In-vitro effect of Peganum harmala total alkaloids on spermatozoa quality and oxidative stress of epididymal ram semen
Hanane Derbak,Mohamed Moussaoui,Amine Benberkane,Abdelhanine Ayad
Department of Biological Sciences of the Environment,Faculty of Nature and Life Sciences,Université de Bejaia,06000 Bejaia,Algeria
ABSTRACT Objective:To determine the in-vitro effect of the total alkaloid extract of Peganum (P.) harmala seeds on ram epididymal sperm.Methods: Semen was divided into six groups according to the following concentrations of the P.harmala total alkaloids:1,5,10,50,and 100 μg/mL,and the control group.The samples were incubated at ambient temperature (21 ℃-24 ℃) for 24 h,and analyzed in terms of motility,membrane integrity,and oxidative status.Results: The sperm kinematic parameters,i.e.straight-line velocity,curvilinear velocity,average path velocity,were significantly higher when treated with P.harmala at concentrations ranging from 1 to 10 μg/mL compared to the control group (P<0.05).In addtion,the highest amplitude of the lateral head displacement value was found in the groups treated with concentrations 1 and 5 μg/mL of P.harmala compared to the control group (P<0.05).Total and progressive motilities showed that the extracts at 1,5,and 10 μg/mL exhibited a high percentage after 24 h of incubation.The effect of P.harmala extracts on the membrane integrity of ram epididymal sperm was concentration-dependent and significantly different compared to the control group (P<0.05).Non-significantly lower lipid peroxidation levels were observed after 24 h of incubation of ram epididymal sperm treated with concentrations 1,5,and 10 μg/mL of P.harmala extracts compared to the control group(P>0.05).Conclusions:Low concentrations (1-10 μg/mL) of P.harmala extracts stimulate sperm motility,preserve membrane integrity and protect ram spermatozoa from lipid peroxidation.
KEYWORDS:Alkaloids;Epididymal sperm;Motility;Peganum harmala;Ram;Semen;Membrane integrity;Lipid peroxidation
1.Introduction
Significance
Several natural extracts are used to preserve animal sperm.The development of a new one in extenders with new natural molecules to improve sperm quality is therefore an interesting goal.This study shows that the addition of the total alkaloid extract ofPeganum harmala
seeds at low doses 1-10 μg/mL can improve the quality of epididymal ram sperm by stimulating velocity,maintaining membrane integrity and preserving sperm cells from lipid peroxidation.The addition of a substance as a seminal component that would stimulate motility and sperm transport in the uterine tract would be desirable when artificial insemination is employed in domestic animals.It is known that plant extracts are excellent sources of natural antioxidants and could be used to prevent free radicalinduced harmful effects.Several studies experimented with the antioxidant effects of plant extract were associated with an improvement in spermatozoa quality[1,2].
The use of medicinal plants is widespread throughout the world.The herbal plants are increasingly recognized as a valuable source of natural products and drugs against various disorders and diseases.Medicinal plants have been used to develop new molecules with potential effects that are considered a very interesting research approach.The beneficial uses of these plant species as an antioxidant to improve fertility were reported[3].More recently,attention has focused on the alkaloid extracts from the plants,e.g.
Glycine soja
[4],Epichlo? coenophiala
[5],Tinospora
cordifolia
[6].Alkaloids are secondary metabolites that have attracted a great deal of research interest due to their different effects on human health[7].They have multiple physiological properties,such as anti-proliferative[8],anti-inflammatory[9],antimicrobial[10],antioxidant[11],and antidepressant effects[12].Peganum
(P.
)harmala
L.,called harmal,is a perennial plant rich in alkaloids of the Zygophyllaceae family distributed in a large part of the world.The seeds ofP.harmala
are the most important part of the plant.They contain a great structural diversity of alkaloids such asβ-carbolines (harmine,harmaline,harmalol,and harman) and quinazoline derivatives (vasicin and vasicinone)[13].There are several reports in the literature indicating a great variety of pharmacological activities fromP.harmala
L.It is usually used to treat lumbago,asthma,colic,jaundice,and as a stimulant emmenagogue[14].Moreover,numerous studies have demonstrated thatP.harmala
alkaloids exhibit antitumor[15],anti-inflammatory[16],antiparasitic[17],and antimicrobial effects[18].On the reproductive system,some studies have been carried outin-vivo
using extracts ofP.harmala
.Nevertheless,their results have been divergent,with improved sperm quality sometimes[19,20] and adverse changes in their characteristics other times[21].In the last time,a large body of research has employed a wide variety of antioxidant herbal extracts to enhance sperm performancein-vitro
and potentially sperm-fertilizing capacity.Notably,polyphenols and essential oils are the most used[2,22].Alkaloid extracts have been poorly studied despite their properties,as mentioned above.This work will shed light on the effects of alkaloid extract on spermatozoa,thus contributing to the veterinary and human reproduction field.To our knowledge,the effect ofP.harmala
alkaloids on sperm cellsin-vitro
has never been reported,and their mode of action is still unknown.For this reason,we aimed in the present study to assess the effects of different concentrations of theP.harmala
extract on motility,membrane integrity,and membrane lipid peroxidation level of fresh epididymal ram spermatozoa extended and incubated in Tris-medium for 24 h.2.Materials and methods
2.1.Plant materials
P.harmala
seeds were collected in June 2016 from areas far from any pollution in the fields of Batna provinces (35°32’N,6°10’E) in Algeria.The scientific authentication of plants was carried out in the Ecology Laboratory,University of Bejaia,Algeria.A specimen was kept in the Herbarium of the Department of Pharmacy at Batna-2(voucher number:PhB080).Seeds were put into drying at room temperature (25 ℃-30 ℃) for four weeks.Thereafter,plant material was pounded by using a coffee grinder resulting in a fine powder and kept in the dark.2.2.Preparation of plant extract
The extraction of alkaloids was carried out according to the method described by Kartelet al
[13] with some modifications.Briefly,dried plant materials (20 g) were crushed and extracted with 200 mL ethanol (96%,v/v) in a Soxhlet apparatus for 8 h.The ethanol extract was concentrated under rotavapor,acidified with HCl (2%,v/v),and extracted with petroleum ether (100 mL) to remove fatty materials.The aqueous layer was brought to pH 9 with ammoniac and extracted three times with dichloromethane (20 mL).The organic layer was dried in the open air to obtain a total alkaloid extract.2.3.Epididymal semen collection
Six testes of mature and healthy rams were obtained from a local slaughterhouse and transported at a cooler temperature to the laboratory.The sperm collection was performed by the retrograde flushing method as previously described by Martinez-Pastoret al
[23].The epididymides were dissected free from the testicles and cleaned of connective tissue.Cauda epididymis blood vessels were cut,and the surface area of the cauda was rinsed and wiped.After isolating the cauda epididymis and the vas deferens,1 mL of the sperm washing solution was injected into the lumen of the vas deferens making a small incision near the junction of the corpus and the proximal cauda with a surgical blade.Sperm were collected in conical tubes and checked,i.e.
samples with volume ≥0.8 mL,concentration >2×10spermatozoa/mL,mass motility >3+and individual motility >70% were selected in this study.2.4.Sperm dilution
Pooled semen was diluted with Tris buffer (3.028 g hydroxymethyl aminomethane,1.70 g citric acid,1.25 g fructose,800 UI/mL penicillin G,100 mL distilled water) in order to reach an approximate concentration of 50×10sperm/mL.The sperm sample was divided into 6 groups according to the following concentrations of the total alkaloids ofP.harmala
:the control group (Tris),group 1 (1 μg/mL),group 2 (5 μg/mL),group 3 (10 μg/mL),group 4 (50 μg/mL) and group 5 (100 μg/mL).All alkaloid solutions were prepared from Tris solution and were mixed with an equal volume of sperm suspensions.The samples were incubated at laboratory temperature (21°℃-24 ℃) for 24 h and then were analyzed in terms of motility,hypoosmotic test,and oxidative status.2.5.Computer-assisted sperm analysis (CASA)
Computer-assisted sperm analysis was performed by using the Sperm Class Analyzer (Version 3.2.0;SCA2014,Microptic SL,Barcelona,Spain);10 μL of sperm of each sample was placed in a pre-warmed Makler cell chamber (Makler Counting chambers,Sefi-Medical Instruments ltd.,Biosigma S.r.l.,Italy).During the spermatozoa analysis,five fields were examined by using a phasecontrast microscope at 10× magnification.Six sperm parameters were measured,namely:straight-line velocity (VSL,μm/s),curvilinear velocity (VCL,μm/s),average path velocity (VAP,μm/s),the amplitude of the lateral head displacement (ALH,μm),total motility (%) and progressive motility (%).
2.6.Membrane integrity
Sperm plasma membrane integrity was evaluated by the hypoosmotic swelling test (HOST)[24].Briefly,30 μL of semen from each sample was incubated at 37 ℃ for 1 h with 300 μL of 100 mOsM hypo-osmotic (9.0 g fructose,4.9 g sodium citrate,1 L distilled water).A total of 200 cells per slide were assessed under phase-microscopy (20× magnification).The spermatozoa showing a coiled and swollen tail was considered to have functional membranes.
2.7.Sperm lipid peroxidation
Malondialdehyde (MDA) has been identified as the end product of lipid peroxidation.Measurement of MDA level was determined by quantifying the thiobarbituric acid reacted substances according to Buege &Aust[25],with some modifications.In order to precipitate protein,0.5 mL sonicated sperm treated at different concentrations ofP.harmala
was added to 1 mL of TBA-TCA-Hcl solution(trichloracetic acid 15%,w/v,thiobarbituric acid 0.375%,w/v in hydrochloric acid 0.25N).The mixture in a glass tube was placed into a boiling water bath (95 ℃) for 15 min and then cooled in ice bath to stop the chemical reaction.Subsequently,the suspension was separated (2 000 ×g
for 10 min) and kept in the ice bath.The thiobarbituric acid reactive substances (TBARS) were then quantified by using a spectrophotometer (Biotech Engineering Management Co.Ltd.UK VIS-7220G) at a wavelength of 532 nm.The molar extinction coefficient for MDA was 1.56×10Mcm.The results were expressed as nmol MDA/10spermatozoa.2.8.Statistical analysis
Data were analyzed by using Statview 4.02 software (Abacus Concepts Inc.,Berkeley,CA,USA).The results of experiments were expressed as mean±standard deviation of mean (mean±SD).The normality of the data was confirmed and the differences between concentrations of motility parameters,sperm membrane integrity,and lipid peroxidation were determined by using one-way analysis of variance,followed bypost hoc
Fisher’s LSD test.Values were considered significant whenP
<0.05.2.9.Ethics statement
This research was approved by the Scientific Council of the Faculty of Natural and Life Sciences (Report of Faculty Science Council #05 dated December 14,2016),University of Bejaia,Algeria.Concerning the ethical aspects,the experimental procedure was performed according to good veterinary practice under farm conditions.
3.Results
3.1.Kinematic parameters
The effects of the total alkaloid extract ofP.harmala
on the kinematic parameters of ram epididymal sperm are illustrated in Figure 1.Over the incubation time,a perfect similarity was observed when comparing sperm velocities (VSL,VCL,VAP) recorded by the CASA.The incubation of spermatozoa with the total alkaloid extract caused a significant increase in VSL in the groups treated with concentrations ranging from 1 to 10 μg/mL compared to the control group (P
<0.05),except for the beginning of the incubation time (0 h).A significant decrease in VCL and VAP was observed in the group treated with 100 μg/mL compared to the control group in all time points (P
<0.05),while a significant enhancement of VCL and VAP was observed with the concentrations ranging from 1 to 10 μg/mL.Also,the highest ALH values were found in the groups treated with concentrations 1 and 5 μg/mL ofP.harmala
ranging from (2.94±0.48) μm to (3.94±0.62) μm.3.2.Sperm motility
Total mobility and progressive mobility showed that the extracts at 1,5,and 10 μg/mL exhibited a high percentage after 24 h of incubation of ram epididymal sperm.At a concentration of 50 μg/mL,the progressive motility percentage was slightly similar compared to the control group [(11.87±8.20)% and(12.96±7.15)%,respectively].On the other hand,the treated group with a concentration of 100 μg/mL ofP.harmala
extract showed a significant low value [(2.92±2.31)%] compared to semen diluted in Tris at 24 h of incubation (P
<0.05) (Figure 2).3.3.Membrane integrity
The variation of the membrane integrity percentage after 24 h of incubation of ram epididymal sperm,according to the concentration ranging from 1 to 100 μg/mL ofP.harmala
extracts,is illustrated in Figure 3.The effect ofP.harmala
extracts on the membrane integrity of ram epididymal sperm was concentration-dependent and significantly different (P
<0.05) compared to the control group.The results indicated a significant alteration of the structural integrity of spermatozoa treated with 100 μg/mL ofP.harmala
extract[(62.86±4.33)%].However,the concentrations 1,5,and 10 μg/mL ofP.harmala
extract seemed to improve the sperm membrane integrity[(73.98±5.17)%,(75.11±4.15)%,and (77.52±1.83)%,respectively]compared to the control group [(66.88±6.87)%],(P
<0.05).On the other hand,there were no significant differences between the groups treated with 50 μg/mL ofP.harmala
extract and the control group.
Figure 1.Values for straight linear velocity (A),curvilinear velocity (B),average path velocity (C),and the lateral movement amplitude (D) after 0,1,2,4,24 h of incubation of ram epididymal sperm in the control group and groups treated with different concentrations of Peganum harmala alkaloid extracts (1,5,10,50,100 μg/mL).Data are expressed as mean±SD.At the same time point,different superscripts (a,b,c,d,e,f) in the different concentration groups denote significant differences,P<0.05.VSL:straight-line velocity;VCL:curvilinear velocity;VAP:average path velocity;ALH:the amplitude of the lateral head displacement.
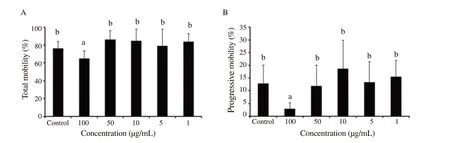
Figure 2.Percentages of total motility (A) and progressive motility (B) after 24 h of incubation of ram epididymal sperm in the control group and groups treated with different concentrations of Peganum harmala alkaloid extracts.Data are expressed as mean±SD.The same superscript letter denotes no significant difference.
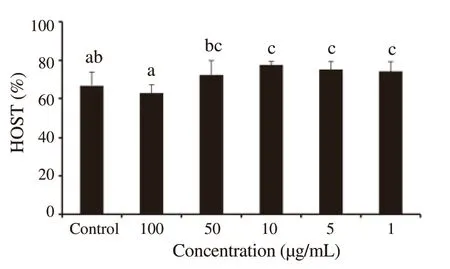
Figure 3.Percentages of membrane integrity after 24 h of incubation of ram epididymal sperm in the control group and groups treated with different concentrations of Peganum harmala alkaloid extracts.Data are expressed as mean±SD.The same superscript letter denotes no significant difference.HOST:hypo-osmotic swelling test.
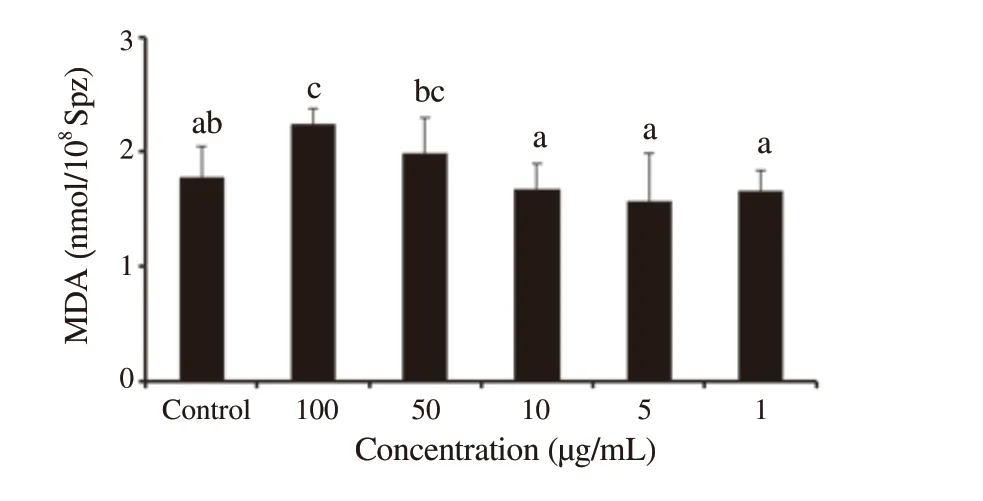
Figure 4.Percentages of lipid peroxidation after 24 h of incubation of ram epididymal sperm in the control group and groups treated with different concentrations of Peganum harmala alkaloid extracts.Data are expressed as mean±SD.The same superscript letter denotes no significant difference.MDA:malondialdehyde.Spz:spermatozoa.
3.4.Lipid peroxidation level
The production of MDA reflects the level of lipid peroxidation of sperm.MDA levels of sperm treated with different concentrations ofP.harmala
extract are shown in Figure 4.After 24 h of incubation of ram epididymal sperm,MDA level non-significantly decreased in the groups treated with concentrations 1,5,and 10 μg/mL ofP.harmala
extract [(1.66±0.22),(1.57±0.42) and (1.68±0.22) nmol/10spermatozoa] compared to the control group [(1.78±0.27) nmol/10spermatozoa] (P
>0.05),while the highest MDA level was observed in the treated group with concentrations 50 and 100 μg/mL ofP.harmala
extract.4.Discussion
Since the 20th century,the consumption of plant extracts known for their rich source of beneficial compounds has increased hugely.However,the interest in herbal products needs thorough research in order to ensure their safety,particularly on the reproductive system.Also,it would be important to establish experiments about plant extracts’ direct effects on sperm performancein-vitro
.According to the literature,numerous scientific papers showed significant biological activity ofP.harmala
extract[15,18].To the author’s knowledge,no study investigating the impact of plant extracts fromP.harmala
L.on ram epididymal sperm was reported.The present study explores the potential effects of total alkaloids fromP.harmala
,in the reproductive field,by analyzing motility,oxidative status,and membrane integrity of ram epididymal sperm.The results of our study have revealed thatP.harmala
extracts have a positivein vitro
effect on sperm motility in comparison with the control group.Low concentrations of the extract ranging from 1 μg/mL to 10 μg/mL stimulate sperm motility,preserve the membrane’s structural integrity,and maintain low lipid peroxidation in ram spermatozoa.In contrast,the highest dose (100 μg/mL) had opposite effects.Our results are consistent with those reported by Hanon[24] in anin vivo
study on male mice,who showed that the alkaloid compounds ofP.harmala
L.had a significant improvement in most parameters of epididymal sperm.A previous study conducted by Tvrdáet al
[26] has suggested that supplementation of berberine to bovine spermatozoa,particularly at concentrations ranging between 1 μmol/L and 50 μmol/L,may protect sperm by enhancing their motility and viability.Ros-Santaella and Pintus[27] reported thatAspalathus linearis
extract significantly affected boar sperm kinetics except for beat cross frequency,ALH,and VCL parameters,which suggests that the rooibos extract enhances sperm velocity.In addition,the lower concentrations (<2.5 μg/mL) ofEurycoma longifolia
have no deleterious effects on sperm functions;however,at very high concentrations,chemical alkaloid compounds may have harmful effectsin-vitro
[28].Recently,one study conducted by Pageet al
[29] reported that overall motility and quality of bovine sperm are decreased by ergonovine and ergotamine,alkaloid compounds found in ergot.A similar observation was reported by Condorelliet al
[30] using the flow cytometry,in which nicotine was suppressed in a concentration-dependent manner in the sperm progressive motility.Otherwise,Haloet al
[31] suggested any dose and/or time dependent significant effect ofViscum albumquercus
on rabbit spermatozoa motility and viability characteristicsin-vitro
.Although some studies are sometimes contradictory in their results,our findings observed an improvement in the performance of epididymal sperm when treated at low doses with alkaloid extracts ofP.harmala
.Nevertheless,the mechanisms of action of alkaloid compounds on sperm motility are still unclear.In the literature,it is noted that mammalian sperm express neuronal receptors on their plasma membrane and are involved in the regulation of sperm function[32].The results obtained concerning the effect ofP.harmala
alkaloid extracts on kinetic parameters,measured by CASA system,of ram epididymal sperm could be due toβ-carboline alkaloids and their ability to bind with sperm membrane receptors.This difference can be explained by the concentration of the active ingredients in the plant extracts and the kind of alkaloid compounds[29].It is important to underline that sperm motility is dependent on many cellular functions,including cyclic adenosine monophosphate and calcium concentrations[33].Also,it should be noted thatP.harmala
alkaloids are lipid-soluble;therefore,they probably can penetrate across sperm membranes and interact with intracellular signaling molecules.However,the adverse effects observed at high concentrations are probably due to harmaline and harmine,which have been previously reportedin-vivo
andin-vitro
on cancer cell lines[34].Moreover,the harmine binds to DNA by intercalation,consequently inducing DNA fragmentation[35],affecting fertilization rates and sperm motility[36].Interestingly,results of both sperm plasma membrane parameters were significantly concentration-dependent when compared to the control group,in agreement with the results reported by Shalawehet al
[37] and Tvrdáet al
[26].A similar observation was reported by Sureshet al
[38],in which the effect of seed extracts ofMucuna pruriens
,rich in alkaloids such as prurienine,prurieninine,and prurienidine,showed remarkable potential reducing sperm damages induced by oxidative stress,with a well preserved epididymal spermatozoa DNA.Our results are corroborated with those reported by Ros-Santaella &Pintus[27],whose alkaloid extracts of rooibos(Aspalathus linearis
) seem to preserve membrane integrity during sperm storage.Likewise,Baghshahiet al
[39] showed that plasma membrane integrity was higher in the semen exposed to a medium containing 75 μg/mL of clove buds extract (Syzygium aromaticum
)extract after cryopreservation processes.Several scientific papers reported that oxidative stress causes defective changes in cellular functions[40].It is generally accepted that antioxidants have shown the ability to reduce free radical production,and their addition to spermatozoa could improve the sperm performances[1,2];a number of studies have shown the biological effects of alkaloid extracts ofP.harmala
,including antioxidant power on oxidative stress in mammalian.This would be due to the fact thatβ-carboline alkaloids ofP.harmala
can act as scavengers of free radicals[41].One possible explanation is the prevention of the oxidation of biogenic amines by inhibiting the monoamine oxidase,which is responsible for the production of toxic substances such as hydrogen peroxide,inducing oxidative stress[42].Indeed,in agreement with our observation,many investigations demonstrated that the essential oils ofRosmarinus officinalis
displayed a high antioxidant activity on sperm quality[3,22].Moreover,the treatment by cholesterol and vitamin E,known for their antioxidant property,preloaded in cyclodextrins,reduces the lipid peroxidation of spermatozoa and increases the membrane integrity[43].It is reported that the antioxidant action at low doses protects spermatozoa from membrane damages[44],thus preserving their fluidity,integrity,flexibility and consequently the conservation of sperm quality.This research represents a preliminary study of the impact of total alkaloids ofP.harmala
on ram semen.Some limitations of this study may include the source of the epididymal sperm collected from rams intended for meat consumption.Further,more intermediate concentrations and a longer incubation time are needed to yield more interesting results in the sperm conservation field.In conclusion,this study reports the effect ofP.harmala
alkaloids on the performance of ram epididymal sperm.The current investigation demonstrated that sperm motility considerably increases at low concentrations (1-10 μg/mL) of alkaloid extracts ofP.harmala
.Also,based on our findings,P.harmala
alkaloids could protect sperm against oxidative damages and improve the membrane integrity of ram epididymal sperm.This plant could eventually be beneficial from infertility therapy.However,future studies should be carried out to understand the biological and biochemical mechanisms underlying this beneficial effect.Conflict of interest statement
The authors declare there is no conflict of interest.
Acknowledgements
The authors would like to thank the management of the Bejaia communal slaughterhouse for their help in sampling ram testicles and for their hospitality.Also,our sincere thanks goes to Dr.R.Kebbi for her the technical assistance in plant alkaloids extraction.This study was conducted in Associated Laboratory in Marine Ecosystems and Aquaculture,University of Bejaia,Algeria.
Authors’ contributions
Hanane Derbak performed the experimental work,the statistical analysis,and wrote the manuscript.Mohamed Moussaoui conducted the testicular samples from the slaughterhouse and analyzed the oxidative stress.Amine Benberkane contributed in the technical aspect of the CASA system and the statistical analysis.Abdelhanine Ayad designed and supervised the experimental study,wrote and revised the manuscript.All authors have read and approved the final version of the manuscript.
 Asian Pacific Journal of Reproduction2021年5期
Asian Pacific Journal of Reproduction2021年5期
- Asian Pacific Journal of Reproduction的其它文章
- Male infertility:A scoping review of prevalence,causes and treatments
- Syngamy,pronucleus,pronuclear breakdown and zygote
- Ameliorative effect of Punica granatum on sperm parameters in rats exposed to mobile radioelectromagnetic radiation
- Excess iodine supplementation aggravates the toxic effects induced by perchlorate on the male reproductive system in rats
- Yq AZF microdeletions in male infertility:An update on the phenotypic spectrum,epidemiology and diagnostics
