Brain plasticity after peripheral nerve injury treatment with massage therapy based on resting-state functional magnetic resonance imaging
Zhen-Zhen Ma Jian-Guang Xu
Abstract Massage therapy is an alternative treatment for chronic pain that is potentially related to brain plasticity. However, the underlying mechanism remains unclear. We established a peripheral nerve injury model in rats by unilateral sciatic nerve transection and direct anastomosis. The experimental rats were treated over the gastrocnemius muscle of the affected hindlimb with a customized massage instrument (0.45 N, 120 times/min, 10 minutes daily, for 4 successive weeks). Resting-state functional magnetic resonance imaging revealed that compared with control rats, the amplitude of low-frequency fluctuations in the sensorimotor cortex contralateral to the affected limb was significantly lower after sciatic nerve transection. However, amplitudes were significantly higher in the massage group than in a sham-massage group. These findings suggest that massage therapy facilitated adaptive change in the somatosensory cortex that led to the recovery of peripheral nerve injury and repair. This study was approved by the Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine of China(approval No. 201701001) on January 12, 2017.
Key Words: injury; massage; model; neuron; peripheral nerve; plasticity; rat; repair
Introduction
Peripheral nerve injury (PNI) results in damaged axons and loss of sensory, motor, and autonomic functions, which can lead to a decline in the quality of life. Surgical repair is the primary treatment for PNI (Sullivan et al., 2016). Although recent years have seen great advances in microsurgery techniques and neural regeneration-promoting methods,functional recovery following reinnervation is still less than satisfying (Miller, 2017; Neumann et al., 2019). Accumulating evidence suggests that brain plasticity-based rehabilitation is essential for the restoration of function in these cases(Sampaio-Baptista et al., 2018). Massage therapy is a popular non-drug intervention that uses mechanical force directly on the human body to alleviate discomfort and promote health(Chou and Huflman, 2007; Dabbour et al., 2018). Massage has been proven to be effective in many clinical trials. For example, perineal massage performed during pregnancy significantly decreased perineal trauma at birth (Labrecque et al., 2001). In other studies, massage reduced the intensity of muscle soreness after exercise-induced muscle damage,and decreased cancer-related fatigue for cancer survivors(Kinkead et al., 2018). Meanwhile, massage has important and bene ficial effects on neurological diseases. Massage has been reported to trigger parasympathetic nervous system activation (and suppress sympathetic nervous system activity)by stimulating skin sensors dominated by vagal nerve fibers,which can improve the neurobehavioral outcome in infants(Diego and Field, 2009). Field et al. (2014) found applying massage therapy to the tender points in patients with carpal tunnel syndrome effectively reduced pain and increased the range of motion of the affected limb. Additionally, Aboodarda et al. (2018) demonstrated that rolling massage could reduce the central excitability of neuronal circuits that innervate the knee extensors. Andrzejewski et al. (2014) reported that the tendons of rats in a massage group exhibited higher expression of proteins important for regulating tendon structure (vascular endothelial growth factor-A, fibroblast growth factor-2, and CD34) than was observed in other groups. Furthermore, Zhang et al. (2019) found that massage therapy changed the status of DNA hydroxymethylation, which can regulate the expression of neurodevelopment-related genes. Most massage-related research has generally focused on the injured region.
We also found that massage therapy facilitated nerve regeneration following sciatic nerve transection and repair in rats, and the model was a typical PNI model that allows the evaluation of neuropathic changes and nerve regeneration(Casals-Díaz et al., 2009; Chawla et al., 2014). According to our previous study (Ma et al., 2016), we con firmed that massage can promote the proliferation of muscle satellite cells, regulate the expression of related myogenic factors, accelerate the recovery of muscle fibers, improve the structure and morphology of skeletal muscle after sciatic nerve transection and repair, and delay the atrophy of skeletal muscle. Our conclusion was similar to that of Wang et al. (2019). In another study (Wu et al., 2018), cortical remodeling occurred in the same PNI-model rats, but these authors used a different intervention method to us. We inferred (hypothesized) that massage therapy might also cause neuroplastic alterations in the brain, but our understanding of brain remodeling mechanisms after massage is still imperfect due to the limited literature.
By combining electro-neurophysiological recordings and functional magnetic resonance imaging (fMRI), studies have shown that low-frequency (0.01-0.08 Hz) fluctuations (LFF)in blood oxygenated-level-dependent (BOLD) fMRI signals are closely related to spontaneous neuronal activity (Logothetis et al., 2001; Goldman et al., 2002). Zang et al. (2007) developed the amplitude of LFFs (ALFF) index (the square root of the power spectrum integrated in a low-frequency range) for detecting the regional intensity of spontaneous fluctuations in the BOLD signal. The ALFF is considered a potentially meaningful index of the brain’s energy and morphology (Aiello et al., 2015; Liao et al., 2019). Furthermore, the ALFF has been widely applied in functional neuroimaging with a slightly higher test-retest reliability and a higher speci ficity (Hoptman et al., 2010). Therefore, the ALFF can be used to evaluate the intensity of spontaneous neuronal activity in the brain (Zang et al., 2007). The present study aimed to test our hypothesis by using BOLD signals and the ALFF to measure brain activity associated with the effect of massage in PNI rats.
Materials and Methods
Animals
Thirty-two healthy female clean-grade Sprague-Dawley rats aged 6-8 weeks and weighing 180-200 g were provided by Shanghai Slack Laboratory Animal Limited Liability Company(Shanghai, China) (license No. SCXK [Hu] 2012-0002). They were kept in a laboratory environment with a 12-hour light/dark cycle. The temperature was set to 20-22°C and rats were provided adequate food and water. The rats were acclimated to their cages for 7 days before beginning any intervention.This study was approved by the Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine (approval No. 201701001) on January 12, 2017. All procedures and protocols were in accordance with the Guide for the Care and Use of Laboratory Animals described by the US National Institutes of Health and followed the Animal Research Reporting:In VivoExperiments (ARRIVE) guidelines.
PNI procedure
The rats were randomly divided into normal, model, shammassage, and massage groups (n= 8 per group). In the model,sham-massage, and massage groups, PNI was established by sciatic nerve transection and direct anastomosis. The rats were anesthetized with intraperitoneal injection of pentobarbital sodium (40 mg/kg; Notlas Co., Ltd., Beijing,China) and placed on a clean operating table in the prone position. We shaved an area about 5 mm below the sciatic tubercle of the right hip and made an incision along the route of the sciatic nerve. While viewing under a 10× microscope(Shanghai Medical Optical Instruments Co., Ltd., Shanghai,China), the trunk of the right sciatic nerve was exposed and separated from the gluteal muscle. It was then transected with a razor blade 1 cm below the lower edge of the piriformis muscle. The epineurium of the sciatic nerve was then sutured with 11-0 sutures (Figure 1A-C). The rats in the normal group were fed normally without surgery.
Massage and sham-massage intervention
Both massage and sham massage were performed 1 week after model establishment (Ma et al., 2016). Interventions lasted 10 minutes and were performed once a day for 4 weeks. The massage group was treated with a massage instrument (Chinese patent No. 2014204820753;Figure 1D).Rats in the sham-massage and massage groups were fixed on a holder and the right gastrocnemius muscle was clamped by the two rubber strips of the massage simulator. The massage group were treated by instrument massage (pressure = 0.45 N,frequency = 120 times/min, velocity = 1 cm/s;Figure 1EandF). The sham-massage group did not receive any massage, but their right gastrocnemius muscles remained lightly clamped for 10 minutes. Normal and model groups did not receive any intervention.
fMRI image acquisition
All fMRI scans of the brain were performed 5 weeks after model establishment by a Bruker 7T magnetic resonance system (Bruker Corporation, Bremen, Germany) with a coil for small animals. After 2.5% isoflurane (RWD Life Science Co., Ltd., Shenzhen, China) induced anesthesia, rats were fixed on the scanner and given continuous 1.5-2% iso flurane anesthesia with ventilator support and respiratory monitoring.Interlayer-scanning echo-plane imaging parameters were as follows: flip angle = 90°, slice thickness = 0.5 mm, repetition time = 3000 ms, echo time = 20 ms, number of averages =1, field of vision = 32 mm × 32 mm, matrix = 64 × 64 voxels.Image acquisition included 200 time points and lasted 200 × 3 seconds; in other words, the duration was 10 minutes.
fMRI data preprocessing
The resting-state fMRI data were preprocessed using the Statistical Parametric Mapping 12 (SPM 12) toolbox (Welcome Department of Cognitive Neurology, University College,London, UK; http://fil.ion.ucl.ac.uk.spm/) and the Resting-State fMRI Data Analysis Toolkit (REST) toolbox (http://www.restfmri.net) toolbox in Matlab version 2014a (MathWorks,Natick, MA, USA). First, we expanded the images by 10 × 10 ×10 times to match the size of the human brain—which made it possible to use/modify processing algorithms originally designed for human data—and stripped the none-brain manually (Gu et al., 2016; Peng et al., 2016). The amplified procedure only changed the dimension descriptor fields in the file header without interpolation. The first five volumes of each functional time series were discarded as the signals had not yet reached equilibrium. The preprocessing steps included slice timing correction, realignment for head motion,spatial normalization in Schwarz’s standard space (resampling with 2.06 mm × 2.06 mm × 2 mm resolution) (Paxinos and Watson, 2005; Schwarz et al., 2006), spatial smoothing (using the Gaussian kernel with 4.12 mm × 4.12 mm × 4 mm fullwidth-at-half-magnitude). Regressing the nuisance signal,detrending, and filtering (0.01-0.08 Hz) were used on the time series of each voxel to reduce the in fluence of low-frequency drift (linear and quadratic), physiological high-frequency respiration, and noise. The images were then corrected for slice-timing and head movement. Rats with head motion greater than 1 mm in any direction or head rotations greater than 1° were excluded. BOLD signals detected by fMRI re flect changes in the activity of neurons (Hillman, 2014; Rogachov et al., 2016). The BOLD signal fluctuates, and the ALFF can tell us how much fluctuation is happening in a given brain region.We calculated ALFF values and standardized ALFF values (the ALFF value of each voxel divided by the global mean values).We then transformed the ALFF values to zALFF values with a Fisher r-to-z transformation, which allowed us to compare them among the groups (Yang et al., 2007).1000 simulations, full-width-at-half-magnitude = 4 mm, using the group GM mask) applied with the AlphaSim procedure in the REST toolbox. In this procedure, a corrected signi ficance level ofP< 0.05 was obtained for a minimum volume of 12 voxels. This process enabled identification of significant differences between each pair of groups.
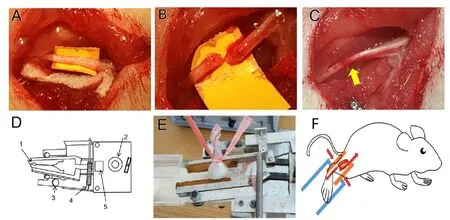
Figure 1 | Demonstration of peripheral nerve transection, repair surgery,and massage therapy.
Statistical analysis
We conducted a one-way analysis of variance of the zALFF values with Group as a factor (normal, model, sham massage,and massage). After the interaction effect analysis, a binary mask was generated using clusters of statistical significance.Furtherpost hoctwo-samplet-tests were then performed within this mask andP-values were corrected for multiple comparisons. The correction standard was determined by Monte Carlo simulations (individual voxelP-value = 0.001,
Results
Effect of massage therapy on the general condition of PNI rats
All rats survived after sciatic nerve transection and repair surgery. Reddening and swelling around the wound was only observed in three rats within the first post-PNI week (two in the massage group and one in the sham-massage group).The wound completely recovered in all rats without obvious infection. No rats needed to be replaced in this study.
One to three weeks following PNI, all rats in the model, shammassage, and massage groups showed different degrees of muscle atrophy, limp gait, ankle joint stiffness, and limited metatarsal flexion in the affected limb. These syndromes had recovered to varying degrees after 4 weeks of intervention.
Massage therapy alters regional ALFF values in the resting-state brain of PNI rats
Regional changes in ALFF value were calculated in the restingstate by performing a whole brain cluster-wise comparison.Significant main effects of Group (F(3,28)= 14.85,P< 0.0001)were distributed in the left somatosensory cortex (Figure 2).The significant clusters were extracted to generate a binary mask forpost hocanalysis. Thepost hocanalysis revealed signi ficantly lower ALFF values in the model group than in the normal group, speci fically in bilateral somatosensory cortices,left parietal association cortex, and bilateral auditory cortices(P< 0.05). ALFF values in the massage group were signi ficantly higher than those in the sham-massage group, speci fically in bilateral somatosensory cortices (P< 0.05). Additionally, ALFF values in the mesencephalic region were signi ficantly lower in the massage group than in the model group (P< 0.05;Figure 3andTable 1).
Discussion
The present study showed a significant difference among the four groups in the left somatosensory cortex. Further,the ALFF values in left somatosensory cortex were higher in the massage group than in the model or sham-massage groups. These results indicate that massage improves the restoration of local brain activity after peripheral nerve injury. According to the literature, massage therapy has been used to treat a number of diseases, such as infant brain maldevelopment, skin diseases, prenatal depression, chronic pain, cardiovascular diseases, immune diseases, cancer,neurodegenerative diseases, sleep, and other uncomfortable symptoms (Field, 2014). Pan et al. (2017) concluded that the effects of massage therapy are based on a mechanism involving the facilitation of blood and lymph fluid circulation,inhibition of erythrocyte aggregation, and decreases in viscosity and substance P levels in the blood, and an increase in β-endorphin levels. Their results demonstrated that massage therapy could increase the expression of nerve growth factor and decrease the secretion of tissue plasminogen activator and plasminogen activator inhibitor-1. These actions could accelerate rehabilitation after PNI (Xian et al., 2015). Wolny et al. (2017) applied massage to the descending part of the trapezius combined wrist mobilization directed at the median nerve. This procedure reduced the subjective symptoms of carpal tunnel syndrome and improved sensory function and sensory nerve conduction velocity. To date, extensive research has reported brain plasticity following PNI (Goswami et al.,2016; Bhat et al., 2017). Interestingly, several studies have focused on changes in brain plasticity following massage therapy. Ouchi et al. (2006) showed that massage therapy in the prone position could significantly increase local cerebral blood flow in the parietal cortex. Sliz et al. (2012) explored immediate neurophysiological effects of different types of massage in healthy adults. They found selective changes in the retrosplenial/posterior cingulate following reflexology massage. The current study has provided powerful evidence that adaptive cerebral plasticity after massage therapy remain even after a relative long period of time (4 weeks).
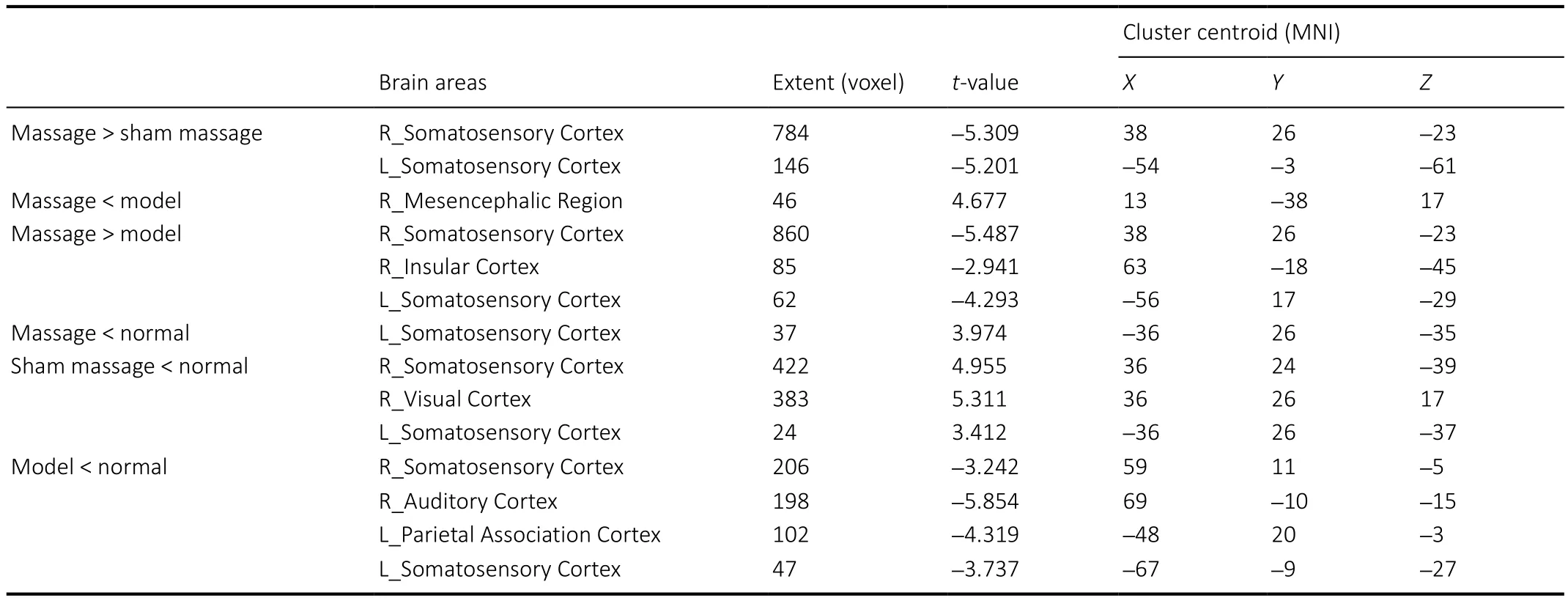
Table 1 |Comparison of amplitude of low-frequency fluctuation between pairs of groups following 4 weeks of intervention
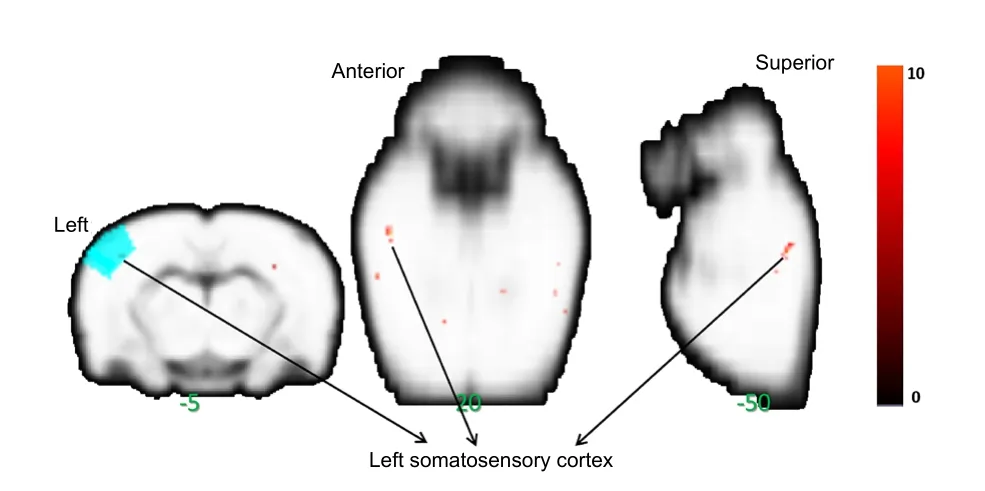
Figure 2| Main effects of massage on a peripheral nerve injury rat.
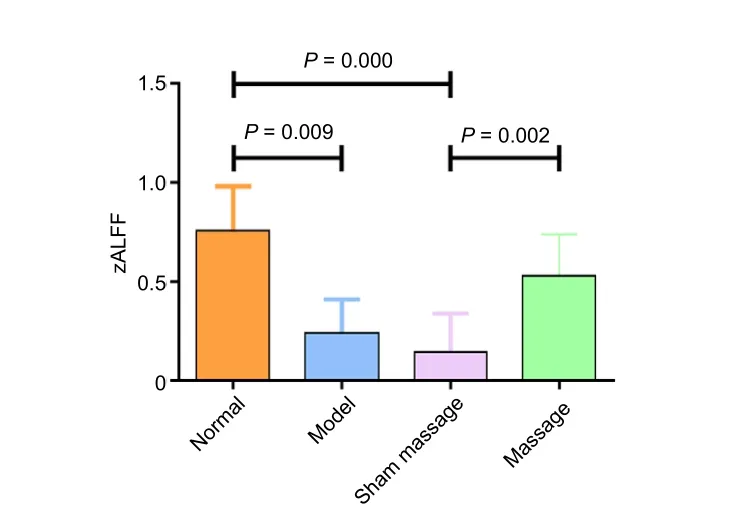
Figure 3| Effects of massage on zALFF values in a peripheral nerve injury rat.
fMRI is considered an important neuroimaging technique for noninvasive observation of brain activity (Guerra-Carrillo et al., 2014). ALFF values are physiologically related to the spontaneous activity of a speci fic brain area and are measured using a method based on the fast Fourier transform of the resting-state time series for each voxel (Mohamed et al.,2004). Viswanathan and Freeman (2007) recorded changes intissue oxygen levels in the visual cortex. They suggested that the BOLD fMRI signal primarily reflects synaptic activity, as well as neuronal activity. This index is highly time-stable in the resting-state and is often used as a biological marker for longterm intervention in various diseases (Küblb?ck et al., 2014).The excitatory/inhibitory balance between synapses is related to hemodynamics and indirectly influences the intensity of the BOLD signal; a negative BOLD signal indicates inhibition of synapse function (Lauritzen et al., 2012). Therefore,decreased ALFF values in the corresponding somatosensory cortex following PNI indicated reduced spontaneous(excitatory) neuronal activity. The changes that we observed in the contralateral somatosensory cortex following PNI were similar to what we found in our previous study (Wu er al., 2018). As the somatosensory cortex loses sensory input from the peripheral nerve, neuronal activity decreases.Evidence shows that input-deprived brain regions can become reactivated after reinnervation (Kaas and Collins, 2003; Wu et al., 2016). The increase in ALFF values following nerve repair demonstrate enhanced activity of related neurons and show the process of peripheral neural regeneration. Following massage intervention, the ALFF values in the somatosensory cortex increased; we can infer that this is related to the facilitatory effect of massage therapy on sensorimotor functional recovery. In our previous study of rats, the recovery of the affected hindlimb following sciatic nerve transection and repair significantly improved after the application of electroacupuncture. In those rats, we also noted an increase in ALFF value of the somatosensory cortex contralateral to the affected hindlimb (Wu et al., 2018). In the present study, after 4 weeks of intervention, the model group showed lower ALFF values than the normal group in the somatosensory cortex contralateral to the affected hindlimb, indicating suppressed spontaneous activity in that region after PNI. Additionally,compared with the model and sham-massage groups, the massage group exhibited significantly higher ALFF values in the contralateral somatosensory cortex. This indicates that massage therapy led to restoration of neuronal activity. The effect of massage on ALFF values in the present study was similar to that of electroacupuncture in our previous study.
The somatosensory cortex processes behavior-related somatosensory information (Kim et al., 2016). It integrates tactile, painful, and proprioceptive sensory information that is projected from the extremities through the thalamus. Thus,the clinical symptoms of PNI manifest as abnormal integration of sensory information. Chao et al. (2018) observed positive BOLD responses to forepaw stimulation in the medial thalamus, hypothalamus, and primary somatosensory cortex of the hindlimb area contralateral to the injured nerve following tibial and common peroneal nerves transection.The sustained activation in the contralateral primary somatosensory cortex, insula, and cingulate gyrus appeared immediately after spared nerve injury. In patients with complete median nerve injury at the wrist and delayed repair 2 years later, the response of the contralateral somatosensory cortex decreased during tactile stimulation of the fingers,while that in the ipsilateral primary somatosensory cortex increased (Fornander et al., 2016). Functions lost in the damaged cortex can be taken over by adjacent regions. Moore and Schady (2000) found that after injury, corresponding somatosensory cortex received sensory information from adjacent areas after PNI or amputation. Using fMRI, Qiu et al. (2014) showed that peripheral afferent dysfunction after brachial plexus injury caused the function of the corresponding cortex to be occupied by adjacent non-speci fic afferent cortical areas. Based on these studies, the function of the corresponding cortex may be partially replaced after PNI,and complete recovery of function is not guaranteed even after immediate repair (Jaquent et al., 2001). Massage therapy provides constant input of peripheral sensory information,which might prevent the corresponding cortex from being occupied by adjacent regions.
In accordance with another study (Philip and Frey, 2014),we found that ALFF in the somatosensory cortex ipsilateral to the affected hindlimb also increased. The increased ipsilateral somatosensory activation may be due to attenuated hemispheric inhibition associated with signal changes in the afferent somatosensory cortex. In this study, unilateral sciatic nerve transection induced suppressed local excitability in the contralateral somatosensory cortex. During the recovery process, massage improved restoration of local brain activity.Therefore, massage therapy may facilitate information integration by restoring the activity of somatosensory cortex to restore the sensory functions.
This study had several limitations. With humans, acquisition of functional images before clinical damage is not possible.Thus, at the beginning of the experiment, to be consistent with the clinical reality, we did not perform pre-surgery fMRI scans in our rats. However, we will explore dynamic changes in the brain before and after surgery in a follow-up study.Molecularly active substances can be used as indexes in future experiments, which will better explain the effect of massage. The difference between humans and rodents limited interpretation of the data. A correlation analysis between behavior assessments and ALFF values is necessary in a future study. We also need to explore the changes in brain plasticity in the acute period after PNI. Because most of the methods used in clinical treatment are implemented with different amounts of force, and the complexity and strength of the manipulation are related to the endurance of the patients and the personal level of the therapist, the application of machinebased massage therapy does not represent actual massage therapy very well.
Acknowledgements:We thank Department of Radiology, Fudan University Shanghai Cancer Center for their equipment support.
Author contributions:Conceptualization: XXX, XYH; methodology: XYH,MXZ, SJM; validation: ZZM; formal analysis: XXX, MXZ; writing-original draft: XXX, MXZ; writing review & editing: MXZ, JGX. All authors read and approved the final manuscript.
Con flicts of interest:The authors declare that they have no competing interests.
Financial support:This study was supported by National Key R&D Program of China, No. 2018YFC2001600 (to JGX); and Shanghai Science and Technology Committee of China, Nos. 18511108300 (to JGX), 18441903800 (to MXZ) and 18441903900 (to XYH). The funding body played no role in the study design, in the collection, analysis and interpretation of data, in the writing of the paper, or in the decision to submit the paper for publication.
Institutional review board statement:This study was approved by the Animal Ethics Committee of Shanghai University of Traditional Chinese Medicine, China (approval No. 201701001) on January 12, 2017. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animal (NIH Publication No. 85-23, revised 1985).
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement:Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-Non-Commercial-ShareAlike 4.0 License, which allows others to remix, tweak,and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Michele R Colonna, Universita degli Studi di Messina, Italy; Gabriele Siciliano, Universita degli Studi di Pisa, Italy; Yu Lei,Huashan Hospital, Fudan University, China.
Additional file:Open peer review reports 1-3.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Gut microbiota: a potential therapeutic target for Parkinson’s disease
- Stem cell heterogeneity and regenerative competence: the enormous potential of rare cells
- Therapeutic potential of neuromodulation for demyelinating diseases
- Glycogen synthase kinase-3β inhibitor SB216763 promotes DNA repair in ischemic retinal neurons
- Dynamic changes in the systemic immune responses of spinal cord injury model mice
- Effectiveness of oral motor respiratory exercise and vocal intonation therapy on respiratory function and vocal quality in patients with spinal cord injury: a randomized controlled trial

