes mellitus (T2DM) is characterized by inability of peripheral tissues to absorb the blood glucose in sufficient amounts despite hyperglycemia and hyperinsulinemia. The appearance of this condition is linked to chronic in flammation.Mechanisms of the insulin resistance include the signaling by the TNF-α in combination with activated TLR2 and TLR4,which can initiate phosphorylation of the insulin receptor 1/2 substrate blocking and in turn the insulin signaling in tissues and thus the glucose transport through the cell membrane(McNelis and Olefsky, 2014). The key role of obesity in T2DM is explained by the senescence of the fat tissue and formation of “necrotic” adipocytes which secrete TNF-α. This results in accumulation of pro-inflammatory M1 macrophages in adipose tissue facilitating even more TNF-α release (Esser et al., 2014) and thereby amplifying the inflammation. These processes form a vicious circle of chronic in flammation that is expected to be a main cause of T2DM.
The insulin resistance in the hypothalamus may result in the appearance of a permanent feeling of hunger that is controlled by the hypothalamic arcuate nucleus. The insulin resistance caused by prolonged free fatty acid exposure may end up even with gliosis in hypothalamus that completely disrupts the insulin regulation and its transport into CNS (Ono,2019). In spite of inflammation development in the adipose tissue the process of lipogenesis and fat accumulation is continued, and the lipogenesis clearly prevails over lipolysis(Kahn and Flier, 2000). Chronic in flammation inhibition could be bene ficial for the unblocking of insulin signaling that may ameliorate the described pathologies. This mechanism is expected to work until the critical degradation of beta cell population in the pancreatic Langerhans islets is reached. It should be mentioned, however, that degeneration of the islets is triggered by the in flammation and usually accompanied by autoimmune reactions (Burrack et al., 2017).
T2DM therapies usually include the use of metformin which is considered as an optimal drug (Sanchez-Rangel and Inzucchi,2017), although the mechanism of its action is still under debate. Metformin is thought to reduce the blood glucose level via attenuation of its production by the liver. It should be mentioned, however, that some adverse effects of metformin were observed (Sanchez-Rangel and Inzucchi, 2017). Potentially effective substances for the treatment of T2DM, at least at early stages of its development, will be discussed below.
MS and chronic in flammation
MS is usually diagnosed when at least three of five criteria are met: 1) abdominal obesity, 2) arterial hypertension, 3)dysglycemia, 4) hypertriglyceridemia and 5) low level of HDL.In developed countries, the prevalence of metabolic syndrome among people over 30 is 10-20%, in the United States - 34%(44% among people over 50; Ford et al., 2002). Among the MS criteria, the enhanced blood pressure seems to be crucial(Sharma, 2011; Patel and Patel, 2015; Bhatti et al., 2017),whereas among many reasons to develop this symptom,increased activity of the sympathetic nervous system is likely dominant (Johnson et al., 2012). MS is considered as a“precondition” for the number of serious pathologies: CVDs,atherosclerosis, T2DM (Bhatti et al., 2017) and chronic renal failure (Prasad, 2014). Substantial proportion of AD patients has been shown to suffer from MS (Vanhanen et al., 2006;Razay et al., 2007).
The mechanisms of MS are not completely understood. The senescence of fat tissue is expected to initiate MS (Palmer et al., 2019) that can explain the existence of overweight people with healthy liver, normal blood pressure, however, having no MS. One of the trigger mechanisms in the inflammatory processes seems to be associated with TLR4 that is activated by Fetuin-A protein (Alpha 2-HS Glycoprotein) in the presence of “free fatty acids” (FFA, Pal et al., 2012). The Fetuin-A protein, which is very important for embryonic development,is generated in the liver. Furthermore, its expression has been shown to persist up to adulthood resulting in substantial protein accumulation in the adipose tissue in T2DM patients,in particular (Khadir et al., 2018). The Fetuin-A/FFA complex activates the TLR4 receptor in adipocytes that in turn triggers the insulin resistance (Pal et al., 2012). Finally, activation of TLR4 on macrophages triggers the inflammatory reactions(McNelis and Olefsky, 2014). FFA amount is controlled by liver,and FFA accumulation in the blood can be evoked by high-fat diets (Liu et al., 2016) resulting in dyslipidemia even in patients with normal weight (Hojland Ipsen et al., 2016). As mentioned above, the fat accumulation in obesity is associated with the appearance of necrotic adipocytes, chronic in flammation and hyperglycemia (Welty et al., 2016), whereas, the hypertension is linked with the sympathetic nervous system activation and triggered by the insulin resistance (Johnson et al., 2012) and,in part, by permanent stress (Hamer and Steptoe, 2012).
Certain lifestyle and nutrition habits seem to be very important for MS development, although pharmacological approaches have many options for the treatment. They include the use of statins (lipid profile), aspirin (as nonsteroid anti-inflammatory drug) and several classes of drugs that lower the arterial blood pressure. Nevertheless,dietary modifications and regular physical exercises are more preferable. Statins, aspirin and angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers should be prescribed as adjuncts rather than alternatives (Sherling et al., 2017). In particular, the dietary impact could be effective by the consumption of products with some natural plant substances like flavonoids (polyphenols) which are characterized by enhanced antioxidant activity (Duluc et al.,2012). Effects of dietary inclusion of flavonoids and some vitamins on MS are reviewed in de la Iglesia et al. (2016).One of the natural functions of flavonoids is the regulation of respiration in plants (Shimoji and Yamasaki, 2005). Given this, there is no wonder that flavonoids can also regulate the energetic process by the inhibition of mitochondrial functions in mammals (Dorta et al., 2005), that explains their apoptotic effects on some actively dividing cancer cells (Abotaleb et al.,2018) and inhibitory in fluences on the in flammation (Sera fini et al., 2010).
Several other potentially effective substances of different origin and specific efficacies towards mitochondria will be discussed below.
AD and chronic in flammation
AD and atherosclerosis share the same genetic basis (in particular, APOE ε4 allele), that seems to be a characteristic for the most of AD and atherosclerosis cases associated with chronic inflammation and vascular pathologies. AD is characterized by concomitant cerebral amyloid angiopathy that is manifested by Aβ deposition on the outer vessels walls expressing proteoglycans Perlecan and Collagen XVIII with heparan sulfate chains, which collect excessive Aβ. Cerebral amyloid angiopathy produces mechanical damage to the vascular endothelium that can lead to hemorrhages and even more dramatic oxygen supply impairment (Grinberg et al., 2012). AD is characterized by neuroin flammation that is diagnosed post-mortem as gliosis. Unfortunately, existing anti-in flammatory drugs for AD are ineffective and traditional corticosteroid therapy can aggravate the disease making it even worse (Machado et al., 2014). The connections AD with atherosclerosis, T2DM and MS are important despite they may not be obvious at first glance as AD is rather isolated from periphery and developed behind BBB. However, AD development has been demonstrated to be very frequently accompanied by atherosclerosis (Lathe et al., 2014).Furthermore, one study demonstrated that the association rate of atherosclerotic deposits in the vessels of AD brains was about 77% (Yarchoan et al., 2012). The risk of AD development is also signi ficantly enhanced in parallel with development of both T2DM (De Felice and Ferreira, 2014) and MS (Vanhanen et al., 2006).
TLR4 receptors of the microglia have been demonstrated to mediate the main immune responses to aggregated Aβ in AD (Walter et al., 2007). Moreover, some mutations in TLR4 elucidated the role of neuroin flammation as a primary cause of AD. TLR4 D299G mutation is associated with the resistance to AD development due to attenuation of the TLR4 signaling pathway which is responsible for the production of proin flammatory interleukin IL-1 (Miron et al., 2019). TLR4 D299G is well known to be hyporeactive to the lipopolysaccharide(Arbor et al., 2000). Surprisingly, TLR4 D299G carriers are resistant to AD development, however, they are more sensitive to bacterial infections (Agnese et al., 2002; Genc et al., 2004),bacterial septic shock (Lorenz et al., 2002) and some viral infections (Tal et al., 2004). Interestingly, the same D299G mutation in TLR4 delays the development of atherosclerosis(Kiechl et al., 2002) and hepatic cirrhosis (Guo et al., 2009).Thus, hyporeaction in TLR pathways may be beneficial for the development of chronic inflammation, but can be lifethreatening in some infectious diseases. In any case, further studies need to be performed as TLR4 is not the only player in the cerebral immune responses.
To date, no effective approaches for AD treatment are known.The immune therapy studies produce only the disappointing results so far (Bachmann et al., 2019) despite the ample amount of available drugs and trials (Hampel et al., 2018).However, in our opinion, several prospective trials with nerve growth factor based therapy might be very promising.Generation ofex vivoautologous fibroblasts genetically modi fied to express the nerve growth factor were implanted in the brains of AD patients and resulted in abrogation of disease development (Tuszynski et al., 2005). However, clinical trials with NGF gene delivery using adeno-associated viral vectors did not show the real effectiveness (Ra fii et al., 2018) although post-mortem analysis of the brains from treated patients demonstrated the response to NGF expressed by adenoassociated viral vectors (Tuszynski et al., 2015). NGF treatment of AD has been studied for a long time, however, NGF/TrkA signaling has been revealed to activate nociceptive perception,in particular, in inflammation (Mizumura and Murase, 2015).In flammation-associated chronic pain could be eliminated by Tanezumab (Hefti, 2019), however, this additional treatment complicates the use of NGF therapy for AD.
Depression and chronic in flammation
Well-defined genetic polymorphisms in IL-1β, IL-6, MCP-1(CCL2), TNF-α and C-reactive protein are related with clinically expressed depression. The existence of these polymorphisms gives an additional immunity in those ethnic groups who live in an ancestral environment. However, the appearance of such mutations in people with modern lifestyles including so-called “Western Diet” makes them vulnerable that in turn triggers the development of the depression associated disorders (Barnes et al., 2017). Up to 80% cases of severe depression are associated with an excessive amount of glucocorticoids released by the adrenal glands into the bloodstream. At abnormally high level of glucocorticoids, the brain activity and synaptic plasticity are suppressed that is aggravated by impairment of the patient’s mood due to the release of corticotropin-releasing hormone, which activates the hypothalamic-pituitary-adrenocortical system (Anacker et al., 2011). Paradoxically, the systemic chronic inflammation can provide a resistance to glucocorticoids that is mediated by in flammasome resulting in the degradation of glucocorticoid receptors (Miller and Raison, 2016) and cessation of the depression. However, in certain patients, the systemic chronic inflammation can also act directly through the proinflammatory cytokines provoking the depression (Miller and Raison, 2016). Interferons, TNF-α, IL-1β and ROS affect the glial cells in the brain and impair the glutamate reuptake that may provoke the excitotoxicity. Pro-inflammatory cytokines can also impair the monoamine-mediated neuronal circuits which are associated with the mood regulation.These negative events can lead to anhedonia, anxiety and depression. Glucocorticoids are frequently used as the antiinflammatory drugs via mechanisms of both the multiple inflammatory gene suppression and regulation of the antiinflammatory gene transcription, however, the higher concentration of corticosteroids has been shown to produce side effects (Barnes, 2006).
It is sure that depression can aggravate the condition of an AD patient. However, it is possible to check it in experimental animal models only. Using the stress-level glucocorticoid administration protocol in a transgenic mouse model of AD, the synthetic corticosteroid dexamethasone has been shown to facilitate the development of both Aβ and tauprotein pathologies instead of expected suppression of the inflammation (Green et al., 2006). In another experimental model the transgenic mice Tg2576 (also model of AD),increased tau protein hyperphosphorylation, insoluble tau inclusions and neurodegeneration were observed, when mice were stressed for one month. These stress-associated changes have been shown to be prevented by an antagonist of corticotropin-releasing hormone (Carroll et al., 2011). More transgenic mouse models of AD also demonstrated aggravation of AD pathologies in stressful environments (Pedersen et al.,1999; Dong et al., 2004, 2008; Pedersen and Flynn, 2004; Lee et al., 2009). Thus, the evident synergic effect of systemic stress on AD development was demonstrated the number of times.That should be taken into account in clinical treatment of excessive in flammation by the use of glucocorticoids (Barnes,1998; Coutinho and Chapman, 2011; Liberman et al., 2018).It should be also mentioned that glucocorticoids are able to target both glucocorticoid receptors and mineralocorticoid receptors (MRs), whose activation can be equally effective (Reul et al., 1990). That explains many side effects of glucocorticoids.Notably, the prolonged activation of MRs can potentiate the senescence in both the fat tissue (Lefranc et al., 2019), kidney(Fan et al., 2011) and cardiovascular system (Gorini et al., 2019).The secretome of senescent cells is able to produce a local chronic in flammation (Newsholme and de Bittencourt, 2014),whereas, the targeting of the senescent cells has been shown to alleviate the obesity-induced metabolic dysfunction (Palmer et al., 2019). Effects of aldosterone, a natural agonist of MR,are manifested in the cardiovascular system by cardiac fibrosis and hypertrophy because of the persistent activation of reninangiotensin-aldosterone axis that leads to vasoconstriction,hypertension and, thus, to oxygen de ficit in tissues (Cannavo et al., 2018). MRs play an important role in obesity where the OS and mitochondrial dysfunction are revealed as well (Lefranc et al., 2018). Finally, another serious complication with excessive use of corticoids is associated with the major depressive disorder (Young et al., 2003; de Kloet et al., 2016; Canet et al.,2018).
In general, depression is a very complicated topic in terms of chronic in flammation. The whole aspects require the separate review, however, the most importantly that these conditions are frequently concomitant to other diseases with chronic inflammation and, hence, are able to trigger and accelerate their development. Interestingly, these conditions can be also treated with some anti-inflammatory agents and, thus, they could be supportive for some depressive people. This topic will be in detail discussed below.
Breaking the Vicious Circle of Chronic In flammation
We propose that the normalization of mitochondria functions could have a key role for treatment of all mentioned disorders and probably many others related to the chronic in flammation in ageing. Certain substances potentially useful for the mitochondrial OS treatment are described in the following sections.
The mitochondria-targeted catalase
Mitochondrial dysfunction and OS are the common hallmarks of most of the metabolic disorders and, thus, the treatments targeting the mitochondria could be potentially very useful(Bhatti et al., 2017). One of the most interesting avenues for the potential therapeutics was a generation of transgenic mice ubiquitously expressing a “mitochondria targeted catalase”(mCAT; Schriner et al., 2005). The open reading frame of this catalase contains a modified signal peptide: an original catalase peptide for peroxisomal localization was replaced by the peptide from ornithine transcarbamylase, that targets the protein to mitochondrial matrix. Thus, the targeted catalase is reallocated to mitochondria upon the translation.This genetic engineering produced a remarkable outcome:the transgenic mice with mitochondria targeted catalase demonstrated substantially increased life span whereas those with nucleus targeted catalase had no health impact. This argues with the statement that “DNA damage contributes to aging”, especially with the development of neurodegenerative diseases (Maynard et al., 2015). In particular, in many familial cases of amyotrophic lateral sclerosis (ALS) the enzyme superoxide dismutase 1 (SOD1) has been shown to have the“gain of function” mutations (Kaur et al., 2016). SOD1 is well known to convert O2-into H2O2and it is expressed in both nucleus and mitochondria; hence, the increased expression and activity of SOD1 will ultimately lead to H2O2accumulation in mitochondria. Thus, these defects in familial ALS with SOD1 mutations seem to be predominantly associated with accumulated damages and harmful structural changes in mitochondria (Son and Elliott, 2014).
mCAT transgenic mice had the delayed developments of cardiac pathology and cataract and reduced ROS production due to aconitase inactivation. It supports the “free radical theory” of ageing and reinforces the importance of mitochondria as a source of free radicals. The transgenic mice were tested in several models of chronic in flammation-related diseases and demonstrated the substantial improvement of cardiac ageing and age-dependent cardiomyopathy (Dai et al., 2009, 2010), ischemic myopathy in high-fat diet mouse model (Ryan et al., 2016), and the lipid/high-fat diet-induced insulin resistance (Lee et al., 2010). Furthermore, when this mouse line was bred with Tg2576 mice, the double transgenic mice were characterized by substantial delay in development of the AD-related pathologies, improved cognitive functions and increased life span (Mao et al., 2012). Other studies in the CNS demonstrated the improved neurovascular coupling in aged mice (Csiszar et al., 2019), reverted toxicity of astrocytes towards motoneurons in an animal model of ALS (Pehar et al., 2014), and, finally, prevention of radiationinduced cognitive dysfunctions (Parihar et al., 2015). Despite that the mitochondria targeted catalase is a remarkably engineered protein, its original kinetics is similar or even more effective to that of glutathione peroxidase (Gaetani et al., 1989; Vetrano et al., 2005; Yu et al., 2005) that makes it very attractive as a potential drug. However, its delivery to targets can be very problematic even when viral vectors for gene therapy will be used. mCAT is dedicated to abrogate ROS production. ROS generation is an essential immune response against bacterial or viral infections, thus mCAT could potentially facilitate the infection development. Another obstacle for the use of transgenic tools for this enzyme expression in humans is linked with possible oncogenic transformation due to the positional effects of recombinant genetic construct integration in genomic DNA. Thus, mCAT seems to be currently unusable for the treatment in humans.
Algae xanthophylls
In plants, the pigment-protein complexes arranged in 3-dimensional “funneling-like” reaction centers, so-called“l(fā)ight harvesting antenna”, in photosystem complexes. They are used for the photosynthesis where the xanthophylls and carotenoids are an integral part of the centers associated with chlorophyll (Horton and Ruban, 2005). Carotenoids and xanthophylls are essential for chlorophyll activation during photosynthesis and for excessive energy dissipation (Young and Frank, 1996). Additionally, plants use a xanthophyll’s cycle mechanism which provides a non-photochemical quenching for ROS elimination when the plants are exposed to excessive irradiation energy or other stressful stimuli (Latowski et al.,2011).
Recently, some xanthophylls received a lot of attention because of their exclusive anti-oxidative abilities and,respectively, their positive effect on human health(Gammone et al., 2015). Certain xanthophylls, synthesized by microalgae and seaweeds (especially, Astaxanthin and Fucoxanthin), are frequently used in studies of NCDs with chronic in flammation. Here, we review possible mechanisms of xanthophylls correction of the mitochondria damaged in chronic in flammation and analyze several diseases in animal and human studies where the xanthophylls have been used for their treatments.
In particular, multiple changes in the enzymatic activities related to mitochondria were demonstrated in aortopathies:in particular, SOD activity was frequently increased in the pathology, whereas the glutathione peroxidase activity was decreased (Soto et al., 2014). It results in the accumulation of hydrogen peroxide in the affected tissues that is normal in the short term in the cases of infections or trauma, but becomes dangerous when it turns into chronic inflammation. The astaxanthin abilities for the protection were demonstrated from both reactive oxygen (O2-; Nishinoa et al., 2016) and H2O2(Wang et al., 2015a) meaning that mechanisms of astaxanthin effects are similar to those of mCAT. However,carotenoids and xanthophylls are able to be as acceptors and donors for electrons simultaneously (Mairanovsky et al.,1975; Faller et al., 2001). The same ability is a characteristic for both astaxanthin (Kidd, 2011) and fucoxanthin that establishes their relations with the chlorophyll photosystem I complex (Ikeda et al., 2013). Compared with the recombinant enzymes, the astaxanthin/fucoxanthin have no anatomical or physiological barriers to be distributed in the organism and can be systemically (orally) applied in form of the oil capsules. Astaxanthin and fucoxanthin are enzymatically generated as a part of the lipid fraction in algae and can be enriched by carotenoids (Sun et al., 2018). Both astaxanthin and fucoxanthin are very prospective medical, nutritional and cosmetic products with an extraordinary potential (Zhang et al., 2015; Shah et al., 2016). These abilities of the xanthophylls most likely are associated with their speci fic transmembrane orientation that displays their reaction centers towards the hydrophilic phases on both sides of the lipid membrane, in contrast to the carotenoids located exclusively within the hydrophobic lipid layers. That provides the unique electron donor-acceptor characteristics and, respectively, biological features of xanthophylls (Grudzinski et al., 2017).
Certain drugs can accumulate in the organism due to ineffective mechanisms of their extraction or biodegradation.Xanthophylls have both acceptable pharmacokinetics and effective pathways for their biodegradation that is characteristic for humans. The metabolism of carotenoids and xanthophylls is controlled by the endogenous beta-carotene oxygenases 1 and 2 (BCO1 and BCO2). These enzymes prevent the over-accumulation of carotenoids because their excess can trigger the OS. In particular, BCO2 with its mitochondrial localization in the matrix membrane, is effectively involved in mitochondrial and cellular metabolism controlling the OS and apoptosis (Amengual et al., 2011; Lobo et al., 2012).Interestingly, BCO2 expression is inactivated in the retina that underlies the accumulation of beta-carotene, lutein and zeaxanthin and, finally, resulting in its photoprotection (Li et al., 2014). The lack of BCO2 has been shown to be associated with malfunctioning of hepatic mitochondria in the BCO2 knock-out mice (Wu et al., 2017). BCO2-mediated elimination of astaxanthin/fucoxanthin in tissues raises the question of their pharmacokinetics. In human plasma, astaxanthin’s halflife has been estimated at 21 hours (Osterlie et al., 2000);fucoxanthin pharmacokinetics was only evaluated in the mouse plasma and tissues. The oral one-week consumption of fucoxanthin resulted in sustained accumulation of fucoxanthin metabolites (fucoxanthinol and amarouciaxanthin A) in the heart, liver and adipose tissue (Hashimoto et al., 2009). So far, no side effects of the xanthophylls treatment have been described in humans yet. Pharmacokinetics of astaxanthin and fucoxanthin and evidence of their positive role in NCDs allow the conclusion that they are very promising drugs. Interactions of recombinant mCAT and the xanthophylls (astaxanthin and fucoxanthin) with the mitochondria are schematically summarized inFigure 1.
Health bene fits of xanthophylls in chronic in flammation
In the “High-Fat Diet” animal model of CVDs/atherosclerosis/type 2 diabetes, significant improvements in the lipid metabolism have been demonstrated after treatment with astaxanthin (Ikeuchi et al., 2007; Yoshida et al., 2010; Xu et al., 2014, 2017; Yang et al., 2014; Kim et al., 2017; Wang et al., 2019) and fucoxanthin (Maeda et al., 2009; Jeon et al.,2010; Woo et al., 2010; Hu et al., 2012; Ha and Kim, 2013).Positive effects of fucoxanthin on the experimental animals were more profound because of evident reduction of their weight. The effect seems to be associated with long-lasting(41-day half-life) accumulation of a fucoxanthin metabolite,amarouciaxanthin A, in the adipose tissue in mice (Hashimoto et al., 2009). The anti-obesity properties of fucoxanthin have been reviewed in Gammone and D’Orazio, 2015.
Besides the lipid profile regulation, astaxanthin affects microbiota composition in the gut (Wang et al., 2019),reduces the OS (Xu et al., 2017), inhibits both inflammation and fibrosis in the liver (Kim et al., 2017), reverses the dietprovoked insulin resistance (Ni et al., 2015), ameliorates the insulin signaling (Arunkumar et al., 2012), prevents the insulin signaling deterioration and improves the glucose metabolism in the liver of insulin resistant mice (Bhuvaneswari and Anuradha, 2012). Furthermore, astaxanthin stimulates mitochondrial biogenesis in insulin resistant muscle (Nishida et al., 2020), protects beta-cells against glucose toxicity in diabetic db/db mice (Uchiyama et al., 2002), eliminates the endothelial dysfunction in streptozotocin-induced diabetes in male rats (Zhao et al., 2011), and prevents nephropathy in diabetic db/db mice (Naito et al., 2004). Fucoxanthin affects glucose homeostasis, lipid metabolism, and liver function in a mouse model of T2DM (Lin et al., 2017) and anti-in flammatory activity in obese mice (Tan and Hou, 2014; Maeda et al.,2015). Health benefits of the fucoxanthin treatment in the prevention of chronic diseases are reviewed in (Bae et al.,2020).
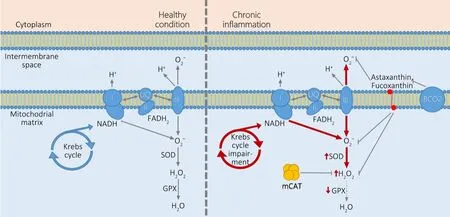
Figure 1 | The healthy and pathologic condition of “chronic in flammation”in mitochondria.
Astaxanthin has been shown to increase the levels of serum HDL-cholesterol and adiponectin in subjects with mild hyperlipidemia (Yoshida et al., 2010) and to improve glucose metabolism and reduced blood pressure in patients with T2DM (Mashhadi et al., 2018).
Xanthophylls and cognition
Well-known interplay between chronic in flammation and stress can tremendously affect CNS causing depression and cognitive impairment. Astaxanthin has been shown to prevent the depression caused by the in flammation in animals (Jiang et al.,2016; Zhou et al., 2017) and to produce an anti-hyperalgesic effect, thus, correcting comorbid depressive like behavior in mice with chronic pain (Jiang et al., 2018). Astaxanthin is able to correct cognitive de ficits and OS associated with diabetes (Xu et al., 2015; Zhou et al., 2015; Al-Amin et al., 2016; Li et al., 2016)and to improve the cognition impaired by traumatic brain injury(Ji et al., 2017). In some studies, the anti-depressant astaxanthin activities have been shown to affect the serotonergic system directly (Jiang et al., 2017). Furthermore, lipopolysaccharideinduced depressive-like behavior in mice was prevented by fucoxanthin via the suppression of inflammatory pathways(Jiang et al., 2019).
Xanthophylls and AD
In vitro, both fucoxanthin and astaxanthin demonstrated evident neuroprotective activities against Aβ42, one of the main hallmarks of AD (Alghazwi et al., 2019). Fucoxanthin ameliorated OS and inflammation in Aβ42-induced BV2 microglia cells and reduced ROS generation (Pangestuti et al., 2013). Astaxanthin neuroprotective properties were demonstrated in many cellular models of neurodegenerative pathologies: AD, Parkinson’s disease, and ALS (Galasso et al.,2018).
The most impressive results were obtained in animal models of AD. Fucoxanthin has been shown to inhibit Aβ assembly,to attenuate Aβ-induced toxicity, and to reduce AD-related cognitive impairments (Xiang et al., 2017). Furthermore,fucoxanthin significantly lowered OS, enhanced the brainderived neurotrophic factor expression and enlarged choline acetyltransferase-positive regions in the hippocampus of mice.In transgenic animal model of AD (APPswe/PS1ΔE9), substantial reduction in Aβ accumulation, reduced Aβ plaque loading and increased APOE expression in the brain, and improved memory were observed after the treatment with fucoxanthin enriched extract from the seaweed Sargassum fusiforme (Bogie et al.,2019). In APPswe/PS1ΔE9mice, astaxanthin alone and particularly in combination with docosahexaenoic acid was extremely effective in reduction of OS and tau hyperphosphorylation, in suppression of neuroin flammation by reducing the expression of in flammasome proteins and in flammasome activation (Che et al., 2018).
Conclusions
AD and other chronic neurodegenerative disorders are characterized by multiple abnormalities at systemic, cellular,macromolecular, and biochemical levels. Neurodegenerationrelated pathologies are well known to be associated with in flammation, mitochondrial malfunctioning, disturbed energy metabolism, and chronic OS. Mechanisms of intracellular in flammation-related processes indicate a pre-programmable character of the “mitochondrial dysfunctions”. However, in the chronic in flammation these dysfunctions become permanent and their correction is essential. The mitochondria-targeted catalase and algae derived xanthophylls, astaxanthin and fucoxanthin, can correct the in flammation related pathologies and provide significant improvements practically in all integrative systems (cardiovascular, nervous, metabolic,immune and others) that are expected to involve similar/identical mechanism(s). Thus, intensive further studies of xanthophylls’ bene ficial effects and their clinical trials could be a promising way for the operative development of therapeutic approaches oriented on actual treatments of the causes of the diseases rather than their symptoms.
Author contributions:The manuscript was conceived and wrote by MAF,edited by VVV, designed by OGT, reviewed and permitted for submission by NVP. All authors participated in the elaboration of the manuscript and approved its final version.
Con flicts of interest:The authors declare no con flicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
 中國(guó)神經(jīng)再生研究(英文版)2021年2期
中國(guó)神經(jīng)再生研究(英文版)2021年2期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Gut microbiota: a potential therapeutic target for Parkinson’s disease
- Stem cell heterogeneity and regenerative competence: the enormous potential of rare cells
- Therapeutic potential of neuromodulation for demyelinating diseases
- Glycogen synthase kinase-3β inhibitor SB216763 promotes DNA repair in ischemic retinal neurons
- Brain plasticity after peripheral nerve injury treatment with massage therapy based on resting-state functional magnetic resonance imaging
- Dynamic changes in the systemic immune responses of spinal cord injury model mice
