Excess iodine supplementation aggravates the toxic effects induced by perchlorate on the male reproductive system in rats
Arijit Chakraborty
Department of Sports &Exercise Science,Somaiya Sports Academy,Somaiya Vidyavihar University,Vidyanagar,Mumbai,Maharashtra 400077,India
ABSTRACT Objective:To investigate the toxicity of excess iodine and perchlorate co-exposure on male reproductive system in rats.Methods: Eighteen male Wistar albino rats were divided into three groups.Group 1 received no treatment and served as the control group.Group 2 received perchlorate alone (130 mg/kg body weight),and group 3 received perchlorate (130 mg/kg body weight)plus excess iodine (0.7 mg potassium iodine/100 g body weight) for 45 days.Urinary perchlorate and iodine excretion pattern,testicular iodine concentration,serum testosterone levels,epididymal sperm count,key enzymes of steroidogenic pathway,reactive oxygen and nitrogen species including total antioxidant profiles in testis with electron microscopic ultrastructure analysis of spermatozoa were evaluated.Results: Co-exposure of perchlorate and excess iodine reduced their excretion pattern,reflecting accumulation with reactive oxygen species generation.It was accompanied by higher lipid peroxidation level with imbalance in the pro-/antioxidant status,inhibiting the activities of Δ5 3β-hydroxysteroid dehydrogenase (HSD) and 17β-HSD rate limiting enzyme activities,and causing reduced synthesis of testosterone,parallel to reduction in testicular and accessory sex organs weight,epididymal sperm-count with deformed ultrastructure of sperm.Perchlorate alone was not a reproductive toxicant;however,in combination with excess-iodine,acute effects were noticed,resulting in a severe deterioration of testicular and spermatozoal structure and function.Conclusions:This study provides a novel insight on the augmentation of the relatively moderate repro-toxic effects of perchlorate to a more severe form in presence of excess iodine on male reproductive physiology,which justifies further investigations.
KEYWORDS:Repro-toxicity;Testosterone;ROS;Spermatozoa;Fertility;Steroidogenesis;Electron-microscopy;Accessory sex organs
1. Introduction
Significance
Excess iodine ingestion results in male infertility by affecting various molecular signaling pathways,culminating into compromised spermatogenesis;however,synergy between iodine and perchlorate is unknown on reproduction.The repro-toxic effect of perchlorate is accentuated by excess iodine as evidenced by deterioration in the structural and functional aspects of spermatozoa,elevated reactive oxygen species generation,pro-/antioxidant and nitric oxide imbalance culminating in altered steroidogenic pathway,hence affecting overall male reproductive physiology.
For correction of iodine deficiency disorders,supplementation of exogenous iodine in salt or by various other mediators results in excess amount of iodine intake,particularly in environmentally adequate iodine regions and is now emerging as universal public health problem[1].However,even in this post salt iodization era,approximately 300 million people throughout the world suffer from thyroid gland dysfunction,while globally the total goitre prevalence in the general population is assessed to be 15.8%[2].Recent data from Iodine Global Network revealed that several countries are now iodine sufficient;however,common households still continue the consumption of fortified iodized salt,making them vulnerable to the negative effects of excess iodine,as both insufficiency and excess of iodine can negatively impact thyroid function hence reproduction.This clearly indicates the etiology of this disease is multifactorial and is affected by environmental factors present in food and water.Our group has already demonstrated the independent role of excess iodine in deteriorating testicular structure and functions as testicular cells also contain iodine channel,sodium-iodide-symporter in addition to thyroid[1,3-5].
In addition to excess iodine,the concern about environmental perchlorate exposure and perturbation of thyroid homeostasis is also increasing[6].Till date,the concern about environmental perchlorate exposure focuses on its inhibition of iodide uptake into the thyroid targeting sodium-iodide-symporter and was regarded as non reprotoxic[7].Perchlorate generally used as an oxidant in solid rocket fuel,fireworks,ammunitions is widely present environmentally and is known to affect thyroid function by blocking iodine uptake,disrupting physical growth and neurological development[8].It is also used as a chemical in plastic packaging and food handling equipment for dry food like cereal,flour,and spices to reduce the build-up of static charges[8].Apart from packaging,this chemical can enter the food system because of the usage of hypochlorite bleach used as a disinfectant in processing and from contaminated water.Therefore,a combination of human activities and natural sources has led to the widespread presence of perchlorate in the environment as such the excessive use of the chemical has reflected in contamination of the groundwater in certain Indian states[9].Perchlorate is also a well-known endocrine-disruptor that can migrate from packaging into food in physiological systems[10].Because of its identical charge and similar ionic radius (I),its uptake by sodium-iodidesymporter can reversibly block the iodine entry into follicular cells,causing anti-thyroidal effects[11].Excess iodine also has been found to be associated with a marked decrease in expression of the sodium-iodide-symporter that is present on the basolateral membrane of thyroid follicular cells;however,this mechanism is not permanent and can be withdrawn after certain durations[12].A statistically significant association between increasing urinary perchlorate concentrations and decreasing serum thyroid hormone concentrations has already been reported using clinical data[8].Excess iodine on the other hand has been a major concern in most of the areas even in post salt iodization phase as the effect of iodine deficiency and excess is almost ‘U’-shaped[1].Both iodine deficiency and excess have been detrimental to the male reproductive system in the long run[4].However,synergistic effects of these important global endocrine disrupters are yet to be established in spite of their natural coexistence naturally in many parts of the world.
Therefore,the aim of this study was to use this idea and develop an experimental setup to investigate whether exposure to a combination of these two important thyroid disrupting factors (perchlorate and excess iodine) has a repro-toxic effect on male reproductive physiology.
2.Methods and materials
2.1.Maintenance of animals
Eighteen male Wistar strain albino rats were used for the experiment.All the rats were housed in clean cages in an airconditioned room and 12 hours light/dark cycles were maintained and rats were allowed free access to drinking water and basal diet.Approximately,10 g of food was fed per 100 g of body weight.The rats were divided into three groupsviz
,the control,perchlorate only treated,and perchlorate+iodine treated groups.All rats were fed on standardized normal laboratory diet containing 20% protein,70% wheat,10% Bengal gram,5% fish meal powder,4% dry yeast powder,0.75% refined til oil and 0.25% shark liver oil with required amount of potassium iodide[4].2.2.Dose and duration
Ammonium perchlorate was given orally at a dose of 130 mg/kg,respectively,in deionized water every day for 45 days[6] which also corresponded to environmental mammalian exposures of this element[8].The third group of rats was administered iodinevia
oral gavage at a dose of 0.7 mg potassium iodine/100 g body weight dissolved in sterile water which corresponded to 100 times of physiological daily dose of iodine[3,4] in addition to the dose of perchlorate as mentioned.Treatment schedule of 45 days was selected to determine the effect of perchlorate and in combination of excess iodine on multiple seminiferous cycles as duration of one seminiferous cycle was 13.2 days in albino rats[13].2.3.Sacrifice of animals and collection of blood sample
At the end of the experimental period of 45 days,body weights were taken,and the rats were sacrificed by cervical dislocation following ethical protocol.Blood samples for hormone assay were collected from the hepatic portal vein of rats.Plasma samples were separated by centrifugation at 448 ×g
and stored at -50 ℃ until assayed[14].2.4.Measurement of urinary iodine and perchlorate
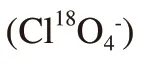
2.5.Epididymal sperm count
Sperm count was determined manually by haemocytometer.Sperm samples were collected from cauda epididymis after sacrifice.Some amounts of cauda epididymis free from fat were placed in a small beaker containing 5 mL 1% (w/v) sucrose in phosphate buffer saline(pH 7.4).The cauda epididymis was punctured with fine needle and spermatozoa was washed with 17β-estradiol.The beaker was shaken gently to give a homogeneous concentration of spermatozoa.A drop of suspension was placed on haemocytometer and covered with cover slip.The number of spermatozoa in 5 small squares was counted.To minimize the error,each count was repeated at least 5 times for each sample[1].
2.6.Assay of Δ5 3? and 17? hydroxy steroid dehydrogenase(HSD) activity
The tissues were homogenized by homogenizing fluid containing 20% spectroscopic grade glycerol,5 mM ethylene diamine tetraacetic acid (EDTA) at a tissue concentration of 10% homogenizing fluid mixture and centrifuged at 10 000 ×g
for 30 min in an ultracentrifuge at a constant temperature of 4 ℃.The supernatant was then used for assay procedure.The activity was determined by optical measurement of rate of reaction of nicotinamide adenine dinucleotide (NAD).The reaction system contained in a final volume of 3 mL:100 μM NAD,30 μg of substrate of 3β HSD or 17β HSD as the case was each in 0.02 mL of purified doxin and a suitable quantity of enzyme (200-500 μL) to initiate the reaction.The final pH of the whole solution was 9.1.The reactions were carried in silica cuvettes of 1.0 cm light path,in a spectrophotometer (SHIMADZU,UV–mini-12400) at 340 nm absorbance.The activities were measured at 15 s intervals against a blank containing all components except steroid linear initial velocities were determined graphically.One unit of enzyme activity was the amount of causing change in absorbance of 0.001/min when the enzyme served as the substrate.Testicular 17β hydroxy steroid dehydrogenase activity was measured by using the same procedure and 500 μL of 5% bovine serum albumin solution[1,18].2.7.Enzymes linked immunosorbent assay (ELISA) of serum of serum testosterone level
Serum testosterone (EC:1.1.1.64) was assayed by ELISA kit obtained from Equipar Diagonstic,Italy (using kit No.MBS495055)in ELISA Reader (Merck) according to the manufacturer’s protocol.Briefly,the assay employed the competitive inhibition enzyme immunoassay technique.Standards or samples were added to the appropriate goat-anti-rabbit testosterone antibody-pre-coated microtiter plate wells with the anti-rat testosterone antibody and horseradish peroxidase (HRP)-conjugate.The competitive inhibition reaction was launched between HRP labelled testosterone and unlabelled testosterone in the sample or standard with the antirat testosterone antibody.Substrate A and B solutions were mixed and then added to the wells,and the color turned blue.The color development was stopped by stop solution,and the intensity of the color was opposite to the amount of dihydrotestosterone in the sample.Sensitivity of the assay was 0.06 ng/dL and the results were expressed in ng/dL.
2.8.Measurement of testicular reactive oxygen species (ROS)
Intracellular ROS levels were measured by fluorimetry in cells loaded with the redox-sensitive dye 20,70-dichlorofluorescin diacetate (DCFDA) using a spectrofluorometer[19].Testicular homogenates from all the groups were separately incubated with DCFDA solution (25 μg/mL) for 30 min at 37 ℃.The excitation and emission wavelengths of the dye were at 492–495 nm and 517–527 nm,respectively.
2.9. Assay of antioxidant enzyme activities
SOD (EC 1.15) enzyme activity was assayed based on the autoxidation of pyrogallol method at 420 nm[20] and inhibition of this auto-oxidation by the enzyme SOD where 50% inhibition corresponded to one unit of enzyme activity.SOD activity was expressed as units/mg of protein.Briefly,100 mg of tissue was mixed with phosphate buffer (50 mM and pH 7.8) solution and tissue homogenate was prepared at 4 ℃.Tissue homogenate was centrifuged at 11 200 ×g
for about 25 min.Supernatant was taken in a cuvette and the assay volume contained 100 mM triethanolaminediethanolamine–HCl buffer (pH 7.4) along with 7.5 mM nicotinamide adenine dinucleotide phosphate (NADPH) and 100 mM EDTA-50 mM MnCl(pH 7.0).Then,the solution was kept at room temperature for about 5 min to stabilize.The decrease in absorbance was noted at 340 nm for 20 min over a 5 min interval at 25 ℃ after the addition of 10 mM mercaptoethanol.In this assay system,1 unit of SOD activity was defined as the amount of enzyme required to inhibit the rate of NADPH oxidation of the control by 50%.Catalase (EC 1.11.1.6) activity was assayed following HOsubstrate method and its activity was expressed as nmoles/mg protein[20].The tissue was homogenized with sucrose (0.25 M)solution at 4 ℃.It was centrifuged and post-mitochondrial supernatant was prepared.100 μL of tissue sample containing postmitochondrial supernatant and phosphate buffer (50 mM,pH 7.8)were mixed in a cuvette and decrease in the absorbance was recorded at 240 nm for 5 min over a 60 s interval before the addition of 60 mM HO.The extinction coefficient of HOat 240 nm was 40 Mcm.
GPx (EC 1.11.1.9) was measured by sodium azide-EDTA method and the activity was expressed as μg of reduced glutathione (GSH)consumed/min/mg protein[20].Briefly,tissue homogenized in 50 mM phosphate buffer (pH 7.8) and tissue homogenate was prepared.Tissue homogenate was centrifuged at 448 ×g
for about 10 min and supernatant was taken for GPx activity.Then,0.4 M sodium phosphate buffer (pH 7.0),10 mM sodium azide,4 mM GSH,and 2.5 mM hydrogen peroxide were mixed.The contents were incubated for 10 min at 37°℃ and 0.5 mL 10% trichloroacetic acid was added to stop the reaction.Mixture was again centrifuged at 252 ×g
for 10 min.The supernatant was assayed for GSH content by using 0.3 M disodium hydrogen phosphate and Ellman’s reagent (5,5’-dithiobisnitrobenzoic acid dissolved in 0.1%sodium citrate).The colour developed was read at 412 nm against reagent blank.GSH (EC 1.11.1.9) was measured by using 5,5’-dithiobis-2-nitrobenzoic acid (DTNB) method.Briefly,testicular lysates (20 μL)were mixed with 200 μL of phosphate-buffered saline and 10 μL of DTNB and after 15 min of incubation,absorbance was taken at 412 nm.esults were expressed as mM/mg protein[19].
2.10.Measurement of lipid peroxide (LPO)
LPO was assayedvia
the adduct formed by malonaldehyde with thiobarbituric acid,which was measured against the blank at 532 nm by using a spectrophotometer (UV-1240 Shimadzu,Japan[21].The level of LPO in the homogenate was measured based on the formation of thiobarbituric acid (TBA)-reactive substances.Malondialdehyde (MDA) forms were adducted with TBA,which was measured spectrophotometrically at 532 nm against the blank containing 50 mM phosphate buffer (pH 7.4).MDA,a product of LPO,was used as a standard.An extinction coefficient of 156 000 Mcmwas applied for calculation.2.11.Measurement of nitric oxide (NO)
NO production was estimated by Griess reaction and was expressed in the form of nitrite accumulation.The results were expressed asμmole/mg protein[20].Tissue homogenate (100 mL) was added into micro titer plate followed by addition of 100 mL Griess reagent (1%sulfanilamide in 5% HPOand 0.1% naphthyl ethylene di-amine di hydrochloride) and incubated at room temperature for 10 min.Later,the absorbance was taken at 550 nm by ELISA Reader (Merck).
2.12.Protein estimation
Proteins were estimated by Folin-phenol reagent method using bovine serum albumin as standard[22].
2.13.Electron microscopic studies
Preparation of rat spermatozoa for scanning electron microscopic studies was performed as described elsewhere[23].Briefly,a drop of cauda epididymal plasma was fixed in 2% glutaraldehyde,centrifuged and washed with 0.1 M sodium cacodylate buffer (pH 7.2),centrifuged in distilled water till the buffer solution was washed out and a thin film was applied on a cover slip,dried,sputter coated with gold and finally observed under scanning electron microscope with magnification 1 000× (Model:Zeiss EVO-MA 10,Source:IASST,Guwahati,India).
2.14.Statistical analysis
Values are expressed as mean±standard deviation (mean±SD).Graph-pad prism software (Version 5.0,San Diego,USA) was used to carry out one-way analysis of variance to determine significant differences between the groups and the intra-group means were compared by using Dunnett’spost-hoc
test andP
<0.05 was considered statistically significant.2.15.Ethics statement
The study was approved by the Institutional Animal Ethics Committee (Ref.1802/GO/Re/S/15/CPC5EA) dated 15th November 2017.
3.Results
3.1.Body weight and organ weights
There was a significant decrease in gain percentage of body weight in the only perchlorate treated group and the perchlorate and iodine co-treated group compared to the control group (P
<0.05).The severity of gain percentage of body weight decrease was found in the perchlorate and iodine treated group (+9.29%).Similar regressive changes were also reflected in organ weightsviz
,testis,prostrate,coagulating gland,seminal vesicles,and epididymis where weight of all those organs were significantly decreased maximal in the perchlorate and iodine treated group,then in the perchlorate alone group in comparison to the control group (Table 1).3.2.Urinary perchlorate and iodine
The urinary concentration of perchlorate was significantly higher in rats of the perchlorate alone group and the perchlorate and iodine co-treatment group than that in the control rats for a duration of 45 days (P
<0.05) (Table 2).However,addition of extra iodine with perchlorate significantly reduced perchlorate excretion.On the other hand,the urinary iodine levels were higher in the perchlorate exposed group compared to the control group.No significant difference was found in urinary iodine level between the perchlorate and co-exposure with excess iodine (P
>0.05).Testicular iodine concentrations were significantly decreased in the perchlorate only exposed group,however,were elevated non-significantly in the perchlorate-excess iodine co-exposed group in comparison to the control group.3.3.Epididymal sperm count
A significant decrease in spermatozoal number was noticed in the perchlorate with iodine group compared to the respective control group (P
<0.05) (Figure 1).3.4.Serum testosterone level
After administration of perchlorate and perchlorate with iodine,serum testosterone levels were found to be decreased significantly(P
<0.05);however,the decrease was more in the perchlorate and iodine co-administered group (P
<0.05) (Figure 2).3.5.Testicular steroidogenic enzyme activities
A significant decrease in the activities of the testicular Δ3β-HSD and 17β-HSD enzyme was found in the only perchlorate group and the perchlorate with iodine co-treated group,compared to the control group (Figure 3A &B) (P
<0.05).However,the group treated with perchlorate and iodine in combination was found to be severely affected (P
<0.05).3.6.Testicular ROS
The generation of total ROS in testicular cells upon exposure to perchlorate alone and perchlorate in combination with iodine increased significantly (P
<0.05) in comparison to the control group(Figure 3C).Maximal ROS was produced after co-administration of perchlorate and iodine in the testicular cells for 45 days (P
<0.05).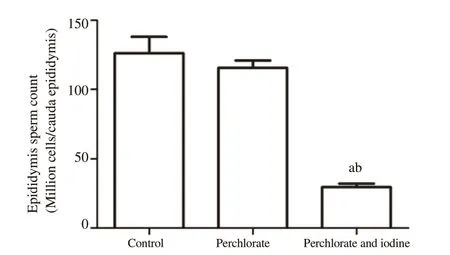
Figure 1.Alteration in epididymis sperm count in the rats subjected to perchlorate and co-exposed with excess iodine.Each bar denotes mean±SD of six rats per group.Values are tested for significance by ANOVA at P<0.05 followed by post hoc test.a:compared to the control group;b:compared to the perchlorate group.
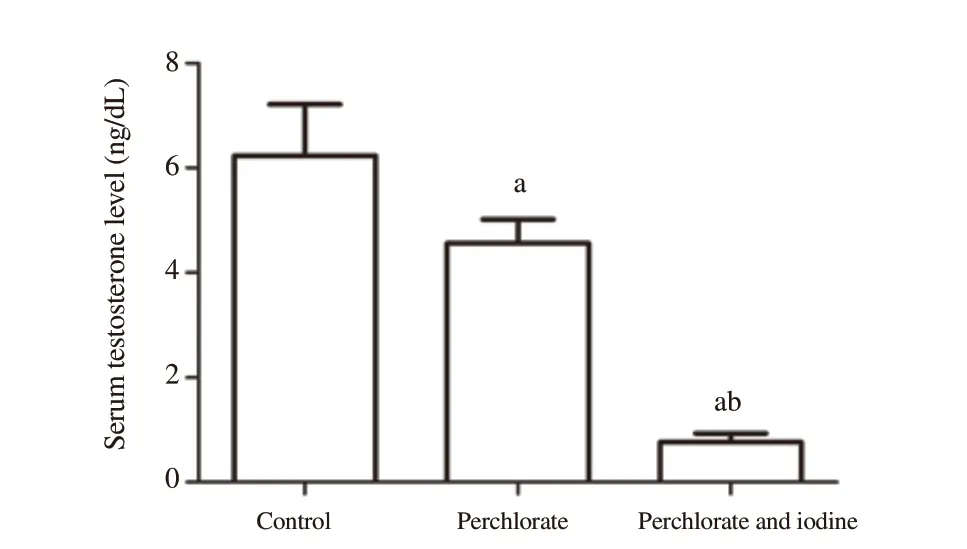
Figure 2.Alteration in serum testosterone level in the rats subjected to perchlorate and co-exposed with excess iodine.Each bar denotes mean±SD of six rats per group.Values are tested for significance by ANOVA at P<0.05 followed by post hoc test.a:compared to the control group;b:compared to the perchlorate group.
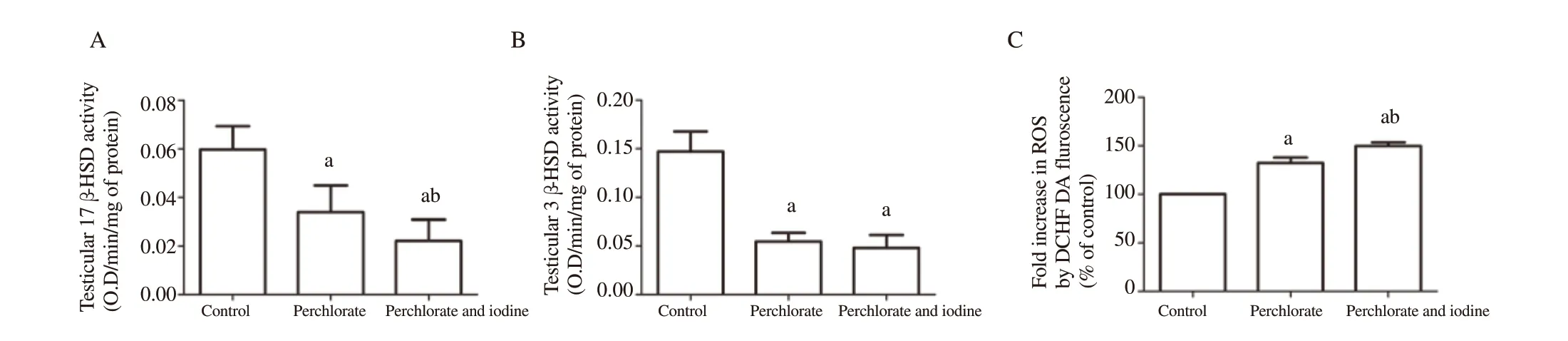
Figure 3.Alteration in testicular 17β -HSD (A),3β -HSD (B) activities and ROS generation (C) in the rats subjected to perchlorate and co-exposed with excess iodine.Each bar denotes mean±SD of six rats per group.Values are tested for significance by ANOVA at P<0.05 followed by post hoc test.a:compared to the control group;b:compared to the perchlorate group.DCFDA:20,70-dichlorofluorescin diacetate.reactive oxygen species;ROS:reactive oxygen species.
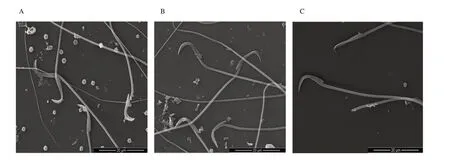
Figure 4.Scanning electron micrograph of spermatozoa of rats at magnification 1 000×.A:The control group shows sperm with normal structure;B:The perchlorate alone treatment group shows a moderate deterioration in the sperm head and neckpiece;C:The perchlorate and excess iodine co-exposed group shows severe effects without head along with structural effects.
3.7.Testicular LPO level and antioxidant enzyme activities
LPO levels were found to be significantly increased in testicular cells upon treatment with perchlorate and iodine in combination(P
<0.05).SOD activity was shown to be decreased in the perchlorate alone treated group and significantly decreased in the group coadministered with iodine.Catalase activity was significantly decreased in the treatment groups wise manner in comparison to the control group with highest effects on the perchlorate and iodine co administered group.GPx and GSH activities both were significantly increased in the treatment groups when compared to the control counterparts (P
<0.05) (Table 4).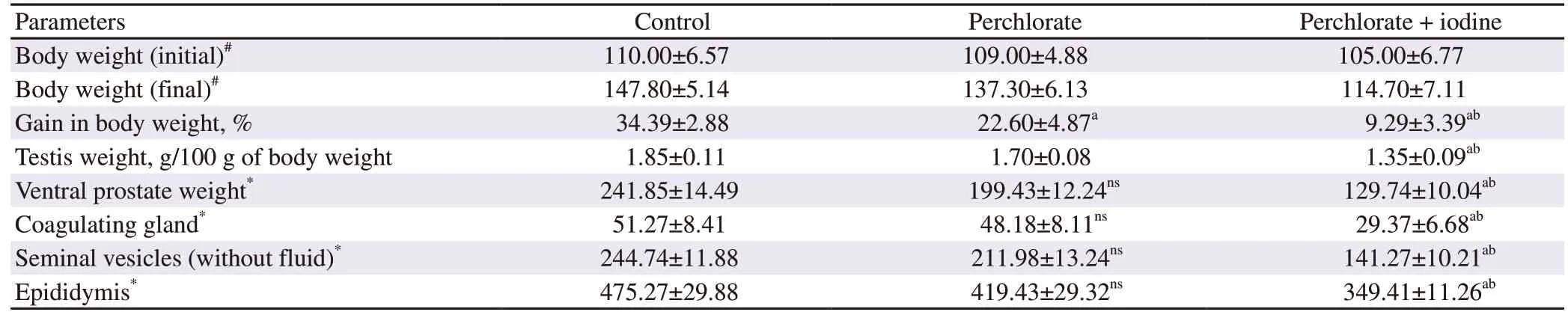
Table 1.Alteration in body weight and testicular,accessory sex organ weight of rats.
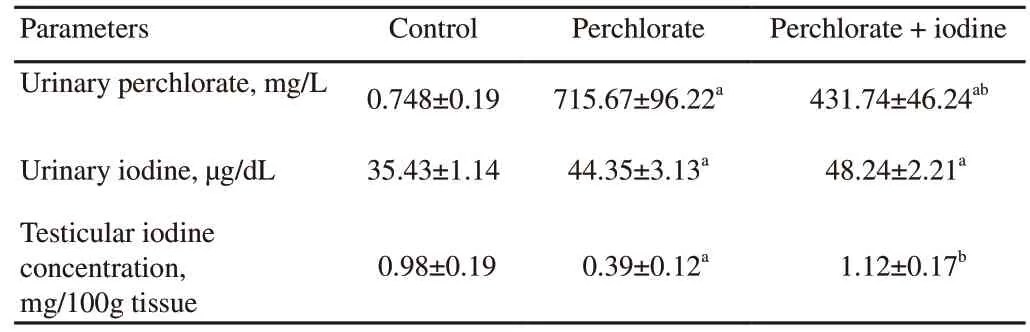
Table 2.Alteration in urinary perchlorate,iodine and testicular iodine concentration of rats.
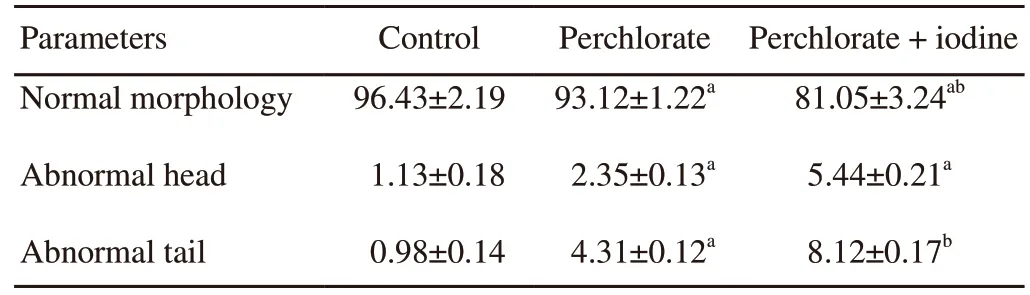
Table 3.Alteration in sperm morphology of rats (%).
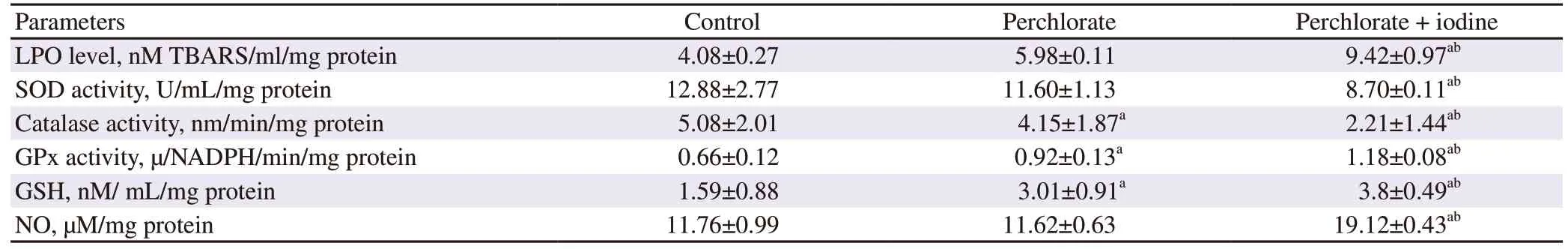
Table 4.Changes in the levels of LPO and antioxidant enzymes of rats.
There was correspondingly a dose-dependent and significant(P
<0.05) increase in lipid peroxidation (Table 4) as evident by increase in thiobarbituric acid reactive substances (TBARS) levels.A significant increase was also observed in NO levels on treatment with perchlorate in combination with iodine (P
<0.05) (Table 4).3.8.Scanning electron microscopic observations of rat sperm from cauda epididymal plasma with sperm morphological analysis
Scanning electron microscopic observations of the cauda epididymal sperm of the control rats showed normal parts (Figure 4A).Plasma membrane was found intact over perforatorium and acrosome.A distinguishable acrosome was covered with acrosomal membrane.The whole spermatozoon was intact with all the membranes and organelles.However,in the perchlorate only treated rats,there were prominent disturbances in the plasma membrane as well as in the acrosomal membrane in the most of sperm heads(Figure 4B).Similar effects were also seen in the group treated with perchlorate and iodine together (Figure 4C) where it was difficult to differentiate the acrosomal membrane as well as the plasma membrane.Serrations in the head region of the spermatozoa were also observed.The shape and size of the sperm head have also changed considerably.There was acute dorsoventrally constriction in the mid-head region of most sperm.The sub acrosomal material was bulged/swelled.Most of the spermatozoa showed a splitting of the head and tail regions,indicating their abnormal morphological structure.A significant increase in gross spermatozoal abnormal morphology was noticed in the perchlorate with iodine group compared to the respective control group (P
<0.05) (Table 3).4.Discussion
Perchlorate only exposure in this study has reduced the net body weight gain percentage in the experimental animals when compared to the control group of rats.These effects were more pronounced when perchlorate was given in combination with excess iodine probably because of their similar mode of anti-thyroidal action inducing hypothyroidism and reflecting on overall level of metabolism[24].Excess iodine on the other hand has been previously found alone responsible for these metabolic effects[1,25].Exclusive perchlorate exposure has been shown to mildly alter the protein and lipid metabolism in experimental animals in addition to lowering the phospholipids and amino acid pool and also influencing lipid composition of mitochondrial membranes[26];however,in our study perchlorate in combination with iodine excess exerted maximum effects on cellular metabolism,reducing weight gain.This study also recorded a significant decrease in relative weight of the testis and accessory sex glands which may be for its negative effect on testosterone bio-synthesis and release[1,20],as continued presence of this androgen is necessary for maintenance of structural and functional integrity of those organs.Combination of both these established anti-thyroidal agents disrupts hypothalamus-pituitarygonadal axis and prevents the secretion and release of testosterone essential for maintenance of shape size and function of accessory sex organs[1,27].Urinary concentration of perchlorate reveals increased excretion pattern in the perchlorate only group;however,in presence of iodine it was reduced.Urinary iodine levels showed obvious increase in the perchlorate only group as it caused effluxes of iodine leading to increased excretion whereas tissue retention of iodine was increased in their co-exposure.Iodine and perchlorate behaviours on sodium-iodide-symporter in extra-thyroidal tissues were not correlated with that of thyroidal sodium-iodide-symporter[28] and that can be the factor for these observable results.These results also indicate that in presence of excess iodine,perchlorate retention was increased and that can be associated observable changes;however,the mechanism involved in this event is still not clear.This study also lacked testicular perchlorate content which could have been substantially added to these present findings.
Anti-fertility effect of perchlorate and excess iodine co-exposure has been reflected by potent decrease in the epididymal sperm count and its morphology in comparison to the perchlorate alone group of experimental animals.Excess perchlorate causes decrease in mitochondrial activity of spermatozoa in amphibians,releasing less energy for the flagellum and impairing movement[29].Excess iodine on the other hand is an established male repro-toxic agent which causes profound change in the concentration,motility,functionality,and morphology of spermatozoa[1,21] by perturbing blood-testicular barrier[3].However,it is to note that perchlorate alone is not a reproductive toxicant[7] yet in combination with excess iodine,the repro-toxic effect of perchlorate and iodine is a considerably augmented raising concern for people living in elevated iodineperchlorate environmental areas.These deteriorating changes were also reflected in ultra-structure of spermatozoa where perchlorate co-exposed with excess iodine sperm showed acrosomal loss,or damage with marked abnormalities in caudal region was observed.This type of change in the rat sperm is suggestive of their inability to fertilize the ovum,leading to male infertility[30].Sperm clearly showed unusual patterns of outer dense fibres,complete absence of plasma membrane,shrinking of the head portion,disorganization of the principal pieces,accompanied by significant decrease in the percentage of normal sperm morphology ascetically in the perchlorate and excess iodine co-exposed group.Oxidative stress generated after administration of perchlorate and excess iodine may be reason for these changes since ROS has a profound effect on the plasma membrane and subsequent functional integrity of the sperm[31,32].
Testicular Δ3β HSD and 17β HSD enzymes are rate limiting enzymes for the synthesis and release of testosterone in steroidogenic pathway,and perchlorate with iodine in combination has exerted maximal effect in downregulation of those enzymes with an obvious decline in serum testosterone level.These endocrine disruptors have been linked as a causative factor for hypothyroidism,which in turn is associated with decrease in the plasma testosterone level and also in testosterone binding globulin[33],which justify those changes.There have been some reports of perchlorate affecting on female reproductive system especially on follicular cells and hindering their structure and function[34];however,in this study it was found that every aspect of male reproductive physiology including spermatozoal structure and function has been severely deteriorated,exaggerated in the presence of excess-iodine suggesting synergistic action,which warrants further experimentation.Iodine in excess has been shown by our group to cause structural and functional alterations in overall physiology of male reproduction in the identical dose and duration;however,in combination with perchlorate,the repro-toxic effects was amplified as seen in this study.
ROS affects negatively on male reproductive functions and possibly induces infertility in the long run by altering hypothalamuspituitary-gonadal axis either directly or indirectly[35].Therefore,in reality,toxicity due to ROS leakage from male gonads during spermatogenesis is kept under tight control by intracellular antioxidant defence systems,including catalases,SOD,peroxiredoxins,and glutathione peroxidases[36] which was also evaluated as a part of this study.As reported earlier,high dose of iodine increased ROS levels and caused symptomatic damage to the plasma and mitochondrial membranes[37] and also to extrathyroidal tissues[21].On the other side,perchlorate exposure in relatively higher doses for 13 weeks did not significantly elevate the ROS level[6];interestingly,in our study much lower doses of perchlorate co-exposed with excess iodine showed increased ROS levels,suggesting the augmenting role of iodine excess on perchlorate when exposed simultaneously.Besides increased ROS production in testicular cells,GPx,catalase,GSH and SOD activities were also altered chiefly after co-exposure of perchlorate and iodine.Both GPx and catalase are major defences against injurious effects of ROS in cellular milieu[38].Studies have shown that decreased activities of catalase and increased GPx were for involvement of plethoric free radicals in addition to classical HOsystem as seen in the study[39,40].Activity of SOD which is the first line of enzymatic defence against intracellular free radicals[41] was decreased though insignificantly in the perchlorate treated group however significantly in the perchlorate and iodine co-administered group implying the vulnerability of the cell membrane and associated organelles to oxidative damage.Destabilized redox potential in the testicular environment has led to free radical chain reactions and consequal suppression on antioxidant capacity[41].Therefore,the increase of free radicals is not recompensed,as anticipated,by a marked alteration of antioxidants in the perchlorate and iodine coexposed group.A high oxidative state in hypothyroid condition has metabolic and biochemical features such as increased mitochondrial enzyme activity[14] and that may additionally influence this present condition.It is thus probable that the testicular cells are damaged by prolonged oxidative stress that surpasses the capability of the organs to synthesize antioxidant molecules or to create them from extra cellular sources as was reported earlier[3].Another hypothesis could be drawn for the competitive entry of iodine and perchlorate in the testicular cells thus overriding the excess iodine-induced deactivation of sodium-iodide-symporter which may further facilitate the entry of more iodine and perchlorate leading to altered intratesticular pro-and antioxidant status,a concept which needs further experimentation and validation.
An elevated LPO level as seen in this study has been previously associated with male infertility and chronic state of oxidative stress[3].Previous findings also indicate that high levels of LPO are a hall mark of degradation of testis and possess severe threat to developing sperm in the testicular environment[3].In this study NO was also found to be elevated in the perchlorate-excess iodine group in addition to oxidative stress markers.Higher concentrations of NO has been linked with male infertility and acute sperm DNA damage[42].Thus,an altered pro-/antioxidant statusvia
increased ROS in the testicular environment associated with increased lipid peroxidation and NO levels under the influence of iodine and perchlorate co-exposure brings about these changes as observed on male reproductive physiology.The study intends to explore the co-exposure of perchlorate and iodine on male reproductive system in adult rats.Addition of an excess iodine only treated experimental group would have given us a clearer picture;however,the same has been published earlier by our group and the result has been correlated on that basis.The paper intended to observe the initial trend in the behaviour of two environmental goitrogens on male reproductive functions;however,more research with finer experimental protocols are needed for the validation of obtained results.
In conclusion,the study provides mechanistic details to predict synergistic role of two established anti-thyroidal agents with analogous mode of action on testicular structure and function.The investigation has been performed inin-vivo
,which adds to the clarity of perceptions regarding long standing public health disorders like infertility and associated male repro-toxic effects in regions where these agents accumulate in the body naturally besides occupational exposure.This study also provides novel insights about augmentation of the relatively moderate repro-toxic effects of perchlorate to a more severe form in presence of excess iodine on male reproductive physiology justifying further investigations.Conflict of interest statement
The author declare there is no conflict of interest.
Funding
The author acknowledges the Science and Engineering Research Board,Department of Science and Technology,Government of India for funding this study (Grant No.LS/PDF/2017/001558).
Author’s contributions
Arijit Chakraborty planned,prepared the draft of the manuscript,and supervised the entire study.
 Asian Pacific Journal of Reproduction2021年5期
Asian Pacific Journal of Reproduction2021年5期
- Asian Pacific Journal of Reproduction的其它文章
- Male infertility:A scoping review of prevalence,causes and treatments
- Syngamy,pronucleus,pronuclear breakdown and zygote
- In-vitro effect of Peganum harmala total alkaloids on spermatozoa quality and oxidative stress of epididymal ram semen
- Ameliorative effect of Punica granatum on sperm parameters in rats exposed to mobile radioelectromagnetic radiation
- Yq AZF microdeletions in male infertility:An update on the phenotypic spectrum,epidemiology and diagnostics
