Incidence of adverse reactions to COVID-19 vaccination: A metaanalysis of randomized controlled trials
Xin-Xin Wu, Jin-Jian Yao, Jin Qian, Qi-Feng Huang, Tang Deng, Shuang-Qin Xu,Hang-Fei Wang, Qi Li, Ji-Chao Peng, Yang Yi, Nan Li, Yue Huang, Xiao-Ran Liu,?
1School of Public Health, Hainan Medical University, Haikou 570100, China
2Emergency Department, Hainan General Hospital Affiliated to Hainan Medical University, Haikou 570100, China
3Emergency and Trauma College, Hainan Medical University, Key Laboratory of Emergency and Trauma of Ministry of Education, Haikou 570100, China
4Department of Intensive Care Medicine, The First Affiliated Hospital of Hainan Medical University, Haikou 570100, China
5Emergency Department, The First Affiliated Hospital of Hainan Medical University, Haikou 570100, China
ABSTRACT
KEYWORDS: COVID-19; Adverse reactions; Safety; Randomized controlled trials
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), whose main routes of transmission are droplets and close contact. COVID-19,featured by a high risk of contagion, rapid and wide-spreading, has swept over the whole world since its outbreak[1]. As of August 2021,the statistics released by the World Health Organization (WHO)reported a cumulative 217 million confirmed cases and more than 4.5 million deaths across the world[2]. Although the current epidemic situation in China has been effectively controlled, the epidemic situation is far from end outside China, and the numbers of infections and deaths have been increasing in some areas. On top of this, some COVID-19 patients are exposed to the risk of reinfection[3].
The ongoing pandemic of COVID-19 has produced multiple variants. Compared with the original virus, the transmission of the mutant strain is much faster, the incubation period is shorter[4].Therefore, the prevention and control measures must be given top priority to contain the pandemic. However, the treatment of COVID-19 is still mainly based on symptomatic treatment, and there is still a lack of effective antiviral drugs[5]. To accelerate the termination of the global pandemic, extensive vaccination is one of the measures at play. Thus, more investments must be pooled into the research and development (R&D) of the vaccines[6].
There is no doubt that vaccination is an effective measure for virus prevention and control. Vaccines currently under R&D or already in the pipeline include inactivated vaccines, attenuated vaccines, nucleic acid vaccines, subunit vaccines, and virus-like particle vaccines.As of April 2, 2021, WHO announced a total of 184 vaccines under preclinical study worldwide, and 85 vaccines under clinical study[7]. From the beginning of the COVID-19 outbreak, the R&D of vaccines has never been stopped. Rapid vaccine development and rapid application have been carried out. Thus, increasing numbers of studies focused on the safety and immunogenicity results of clinical trials of various vaccines have been published[8-12]. The safety of vaccines developed in an emergency (short research and development period) must be monitored for the long haul, especially the adverse reactions. As more vaccines are going into service, the adverse reactions must be closely watched. Adverse reaction of COVID-19 vaccines has already been reported, for example, local adverse reactions such as pain, erythema, itching at the injection site,and systematic adverse reactions including fatigue or weakness, and fewer cases of severe fever or allergies. Therefore, 30 min of onsite observation is requested after vaccination[8]. This paper aims to evaluate the safety of some widely used COVID-19 vaccines through evidence-based medicine and provide a reference for the clinical application of the vaccines.
2. Patients and methods
2.1. Search strateg y
We systemically searched PubMed, Embase, The Cochrane Library, Web of Science, CNKI, WanFang Data, and VIP Database.The references of the included papers were also searched and reviewed. Randomized controlled clinical trials (RCTs) on the safety of different types of COVID-19 vaccines in the population were searched from the inception of each database to August 31,2021. The following keywords were used, both separately and in combination, “COVID-19”, “SARS-CoV-2”, “2019-nCoV”,“vaccine”, “vaccines”, “safety” or “adverse reactions”.
2.2. Inclusion and exclusion criteria
Inclusion criteria: (1) RCTs; (2) Healthy individuals with no prior history of COVID-19 infection; (3) The vaccination group received COVID-19 vaccine and the placebo group received placebo; (4)Outcome indicators: ① Incidence of local adverse reactions related to the vaccine injection site, including swelling and pain;② Incidence of systemic adverse reactions after a vaccine injection,including fatigue, fever, headache, etc.
Exclusion criteria: (1) Studies with the same research subjects; (2)Repeated publications or publications not in Chinese or English; (3)Incomplete or missing data information, unable to obtain complete data and full-text literature; (4) Animal experiments, literature reviews, case reports, and studies inconsistent with this study's aims.
2.3. Data extraction
Literature screening and quality evaluation were performed independently by two investigators. All the retrieved data were input into EndNote X9.0 software. Duplicate articles were first deleted,and then articles that were inconsistent with this study's aims were further eliminated by reading the titles and abstracts of the articles.After cross-checking, the literature screening was completed.When there was disagreement about a study, the third investigator will join the evaluation. The extracted data were as follows: First author, publication date, study area, study phase, type of vaccine,vaccine manufacturer, vaccine registration information, baseline characteristics (sample size, sex, and age), and the number of adverse events.
2.4. Quality evaluation
The quality assessment and cross-check of the included literature were independently conducted by two researchers using a unified form. Disagreements can be resolved through discussion or consultation with a third person. The methodological quality of each study was analyzed as follows: (1) Whether random allocation method was used correctly; (2) Whether the blind method is used and whether the blind method is correctly implemented; (3) Whether to report the situation of lost follow-up; (4) Distribution of hidden situations; (5) Whether to use intentionality analysis for quality evaluation.
2.5. Statistical methods
Meta-analysis was performed using Revman 5.2 software,and relative risk (RR) and 95% confidence interval (CI) were used as effect analysis statistics for dichotomous variables.The heterogeneity test was performed on the included studies. When P≥0.1 and I2≤50%, there was no significant heterogeneity between the results of the studies, so the fixed-e ffects model was used for analysis; when P<0.1 and I2>50%, the meta-analysis was performed using the random-effects model. If there is obvious heterogeneity, subgroup analysis or sensitivity analysis was applied.
3. Results
3.1. Literature search results
A total of 862 articles were screened at first, and 57 articles with greater relevance were selected after removing duplication and initial screening. By carefully reading the full text to determine if there were any data related to an adverse vaccine reaction, 13 articles were finally included. The flowchart of the literature screen is shown in Figure 1.
3.2. Basic characteristics of the included studies

Figure 1. The study flowchart.

Table 1. Basic characteristics of the included studies.
A total of 13 studies included in the literature were randomized,blind, controlled trials, including 7 inactivated viral vaccines, 4 mRNA vaccines, and 2 adenovirus vector vaccines. A total of 81 287 participants were randomly assigned to either COVID-19 vaccine or placebo for quantitative analysis. Among them, 42 392 were COVID-19 vaccinators, with 22 699 adverse reaction events.The sample size varies greatly among different studies, with 36 cases at the lowest level and 15 181 cases at the highest level.Among the included studies, 2 RCTs were conducted of minors and the remaining 11 of adults. The baseline characteristics, vaccine characteristics, and developer information of study participants are shown in Table 1.
3.3. Quality of the included literature
The quality of the 13 included studies was evaluated using the bias risk assessment tool of the Cochrane Systematic Review Manual. The results are shown in Figure 2. The quality of the studies included allocation concealment, blindness, incomplete outcome data, good selection reporting, and low risk of deviation.
3.4. Total incidence of adverse reactions
A total of 13 RCTs were included. Meta-analysis of the random effects model showed that the incidence of total adverse reactions was significantly higher in the vaccination group than in the placebo group (RR=1.67, 95% CI: 1.46-1.91, P<0.01) (Figure 3).
3.5. Incidence of local adverse reactions
A total of 11 RCTs were included. Meta-analysis of the random effects model showed that the incidence of local adverse reactions was significantly higher in the vaccination group than in the placebo group (RR=2.86, 95% CI: 2.11-3.87, P<0.01) (Figure 4A). Local adverse reactions in the vaccination group were higher than those in the placebo group (77.3% v.s. 20.4%, χ2=208.79,P<0.01). The most common local adverse reactions were injectionsite adverse reactions, and most of the injection site adverse reactions were mild and moderate, mainly pain and swelling at the injection site, with the incidence of 77.5% and 7.0%, respectively.Symptoms usually last for a short time and resolve spontaneously within 3 to 5 d.
3.5.1. Incidence of painA total of 9 RCTs were included. The randomized effects model meta-analysis showed that the incidence of pain in the vaccination group was significantly higher than that in the placebo group(RR=2.55, 95% CI: 1.75-3.70, P<0.01) (Figure 4B).
3.5.2. Incidence of swelling
A total of 9 RCTs were included. The random-effects model meta-analysis showed that the incidence of swelling was significantly higher in the vaccination group than in the placebo group (RR=4.16, 95% CI: 1.71-10.17, P=0.002) (Figure 4C).

Figure 2. Risk of bias of the included studies.

Figure 3. Forest plot of the incidence of total adverse reactions.
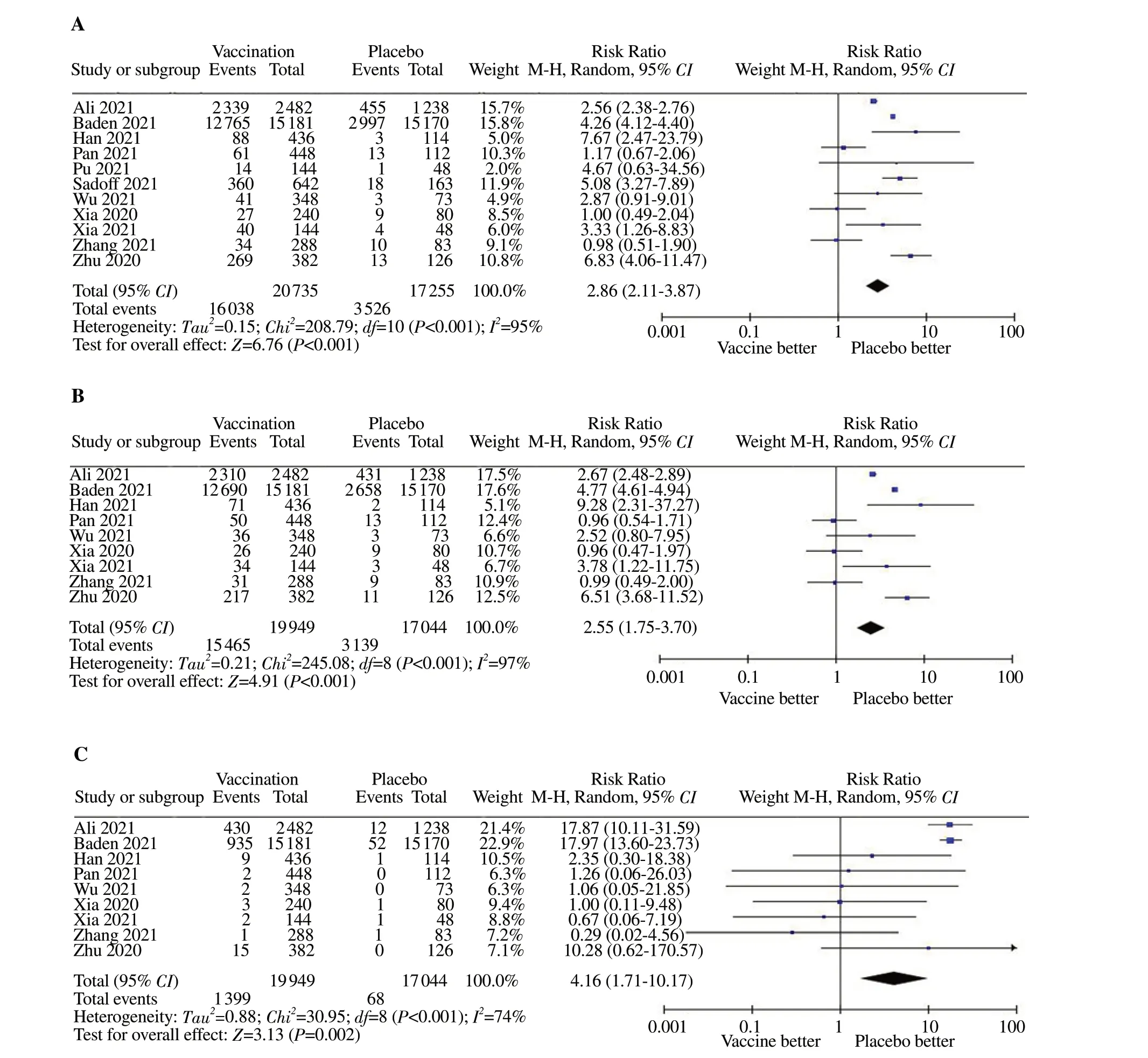
Figure 4. Forest plot of the incidence of local adverse reactions, pain and swelling. A: The incidence of local adverse reactions; B: Incidence of pain; C:The incidence of swelling.
3.6. Incidence of systemic adverse reactions
A total of 10 RCTs were included. Meta-analysis of the randomeffects model showed no significant difference in the incidence of systemic adverse reactions between the vaccination group and the placebo group (RR=1.25, 95% CI: 0.92-1.72, P=0.16) (Figure 5A). The most common systemic adverse reactions were fatigue, headache,and fever, with incidence rates of 35.2%, 31.1%, and 1.71%,respectively. These reactions were general adverse reactions and mostly self-cured within a few days.
3.6.1. Incidence of fever
A total of 9 RCTs were included. Meta-analysis of the fixedeffect model showed that the incidence of fever was significantly higher in the vaccination group than in the placebo group, and the difference was statistically significant (RR=2.34, 95% CI: 1.84-2.97, P<0.01) ( Figure 5B).
3.6.2. Incidence of fatigueA total of 9 RCTs were included. Meta-analysis of the fixed-effect model showed that the incidence of fatigue was significantly higher in the vaccination group than in the placebo group (RR=1.36, 95%CI: 1.32-1.41, P<0.01) (Figure 5C).
3.6.3. Incidence of headache
A total of 9 RCTs were included. Results of the fixed-effect model meta-analysis showed that the incidence of headache in the vaccination group was significantly higher than that in the placebo group (RR=1.22, 95% CI: 1.18-1.26, P<0.01) (Figure 5D).

Figure 4. Forest plot of the incidence of systemic adverse reactions, fever, fatigue and headache. A: The incidence of systemic adverse reactions; B: The incidence of fever; C: The incidence of fatigue; D: The incidence of headache.

Table 2. Analysis results of different subgroups.

Figure 6. Funnel plot of the incidence of total adverse reactions after injection of COVID-19 vaccine. SE: Standard error; RR: Risk ratio.
3.7. Subgroup analysis
3.7.1. Subgroup analysis of different vaccinesAccording to the types of vaccines, the subgroups were divided into inactivated vaccines, mRNA vaccines, and adenovirus vector vaccines. Meta-analysis results showed that the incidence of adverse reactions after injection of the three COVID-19 vaccines was higher than that of the placebo group, and the difference between the mRNA vaccine subgroup and the adenovirus vector vaccines subgroup was statistically significant (P<0.01), as shown in Table 2.
3.7.2 Subgroup analysis of different age groups
Subjects were divided into two groups according to age: The juvenile subgroup (<18 years old) and the adult subgroup (≥18 years old). Meta-analysis results showed that the incidence of adverse reactions after injection of COVID-19 vaccine in subgroups of different ages was higher than that in the placebo group, with a statistically significant difference (P<0.01), as shown in Table 2.
3.8. Publication bias
Funnel plots were drawn from the data extracted from the included studies. As shown in Figure 6, most of the large samples were concentrated in the middle and top of the graph, while the small samples were concentrated on the lower right side. The overall distribution showed incomplete symmetry, suggesting a certain degree of publication bias, which may be related to the methodological quality of the included literature studies.
4. Discussion
A total of 13 studies were included in this retrospective metaanalysis to evaluate the safety of COVID-19 vaccines. A total of 81 287 subjects were included in the study, and none of them reported serious abnormal reactions after receiving the COVID-19 vaccine. The symptoms usually last for a short time and resolve spontaneously within 3 to 5 d, similar to other widely used vaccines.Vaccine-related serious adverse events are rare. Overall, this suggests that COVID-19 vaccines have a good safety profile.
To better understand the incidence of adverse reactions to COVID-19 vaccines, subgroup analysis was conducted from two aspects of vaccine type and age of patients. In terms of safety, the incidence of adverse events of all three types of vaccines was higher than that of the placebo group, and the incidence of adverse events of adenovirus vector vaccines was higher than that of mRNA vaccines and inactivated vaccines. The 2 included RCTS[19,22] showed that grade 1 (the least serious) adverse reactions occurred mainly after the injection of COVID-19 vaccine in minors, and no serious adverse reactions were observed, and their ability to induce antibody production was very strong, indicating that the injection of COVID-19 vaccine in minors is also safe.
Among the 13 studies included in this meta-analysis, 8 were done in China[13-19,24], which to some extent validates the safety of the Chinese vaccines. A recent Chinese study on a survey of 8 742 people's willingness, hesitancy, and refusal to receive vaccinations showed that 9.0% of them explicitly refused vaccination, while 834(35.5%) reported vaccine hesitancy. Further questioning of those 834 people found that 48.8% expressed doubts, 39.4% were refusers,and 11.8% were delayers[26]. Among the 8 742 patients, only 69.7%believed that the Chinese vaccines were reliable. People's acceptance of vaccines and willingness to vaccinate is largely related to their uncertainty about the safety of the vaccines and the incidence of adverse reactions. For these reasons, meta-analyses of adverse reactions to vaccines are beneficial to strengthen public trust in vaccines. They can increase the willingness of people to vaccinate and can facilitate the earlier establishment of herd immunity.
Vaccines are heterogeneous macromolecular substances in the body that induce immune cells to produce humoral immunity and cellular immunity. In addition to the normal immune response, there are often some unfavorable physiological reactions in the body[27].Adverse reactions to vaccination are divided into general reactions and abnormal reactions. The general reactions of vaccination are normal reactions, which can be cured on their own within a few days without treatment. Local reactions are generally mild and limited. Systemic reactions can be caused by vaccination, though some reactions have nothing to do with the vaccine itself[28]. On the one hand, the causes of adverse reactions may be related to the health condition of the vaccine recipients[29]. Vaccine recipients have different allergic histories or combinations with underlying diseases,and long-term treatment with certain types of drugs leads to immune suppression or immune deficiency. Therefore, adverse reactions may occur. In addition, to improve the immunogenicity of antigens, it is necessary to add adjuvants to the antigens to enhance their ability to induce an immune response in the human body. Currently, aluminum adjuvants are the only approved adjuvants[30]. Inactivated COVID-19 vaccines all contain aluminum hydroxide adjuvants. Aluminum adjuvants may cause local irritation and adverse reactions, but these adverse reactions are usually temporary and self-healing[31].Currently, there is no evidence that aluminum salts in vaccines can cause severe or persistent adverse reactions[32]. If the ambient temperature of the vaccination site is too hot or cold, this will have a certain impact on the body of the vaccine recipient, leading to the occurrence of adverse reactions. Insufficient sleep and vigorous exercise after vaccination is important reason for adverse reactions after vaccination[29]. Therefore, to ensure the effectiveness and safety of vaccination, medical personnel must pay more attention to the above factors. They must be trained in managing different adverse reactions to vaccinations. Otherwise, improving patients' knowledge about vaccines, standardization of vaccination procedures, and quick response to adverse reactions are also effective measures.
This study has the following limitations. First, due to the differences in the types of COVID-19 vaccine candidates, their characteristics,their immune mechanisms, their immunogenicity, and the study populations, there is heterogeneity in the combined analysis of the literature. Second, many of the included studies did not report the overall incidence of local adverse reactions or systemic adverse reactions, but the incidence of redness, swelling, heat, and pain or the incidence of fever and fatigue were individually statistically analyzed. This resulted in a limited number of studies satisfying the inclusion criteria, so only 13 were included. Finally, different researchers used different standards in the recording of adverse reactions after the COVID-19 vaccination, which may impact the final study results. Due to these limitations, the final study results must be interpreted carefully. However, the combined findings can reflect the actual level of the occurrence of adverse reactions after the current COVID-19 vaccine candidate vaccination to a certain extent, giving the study clinical reference value.
5. Conclusions
In conclusion, the key to curbing the COVID-19 pandemic is to implement the COVID-19 vaccination program worldwide. To ease the uncertainness and safety of vaccination, adverse reactions caused by vaccines need to be treated objectively. Inactivated vaccines, mRNA vaccines, and adenovirus vaccines all can induce immune responses through similar viral proteins, but different types of vaccines may cause different vaccine-related adverse reactions.Adverse reactions caused by the same type of vaccine are related to the individual status including underlying diseases and allergic history of the vaccine recipients, because not only COVID-19 vaccines but other vaccines may also occasionally elicit adverse reactions[33]. Before during, and after vaccination, attention should be given to the possibility of adverse reactions, and the inoculation procedure should follow standardized operation and procedures that reduce the incidence of adverse reactions and improve the safety of vaccination[8]. The controllable and treatable characteristics of adverse reactions to the vaccines will reduce the public's concerns about the safety of the vaccine and increase their willingness to vaccinate. The global COVID-19 situation is still worsening, and the population is generally susceptible to SARS-CoV-2. Vaccination is the only way to get out of the pandemic or decrease the loss of public, we should encourage more citizens to vaccinate to rapidly establish an immune barrier in the local population, thereby effectively blocking the resurge of the COVID-19 epidemic.
Conflict of interest statement
The authors report no conflict of interest.
Funding
This research was funded the Province Natural Science key Foundation of Hainan (No: ZDYFXGFY2020004 and No: ZDYF 2019125); the National Natural Science Foundation of China (No:81960351), and Hainan Province Clinical Medical Center.
Authors'contributions
X.X.W, J.J.Y, J.Q. and T.D., were responsible for the conception and designing of the study, Q.F.H., S.Q.X., H.F.W. and Q.L.was responsible in data gathering, J.C.P, Y.Y., N.L., Y.H. was responsiblefor data analysis, X.X.W. and J.J.Y. drafted the manuscript, X.R.L. responsible for thecritical review of the paper.All authors participated in interpretation of the data.
 Journal of Acute Disease2022年1期
Journal of Acute Disease2022年1期
- Journal of Acute Disease的其它文章
- Resuscitative cardiac arrest during a Caesarean section-When every second counts: A case report
- Influential factors of healthcare provider resilience in disasters: A thematic analysis
- Acute and sub-acute toxicities of hydroalcoholic extract of Allium affine aerial parts in rats
- Arrhythmia and its risk factors post myocardial infarction: A prospective study
- Health literacy, behavioral and psychosocial characteristics in coronary artery patients: A hospital-based study in Turkey
