lnflammatory bowel disease-related colorectal cancer:Past,present and future perspectives
lNTRODUCTlON
Chronic inflammation is known to be a major risk factor in the pathogenesis of cancer.Inflammatory bowel disease(IBD)is a chronic inflammatory condition affecting the gastrointestinal(GI)tract and IBDrelated colorectal cancer(IBD-CRC)is one of its major and serious complications.Although only 1%-2%of IBD patients develop IBD-CRC,it contributes to about 15% of IBD-related mortality[1].As per current long-term epidemiological data,the risk of CRC in IBD patients is high,particularly in patients with extensive ulcerative colitis(UC).Eaden
[2]in their meta-analysis of 116 studies found that the incidence of IBD-CRC was 2%,8% and 18% at 10,20,and 30 years after the onset of UC,respectively.The role of Crohn’s disease(CD)in the development of CRC is debatable and considered modest in comparison to UC.Canavan
[3]in their meta-analysis estimated cumulative risk of 2.9% at 10 years,5.6% at 20 years,and 8.3% at 30 years in patients with CD.However,factors like patient selection,sample size,duration of follow-up,completeness of case recruitment,and geographical differences may have influenced these estimates.
The severity of inflammation is a significant risk factor that increases the risk of IBD-CRC.Inflammation-related oxidative stress leading to genomic instability is considered the main trigger for the development of CRC.Studies on colonic tissue in IBD-CRC at the cellular and molecular level have found that the sequence of development of carcinogenesis is different from that observed in sporadic cancer in the non-inflamed colon.IBD-associated carcinogenesis follows an ‘inflammation-dysplasiacarcinoma’ sequence instead of the ‘adenoma-carcinoma’ sequence seen in sporadic CRC[4].
In this review article,we present a comprehensive overview of the literature on the epidemiology of IBD-CRC over decades,risk factors and pathogenesis including molecular pathways implicated.In addition,we present preventive strategies,current evidence for surveillance,the evolution of surveillance techniques with time,chemoprevention and explored which endoscopic technologies are likely to become standard for surveillance in the future.We have also touched upon the emergence of using diet and faecal microbiota modulation as a potential strategy in the future.
Trends in the incidence of IBD related CRC over decades
Many recent population-based studies and meta-analyses have shown that the risk of IBD-CRC is lower than what has been previously reported,most of which were from studies done in tertiary referral centres.One probable reason for this difference could be that recent studies are more focused on selecting the right study population(included more severe cases),sample size and are more thorough with follow-up;completeness of study recruitment and geographical differences were perhaps taken into consideration while analysing their findings.The details of the study results that reported the incidence of IBD-CRC over the last four decades are summarized in Table 1.
Then a young man better dressed and better looking than any of us presented himself at our table. “Good evening, my name is Paul, and I’ll be your waiter this evening. Would you like a few minutes before I take your order?”“No,” I said, “I’m just a meat-and-potatoes guy, so I’ll have the filet3 mignon and baked potato.”
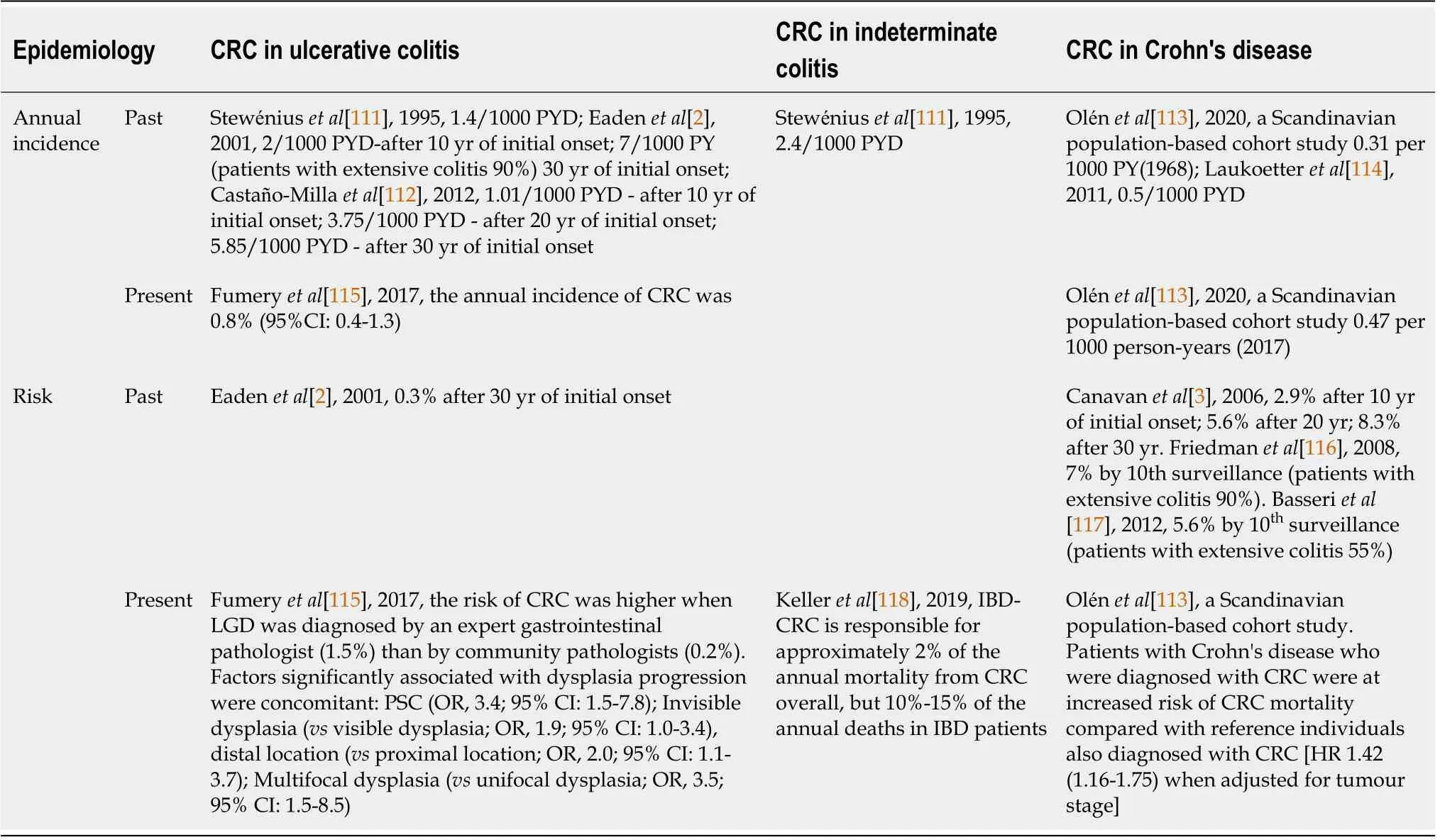
RlSK FACTORS OF lBD RELATED CRC
The risk factors of IBD-CRC can be broadly classified as factors that are genetic or familial and factors related to diet and lifestyle.These are illustrated in Figure 1A.
Dietary therapy is also considered to be helpful,especially in children with CD who receive exclusive enteral nutrition[95,97].Therefore,microbiome-modulating interventions like the application of probiotics[98,99],prebiotics[97,100,101],antibiotics,faecal microbiota transplantation(FMT)[102,103],and gene manipulation is being widely explored as new treatment options for a large number of chronic inflammatory diseases including UC,CD,and CRC.Genetic studies involving IBD patients reported 163 IBD susceptibility gene loci.These loci were found to be involved in regulating the host and gut microbes' interactions[104,105].Mechanistically,it is plausible that by correcting the gut microbiota composition,the innate immune system can be modulated,leading to lesser inflammatory damage to the gut epithelium.This could enhance gut barrier function,prevent pathogen colonization and exert selective cytotoxicity against tumour cells[92].These actions could break the vicious cycle of inflammation-mediated dysplasia.
I...I thought you could use it for something. Susan s stammered3(,) explanation did nothing to help us understand why a twelve-by-eighteen-inch dark blue carpet remnant() was being presented as a birthday gift.
Age and disease duration
colon with quiescent disease[68].
Geographic variation risk
In a meta-analysis,Zhou
[10]found that Oceania has a higher incidence than other continents.In Asia,it was found that the risk of CRC among UC patients increased after 10-20 years of disease duration,whereas in Europe,the risk of CRC in UC showed no statistical difference in disease duration for 1-9 years,10-20 years,21-30 years,or more than 30 years.In North America,the risk of CRC among UC patients increased significantly after more than 30 years of disease detection.
The fisherman slept well and soundly, for he had done a great deal that day, but his wife could not sleep at all, and turned from one side to another the whole night long, and thought, till she could think no longer, what more she could become
Gender
Gender is reported to be an important risk factor for IBD-CRC.In a large population-based cohort(
=7607)of individuals diagnosed with IBD from 1954 to 1989,the risk of CRC was found to be 60% higher in males aged < 45 years at diagnosis,with a relative risk(RR)of 1.6[95% confidence interval(CI):1.2-2.2]compared to females[11].Similar findings were noted in a meta-analysis conducted by Jess
[12]where men had a greater risk with a standardized IR(SIR)of 2.6(95%CI:2.2-3.0)compared to women(SIR of 1.9;95%CI:1.5-2.3).
Extensive UC
In a study published in 1994,Gillen
[13]reported a 19-fold increase in the risk of CRC in extensive UC compared to the general population(matched for age,sex,and disease duration).Similar findings were reported by Zhou
[10]in a large meta-analysis that included 58 studies and 267566 UC patients;they found that disease extent-specific risk estimates for CRC in UC were reported in 21 of the 58 studies and that extensive UC and left-sided UC had a higher risk of CRC(SIR:1.42,95%CI:0.83-2.42;SIR:0.56,95%CI:0.38-0.83 respectively)compared to proctitis(SIR:0.18,95%CI:0.01-0.03).
43.She did not recognize the Princess in her glittering garments: A suspension of belief is required for this frequent fairy tale plot device. The sisters in Cinderella do not recognize their sister in her splendor99 and now the waiting-maid does not recognize the princess despite having seen her in royal attire100 previously101. But then again, no one ever recognizes Superman behind Clark Kent s glasses either.Return to place in story.
Primary sclerosing cholangitis
Primary sclerosing cholangitis(PSC)is a chronic cholestatic liver disease and a significant proportion of patients with PSC also develop IBD[14],often characterized by pancolitis,rectal sparing,backwash ileitis,and importantly,a threefold increased risk of colorectal dysplasia[15,16].PSC with CD phenotype has been observed to be less severe than PSC with underlying UC[15-17].
A multicentric retrospective cohort study involving 277 PSC-IBD patients found that the IR of CRC since PSC diagnosis at 3.3 cases per 1000 patient-years(95%CI:1.9-5.6),with an IR of 61 PSC cases per 100000 IBD patient-years.Of these,69.7% were male,67.5% had UC,and the mean age at PSC diagnosis was 40 ± 16 years.PSC-IBD patients with symptoms of PSC at diagnosis were noted to have an increased risk of CRC[16].
Gut microbial dysbiosis
Recent evidence suggests that intestinal microbiota;particularly the bacterial component plays a fundamental role in the health and progression of diseases such as IBD and CRC.The factors that are known to influence the gut microbiome are illustrated in Figure 1B.
The development of IBD is often associated with altered microbial communities(dysbiosis)in the gut,interaction of genetic and environmental factors leading to chronic inflammation in the intestine.According to the “common ground hypothesis”,microbial dysbiosis and a leaky gut(due to intestinal barrier impairment)[18-20]are at the core of the chronic inflammatory process associated with IBD-CRC[21].Several studies involving patient and gnotobiotic mouse models[22,23]have substantiated this hypothesis[24].
Studies have shown high densities of mucosa-associated bacteria[21,25],with the ability to produce a greater mass of biofilm and extracellular matrix were present in IBD patients[26].These mucosae associated highly virulent bacteria are suspected to play a pivotal role in gut inflammation and tumorigenesis[21].Some of the common gut commensals like
[27],
[28],
[29],and
species[30]have been implicated in gastric tumorigenesis and CRC[23].In a study by Gevers
[31]on new-onset treatment-na?ve pediatric patients with CD and UC,an abundance of
,
/
,
,and
were seen in ileal and colonic biopsies.Although the role of microbial and host factors in disease pathogenesis has not been established in chronic gut inflammation and IBD-CRC,it can be hypothesized that the combined effect of host barrier defects and bacterial invasiveness may evoke a massive amount of immune hyperactivation in the gut mucosa.This is likely to ultimately lead to a vicious cycle of chronic inflammation driven by the malignant transformation of the gut epithelium[21].
PATHOGENESlS OF CRC lN lBD
Molecular pathways and mechanisms
The molecular pathogenesis of IBD-CRC is very different from sporadic CRC[32].With the advent of molecular technology in recent years,the pathophysiology of the development of IBD-CRC has been extensively studied,and has led to better understanding of molecular mechanisms and identification of new biomarkers[32-34].
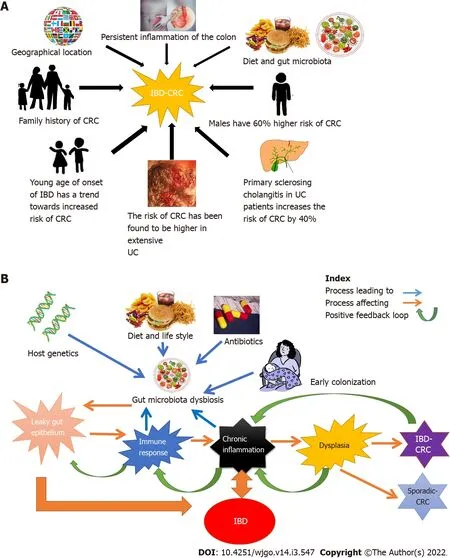
Numerous positive and negative regulators in the development of IBD-CRC have been identified which are illustrated in Figure 2.The process of development of IBD-CRC is triggered probably due to chromosomal and microsatellite instability through well-defined pathways(
pathway,CIMP pathway),causing mucosal dysplasia[32].The involvement of these pathways suggests that persistent inflammation plays a prominent role in carcinogenesis.The changes occurring in the microenvironment due to chronic inflammation are thought to be responsible for the increased risk.The chronic proliferation necessary to repair epithelial layer damage(caused by constant inflammation)enhances the risk of dysplasia[35].Although multiple cytokines and pathways have been identified in the pathogenesis of IBD-CRC[32-34],it continues to be a topic of ongoing research.Further research will not only enhance our understanding but also help identify non-invasive biomarkers and targets of therapy.A summary of currently known molecular mechanisms is summarized in Table 2.
The big old monster greedily accepted my dime, and I heard the bottles shift. On tiptoes I reached up and opened the heavy door. There they were: one neat row of thick green bottles, necks staring directly at me, and ice cold from the refrigeration. I held the door open with my shoulder and grabbed one. With a quick yank() , I pulled it free from its bondage13. Another one immediately took its place. The bottle was cold in my sweaty hands. I will never forget the feeling of the cool glass on my skin. With two hands, I positioned the bottleneck14 under the heavy brass15() opener that was bolted to the wall. The cap dropped into an old wooden box, and I reached in to retrieve16 it. I was cold and bent17 in the middle, but I knew I needed to have this souvenir(,) . Coke in hand, I proudly marched back out into the early evening dusk. Grampy was waiting patiently. He smiled.
TP53 and KRAS mutations
Earlier studies have found
and
mutations in IBD-CRC and sporadic CRC.However,the molecular pathway towards the progression of carcinogenesis is different[32,36,37].Recent studies have shown that
mutations were detected among 70% of sporadic colorectal carcinomas[38]and that both loss and gain of function of
might promote malignancy at the late phase of carcinogenesis[39].
Mesalazine or 5-aminosalicylic acid(5-ASA),a structural analogue of aspirin,has been used for many decades as first-line therapy for mild-to-moderate UC in oral and topical forms.In addition to its anti-inflammatory properties,it has received much attention for its chemopreventive effects.The drug appears to exert its effects through multiple mechanisms.A systematic review that looked into molecular mechanisms of chemoprevention of CRC was published in 2009.Lyakhovich and Gasche[74]in this study summarised that 5-ASA inhibits cyclooxygenase-2(COX-2)/prostaglandin E2 synthesis,decreases the transcriptional activity of NF-κB by modulating RelA/p65 phosphorylation,and interferes with the Wnt pathway through protein phosphatase 2A.Multiple other systematic reviews have reported on the chemoprotective effects of 5-ASA.Velayos
[77]included nine studies with 1932 UC patients in their systematic review and meta-analysis and reported a protective effect of 5-ASA in IBD-CRC and CRC/dysplasia.A large meta-analysis by Qiu
[76]comprising of 26 studies with > 15000 patients(UC + CD)reported a chemopreventive effect on CRC but not dysplasia.A dose of> 1.2 g/d was effective to reduce the risk.Another meta-analysis reported that 5-ASA was protective against CRC and dysplasia with a strong protective effect noted in UC but a non-significant effect in CD[78].
The waves rose mountains high, as if they would have overtopped the mast; but the ship dived like a swan between them, and then rose again on their lofty, foaming50 crests52
STAT3 and IL-6/p-STAT3 pathway
The signal transducer and activator of transcription 3(STAT3)pathway has been identified as an important one in the development of both sporadic CRC as well as IBD-CRC(Figure 3A).The exact mechanism is still not very well understood but it is reported to be due to signalling protein dysregulation and constitutive activation of STAT3[33,40].Corvinus
[41]showed in their murine model that constitutive STAT3 activation was persistent and important in CRC cells,possibly triggered by IL-6.Further,studies by other investigators have reported subsequently that STAT3 activation is associated with invasion,survival,and growth of CRC cells in mice
[40,42].Lin
[40]have also demonstrated using both
and
models that blockade of IL-6/p-STAT3(phosphorylated-STAT3)using an inhibitor suppressed tumour cell growth in colon cancer cells.
In human colonic tissue,patients with active colitis had significantly more IL-6 and p-STAT3-positive epithelial cells than both inactive UC and controls;in addition,they found that the proportion of suppressor of cytokine signalling 3(SOCS3)-positive cells was lower in patients with dysplastic lesions and CRC.A study by Gui
[43]that compared the expression of IL-6,STAT3,and SOCS3 in adenomas from IBD and non-IBD patients found significantly lower IL6,lower IL6R,higher STAT3,and lower SOCS3 expression in IBD associated dysplastic lesions.
Overall,dysregulation of this signalling pathway plays an important role in triggering neoplasia.STAT3 pathway driven by IL-6 continues to be a topic of ongoing research and likely to be an attractive target with therapeutic potential.
The Wnt pathway
The canonical
pathway(β-catenin mediated
-signalling)regulates the proliferation and differentiation of colonic stem cells in the normal colon[44,45].However,the loss of
gene results in the shift of β-catenin from membrane to the nucleus.This causes increased transcription of cyclin D1 and
genes,leading to carcinogenesis(Figure 3B)[32,46].Claessen
[32]in their study reported that the
pathway was activated in the early phase of colitis-associated CRC,and in about 50% of IBDassociated neoplasia cases.Another significant finding was that the pathway was also activated in the surrounding regions of dysplasia associated with IBD,a phenomenon termed as “field-cancerization”[32].They suggest that estimation of β-catenin can be used as a biomarker for colonic field cancerization,facilitating early detection of neoplasia during colonic surveillance[32].It has been shown in other studies that β-catenin could potentially be used as a marker of survival[47,48]and prognosis[47].
32.Promise me never to talk with your mother alone: Promises, while important today, were more powerful in the past when honor was a great motivator. Also, before the time of literacy among the masses and written contracts, verbal promises were given greater weight. A promise was a contract and actionable by law if broken. Folklore emphasizes the importance of a promise by meting89 punishment upon those who do not keep their promises. Return to place in story.
Dysplasia
CRC results from a series of genetic mutations that alter the normal growth pattern of cells,as a consequence of which,affected cells acquire a growth advantage over other cells.This aberration leads to morphological changes termed dysplasia.It was postulated that colorectal dysplasia could represent a premalignant lesion in IBD as early as 1949 by Warren and Sommers[49]and some years later in 1967 important observations that dysplasia originated from nonpolypoid mucosa were also reported by another group.
Historically,an elevated lesion containing dysplasia was referred to as a dysplasia-associated lesion or mass(DALM)[50].The diagnosis of DALM became complicated over time because of the inconsistent criteria used in describing IBD-related dysplasia.The term DALM was also was being inaccurately always linked to colectomy[50].However,with the advent of fibre optic endoscopic visualization techniques and improvement in localized surgical resection procedures the definition,classification,and management of dysplasia became more systematic[50-52].The SCENIC guidelines in 2015 made important recommendations to standardize how lesions are described during surveillance.It was recommended that the term DALM be abandoned[53].The term dysplasia redefined as an abnormal growth of cells,tissues,or organs leading to the development of abnormal histological or anatomical structures has now replaced the previously used term DALM[52]dysplasia is categorized as low-grade dysplasia(LGD)and high-grade dysplasia(HGD)based on the degree of histological abnormalities.
The identification of dysplastic changes is important as this is an important stage in the development of cancer and considered a strong predictor of CRC in IBD.Chronic intestinal inflammation is the primary risk factor that leads to LGD,which can then progress to HGD and eventually CRC[54](Figure 4).This sequence of events is thought to be accelerated in IBD-CRC compared to sporadic CRC[54].In a study de Jong
[55]investigated the long-term risk of HGD and CRC following the development of LGD using a nationwide database identifying a large IBD patient cohort.The risk factors for advanced neoplasia progression were found to be age > 55 years at the time of LGD detection,male gender and follow-up at a tertiary IBD referral centre.The study also found that the incidence rate of progression to advanced neoplasia was 22% after 15 years of detection of IBD.Dysplasia in colonic strictures and epithelial dysplasia are both well-documented risk factors and considered to be precursors to the development of IBD-CRC[55,56].In a case-control study among 53568 IBD patients undergoing colonoscopy,Sonnenberg and Genta[56]found that the prevalence of dysplasia was 3.22% and 2.08% in UC and CD respectively[odds ratio(OR)= 0.75,95%CI:0.65-0.86],with a small increase in the prevalence of dysplasia within a stricture.The prevalence of cancer was higher in IBD patients with stricture compared to that without-0.78% and 0.11%,respectively(OR =6.87,95%CI:3.30-12.89).A thirty-six-year analysis of a colonoscopic surveillance program found that in patients with UC who had undergone a colectomy due to HGD,46% had a cancerous growth in the colon[57],thereby suggesting that the presence of HGD confers a high risk of synchronous cancer in the colon.Overall,dysplasia is a well-established histological stage in the development of IBD-CRC,and its detection during colonoscopy should prompt appropriate management to prevent progression to CRC.Surveillance programs are intended for the early detection of dysplastic lesions and strictures.In highrisk patients,surveillance helps in tracking disease progression in IBD patients.The intervals at which surveillance should take place vary from region to region,and based on guidelines by national societies.The British Society of Gastroenterology(BSG)recommends an intensified surveillance endoscopy program or a colectomy after the first 5 years of detection of LGD[58].Other recommendations include re-evaluation by a second pathologist if LGD is detected and further assessment by an expert endoscopist.The details of surveillance techniques are discussed in detail in the next section.


STRATEGlES USED TO REDUCE THE lNClDENCE OF CRC lN lBD
Surveillance colonoscopy
Surveillance in IBD could be described as the process of careful examination of the colon to detect early mucosal changes that may herald possible neoplasia.The mucosal changes/lesions(dysplasia of varying degrees)or adenomas provide an opportunity for early diagnosis and management of these lesions.There have been multiple studies in the past which have supported the use of surveillance as a tool to reduce cancer incidence in IBD.With the wider adoption of surveillance programmes over many years,long-term data have been in favour of regular surveillance of at-risk patients[7,59].Over the last 2 decades,the practice of surveillance in IBD has largely been in line with guidance,which was mainly based on their large metaanalysis on the risk of IBD related CRC in 2001[2].This landmark study,in particular,helped strengthen guidelines for regular surveillance.The summaries of recommendations are:Screening colonoscopy after 8-10 years that will also clarify disease extent for all patients;Regular surveillance to begin after 8-10 years for pancolitis and after 15-20 years for the left-sided disease;Reduced screening interval with increasing disease duration(due to increased risk in pancolitis);In the second decade of disease a colonoscopy to be conducted every three years,every two years for the third decade,and yearly by the fourth decade of disease;Two to four random biopsy specimens every 10 cm should be taken from the entire colon with additional samples of suspicious areas;Patients with PSC(including those with an orthotopic liver transplant)represent a subgroup at higher risk of cancer and they should have an annual colonoscopy.
Aspirin and non-steroidal anti-inflammatory drugs(NSAIDs)have been studied for their chemo-preventive properties in the context of sporadic CRC.The elevated levels of COX-2 expression found in most CRC meant that NSAIDs and selective COX-2 inhibitors(COXIBs)[81]carry the potential for use in chemoprevention.A large,randomised study reported a reduction in metastatic disease in CRC with aspirin use[82].Although the mechanisms make these medications attractive options,there have been no prospective studies done to study their efficacy in the context of IBD-CRC.A systematic review and meta-analysis by Burr
[83]reported on the effect of aspirin and NSAIDs on IBD-CRC.They found only 9 retrospective studies in IBD which included the use of either or both these drugs with CRC as one of the outcomes.The authors concluded that the studies presented several limitations including selection bias as well as confounding.Also,the number of patients included was a small and overall large variation in studies that led to no strong conclusions[83].
Meantime the Fairy had prepared a chariot, to which she harnessed two powerful eagles; then placing the cage, with the parrot in it, she charged the bird to conduct it to the window of the Princess s dressing-room
Although the SCENIC guidelines do not recommend routine use of Narrow Band Imaging(NBI)for surveillance,recent studies have shown that this could be a reliable modality.A large multicentre study by Watanabe
[63]randomised 263 surveillance patients to either chromoendoscopy or surveillance using NBI.The results showed no significant difference in lesion detection rates(10.7%
11.9%)and the duration of procedure was shorter with NBI(by 4 min;
< 0.001)[63].Further,a study by Bisschops
[64]found NBI to be significantly better than high definition chromoendoscopy images to differentiate neoplastic from non-neoplastic lesions among experts.The results of these studies indicate that the NBI may have a potential role in surveillance in the future and is likely to find a place in updated guidelines.
How effective is surveillance?
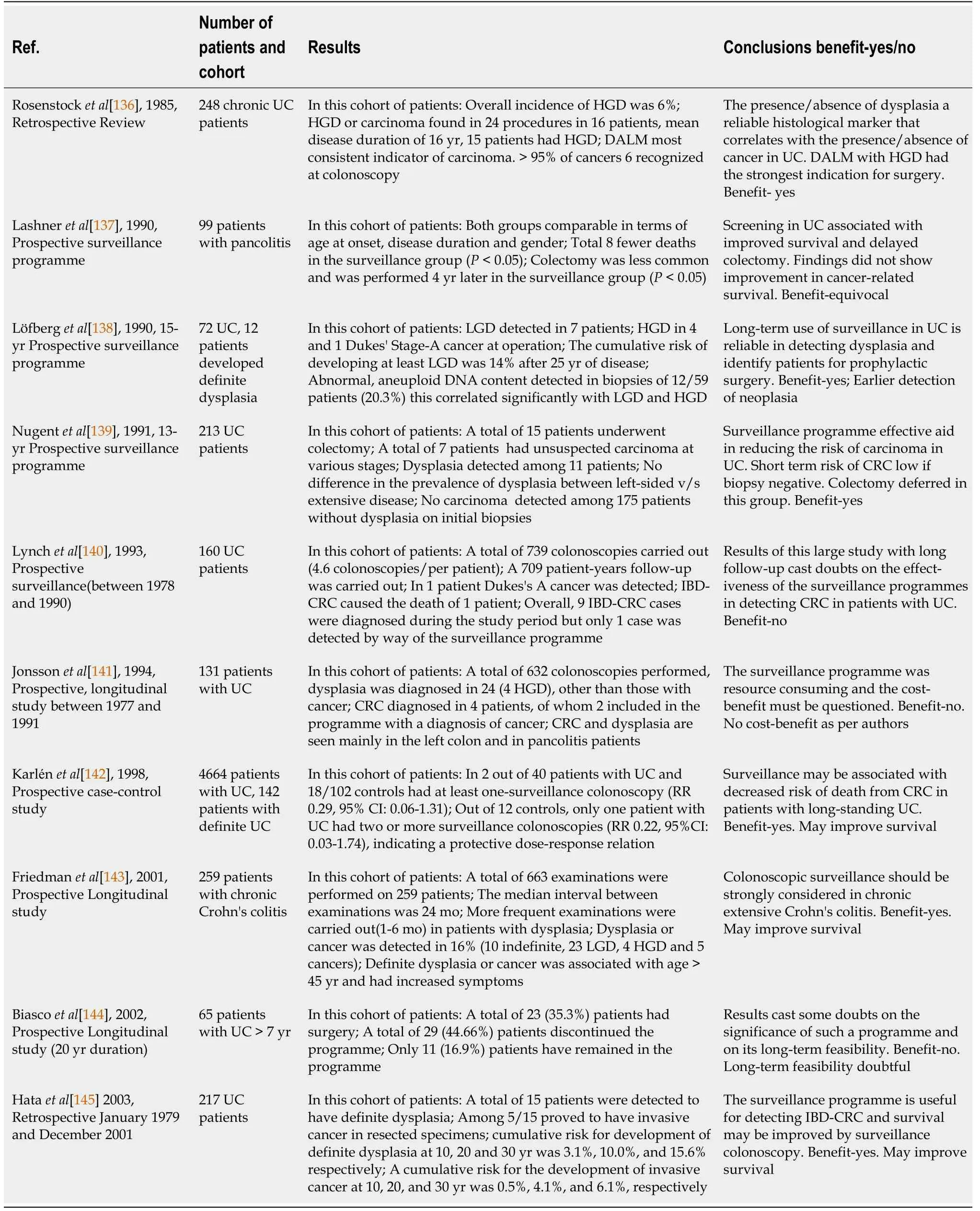
The effectiveness of surveillance in IBD has been a topic of much debate over years for multiple reasons— cost-benefit to health systems,resource requirements,and also because studies show many conflicting data.
A Cochrane review by Collins
[65]from 2006 looked into the effectiveness of surveillance in reducing the death rate from CRC in IBD.This study included a combination of prospective and retrospective studies that looked at the impact of surveillance on IBD-CRC.They reported on direct and indirect evidence to answer the question of the effectiveness of surveillance.The details of the studies included are given in Table 3.In summary,one study showed a dose-response to survival wherein a higher number of surveillance procedures were protective and increased survival,one showed that surveillance picked up CRC at an earlier stage and 5-year survival was better in the surveillance group compared to the non-surveillance group and another showed improved survival in the surveillance group compared to non-surveillance,but no improvement in mortality due to CRC.Some other studies have tried to estimate the economic benefits of surveillance.However,these models were calculated for sporadic cancers,and conclusions extended to IBD-CRC.It was shown that screening programs for normal individuals in the community have financial gains and therefore an argument has been made in favour of surveillance of high-risk patients with IBD.A more recent systematic review and metaanalysis by Bye
[66]included observational studies of patients that included patients undergoing surveillance.Their pooled analysis showed a reduction in IBD-CRC in patients undergoing surveillance by 42% and IBD-CRC-related death by 64%,compared to those who did not undergo surveillance[66].Current literature appears to favour surveillance and therefore it is part of standard service provision in many endoscopy centres.
Impact of using different biopsy techniques and endoscopic modalities
Random biopsies during surveillance colonoscopy had been standard practice,which was a labourintensive process not only for the endoscopist but also the pathologist.Studies that looked at accuracy of targeted biopsies changed the landscape of surveillance making it more efficient without compromising on the accuracy of detecting neoplasia.
Targeted biopsies and white light endoscopy
In a key prospective exploratory trial,Watanabe
[67]randomised chronic UC patients undergoing surveillance to either have targeted biopsies(from lesions detected)or step-wise multiple biopsies(random biopsies every 10 cm).The patients underwent high-definition white-light endoscopy(HDWLE)in most cases.The investigators found that the detection of neoplasia was significantly higher in the target biopsy group compared to random biopsies(6.9%
0.5%),with a lower mean number of biopsies in the targeted group(34.8
3.1;
< 0.001)and shorter examination time,concluding that targeted biopsies were as effective as random biopsies and more cost-effective[67].This finding has been suggested in other studies,thereby indicating random biopsies could still be useful in select high risk patients,in line with the 2019 European Society of Gastrointestinal Endoscopy(ESGE)recommendations[68].
Dye-chromoendoscopy
Dye-chromoendoscopy(DCE)is currently the standard of care for surveillance colonoscopy in IBD as it has been reported to aid the detection of subtle mucosal lesions.A prospective randomised trial that compared DCE using methylene blue with conventional endoscopy reported more accurate findings with better ability to differentiate between neoplastic and non-neoplastic lesions in patients with longstanding UC.Another prospective study by Marion
[69]in 2008 compared the same techniques with randomised and targeted biopsies in a cohort of 102 patients with IBD.DCE detected significantly higher number of dysplastic lesions compared to random biopsies[69].A large systematic review and network meta-analysis found DCE to have a significantly higher diagnostic yield for neoplastic lesions compared to WLE[70].This technique is therefore recommended for surveillance endoscopy by the ESGE.
She went out and told the servants that the scullery-maid was an impostor, and that they must take her out into the court-yard and strike off her head
Virtual chromoendoscopy
Virtual electronic chromoendoscopy(VCE)or dyeless virtual chromoendoscopy uses image enhanced technology(I scan)that has been introduced in recent years but already increasingly adopted by expert endoscopists for surveillance colonoscopy.A retrospective study by Gasia
[71]compared various technologies namely standard WLE,high definition WLE,DCE,VCE,and also strategies of targetedbiopsies
random.They found targeted biopsies to be better at neoplasia detection across all technologies except standard WLE.In a prospective randomised trial by the same investigating group,Iacucci
[72]randomised patients with long-standing colitis into three arms:WLE,DCE,and VCE.In this non-inferiority study,VCE was found to be non-inferior to DCE in the detection of all neoplastic lesions.ESGE now strongly recommends the use of VCE or dye-spray with targeted biopsies for surveillance of
The association between disease duration in IBD and probability of CRC is controversial.Studies published over the decades report different conclusions.Several studies have found that the incidence of IBD-CRC is higher among patients who develop IBD at a young age making duration of disease an important risk factor[2,5,6].Another surveillance study published in 2015 by investigators at St Mark's Hospital,London followed up 1375 UC patients for 15234 patient-years(median,11 years per patient)and IBD-CRC was detected in 72 patients[incidence rate(IR),4.7 per 1000 patient-years].Although the IR of early IBD-CRC was noted to have increased by 2.5-fold in the current decade compared with the past decade(
= 0.045)it is reassuring that the 10-year survival rate was high(79.6%)[7].A number of studies have concluded that in Crohn’s colitis risk of CRC is similar to UC if the extension and duration of the disease are comparable[8,9].
Chemoprevention of CRC
Chemoprevention in cancer is a term used for the use of pharmacological agents to reduce or delay the risk of carcinogenesis or progression of the disease[73,74].Although there have been multiple drugs investigated for their potential,mesalazine currently has the largest evidence base to support its use for chemoprevention in CRC[74-76].
It has been reported that the adenomatous polyposis coli(
)and
mutations were significantly less common in IBD-CRCs than in sporadic CRCs(15%
53%,
< 0.001 and 20%
38%,
= 0.02,respectively)[38].
With many reporting on the mechanisms of 5-ASA in reducing the risk of CRC,it is plausible that it has a chemopreventive effect in IBD and can be used in this cohort of patients.
Thiopurines have been used for many decades in the management of IBD.There have been no randomised studies to investigate the efficacy of thiopurine therapy and current evidence is from cohort,case-control or population-based studies,with conflicting reports.A systematic review by Jess
[79]in 2014 reported no protective effect of thiopurine therapy on CRC in IBD patients.The studies included carried heterogeneity and included clinic-based cohort and case-control studies,but no population-based studies.The lack of protective effect may be explained due to the inclusion of studies with patients at a severe spectrum of disease[79].
Another systematic review and meta-analysis by Lu
[80]reported in 2018 on 24 observational studies involving 76999 participants to evaluate the risks of developing CRC in IBD patients on thiopurines.The authors found an overall protective effect of thiopurine use on CRC in patients with IBD(OR = 0.63,95%CI:0.46-0.86)in a pooled estimate and the effect was significant in UC patients(OR= 0.67,95%CI:0.45-0.98),but not in CD patients(OR = 1.06,95%CI:0.54-2.09).Interestingly,the authors also reported that the protective effect was limited to clinic-based and case-control studies but no population-based studies.
These recommendations have been adopted by both the BSG and European Crohn’s and Colitis Organisation(ECCO),with some minor differences and recent updates[60,61].The core recommendations for surveillance remained stagnant for about twenty years.Recent advances in endoscopic technology and the use of new methods have meant that surveillance practices have started to change but can vary depending on the centre,availability of equipment,and expertise.The introduction of new technology has been matched by sound recommendations by the SCENIC guidelines and availability of newer endoscopic classification systems to help clinicians describe IBD-related dysplastic lesions whilst using these techniques.
,the Frankfurt Advanced Chromoendoscopic IBD LEsions(FACILE)classification that has been developed,validated,and shown to be reproducible[62].
And now, because he was afraid that their stepmother might not treat them well and might do them harm, he put them in a lonely castle that stood in the middle of a wood
At present,the use of aspirin or NSAIDs as chemopreventive agents is not part of any guidelines.This is unlikely to change as large prospective studies that study IBD-CRC are unlikely to be carried out.
IBD leads to impaired folate absorption.Folate is involved in DNA methylation and may produce epigenetic changes that affect the gut microbial and host immune interactions[84].Folic acid has been investigated in the past as a chemopreventive agent.The effect of folate supplementation on dysplasia and cancer in IBD was first reported by Lashner
[85]in a case-control study.In this study,all patients with pancolitis of > 7-year duration(except those with known HGD and invasive cancer)in the surveillance program exposed to folate supplements were compared to the control group(patients in the surveillance program,no dysplasia and not exposed to folate).Although folate supplementation was associated with a 62% lower incidence of dysplasia or cancer,the duration,and dose of folate intake were unclear,and results did not reach statistical significance probably because it was underpowered.Another retrospective study by the same group reported that the relative risk of neoplasia was lower(0.54)with folate supplementation(after at least 6-mo of exposure).The authors concluded that daily folate supplementation may protect against the development of neoplasia in UC,although the results did not reach statistical significance.
The effects of folate supplementation were best summarised by a systematic review and metaanalysis by Burr
[86]in which they included ten studies with low to moderate heterogeneity and a total of 4517 patients.The authors concluded that the results showed a pooled hazard ratio of 0.58(95%CI:0.37-0.80)suggesting an overall protective effect for folate supplementation on the development of IBD-CRC[86].
The presence of visible dysplasia perhaps is relatively straightforward with the grade of dysplasia determining the intensity of future surveillance or need for surgery,but invisible dysplasia poses a challenge.Although the proportion of invisible dysplastic lesions is low due to the use of advanced endoscopic techniques[87],the detection of such lesions can present a dilemma in management,particularly because patients do not readily accept colectomy despite physician recommendations[88].
Surgery
Surgery in the form of colectomy remains an important and effective strategy in preventing IBD-CRC,particularly in patients who have HGD or ‘indefinite’ dysplasia or invisible dysplasia detected on biopsies.Among visible lesions seen during endoscopy,polypoid lesions and some non-polypoid lesions with LGD in selective cases can generally be managed with endoscopic resection if full resection can be achieved,and further surveillance may be a reasonable option as per current guidelines[61].
However,non-polypoid lesions that cannot be managed endoscopically or the presence of invisible dysplasia regardless of degree are considered high-risk for progression to cancer and therefore recommended to undergo surgery[61].
While there is weak evidence from retrospective studies in favour of folate supplementation,in the absence of prospective randomised data to support this,it is unlikely that folic acid will be used routinely for chemoprevention.At present,it is not part of guidelines by most national and international societies despite it being a cheap,safe,and well-tolerated supplement.
The risk of cancer with visible LGD has been known to be low with a larger body of evidence.In a retrospective study by Ten Hove
[89],the incidence rate of advanced cancer was low at 1.34 per 100 years in patients with LGD after a follow-up of nearly 5 years,with no significant difference between chromoendoscopy and WLE and a systematic review by Kabir
[90]reported a pooled estimated rate of cancer in visible LGD at 2.7%.However,there is very little data available on invisible dysplasia.In their systematic review,Kabir
[90]reported that pooled estimates of cancer due to invisible HGD and invisible LGD were 11.4% and 2.4% respectively,based on two cohort studies and one case series.With such a high risk of progression to cancer,surgery should be considered as a serious and realistic option in reducing the risk of IBD-CRC.
Diet therapy and gut microbiota modulation
Our better understanding of the human gut microbiome has opened up a new possibility of treatment for IBD and IBD-CRC[91].Recent molecular level research on the gut microbiota using whole-genome sequencing technology has proved that some factors can alter the microbiome and the pathogenesis of IBD[92].
It has been hypothesized that diet plays a key role in the modulation of the gut microbiota composition.Gut microbiota in turn plays a major role in maintaining gut homeostasis and is associated with the modulation of host inflammatory and immune responses[93].Studies have shown that nutritional components(added sugars,trans-fats,omega-6 fatty acids,red processed meat
)contribute to a chronic inflammatory condition by regulating various immune and inflammatory pathways[94,95].Diet has been identified as one of the vital factors associated with CRC etiology[94,96].
Mother knew I d need the shirt as a reminder33 that a sense of humor, spiced with love, is one of the most important ingredients in a happy marriage. In a pocket was a note: Read John 14:27?29. I love you both, Mother.
From what one person and another have let fall, he exclaimed, I have contrived13 to learn that he is in the palace of the king, who keeps him hidden lest anyone should see him; and that to-morrow he is to marry the princess, who, ugly creature that she is, has not been able to find any man to wed14 her
Future directions
There have been several recent advances made with novel endoscopic technologies such as endocytoscopy,confocal laser endomicroscopy(CLE),both of which allow examination of the bowel mucosa with histology-like images at 500-fold to 1000-fold magnification,allowing
evaluation in real-time.
Endocytoscopy has been reported to be effective in recognising low-grade adenoma in the colon[106].Its utility in IBD surveillance has not been evaluated thoroughly yet and is a subject of research.There is evidence that CLE is a useful tool in assessing dysplasia,with a stronger evidence base in the evaluation of Barrett’s oesophagus.It has been studied in the context of IBD and shown to increase the rate of detection of neoplastic lesions.In a consensus-based report on the applications of CLE,although there was wide agreement that CLE can detect dysplasia effectively in IBD[107],its adoption is limited by cost and lack of expertise.This is likely to change in the future as endoscopists become more familiar with the technology and wider use may drive down costs.
Full-spectrum endoscopy(FUSE)is an emerging technique that employs two lateral additional cameras to a standard colonoscope,allowing operators to view behind folds and blind spots.Leong and Koo[1]investigated its ability to detect dysplastic lesions in a robust study design involving patients undergoing surveillance.They prospectively randomised 52 patients to either standard colonoscopy or FUSE and then crossed over to the other group for a repeat procedure.FUSE missed significantly fewer dysplastic lesions compared to standard(25%
71.4%)with a slightly longer withdrawal time.Kudo
[108]reported similar findings in their tandem colonoscopy trial.The advantages of this technique are apparent but are currently not part of guidelines and recommendations by relevant societies.Further research and familiarity with the technique are likely to encourage more clinicians to use this for surveillance.
The next generation of advancement comes in the form of using artificial intelligence(AI)in endoscopy.AI is currently being used widely in innumerable areas and its applications are seemingly unlimited.AI in IBD has been evaluated by Stidham
[109]where they found that performance of deep learning models was similar to experienced human reviewers when grading endoscopic severity in UC.AI built into endoscopic systems to aid detection of dysplastic lesions is currently a subject of research globally,with few early reports available in literature[110].
The discovery of microbiota-regulated mucosal and systemic immune response pathways have opened up avenues to explore the impact of this response on the development of cancer immunotherapies.However,it should also be considered that an individual’s commensal gut microbiota keeps evolving and changing throughout the lifetime based on various environmental factors[23].This phenomenon plays a pivotal role in phenotypic variation in disease development,progression,and therapeutic success among individuals.Therefore,it will not be wrong to hypothesize that future gut microbiota modulating therapies need to be personalized according to an individual’s microbiota.
CONCLUSlON
IBD-related CRC is a serious complication that deserves attention.The evolution of strategies in reducing this risk over decades is interesting.Although surveillance is now the cornerstone of early detection of neoplasia,the key to reducing this risk is keeping patients in remission.It is encouraging that there are some signals of lowered risk of IBD-CRC recently but with increasing disease burden,we have to remain vigilant.Further research into exploring pathways involved in CRC will provide a better understanding and potential new targets to exploit,be it for new or repurposed drugs.The expansion in the use of advanced endoscopic techniques is likely to improve neoplasia detection and help patients.AI carries the potential to bring about a paradigm shift in endoscopy and surveillance but needs rigorous evaluation before it is deployed for routine clinical use.Lastly,modulation of microbiota may well be something to watch out for in the future as a reliable intervention in this cohort.
FOOTNOTES
Majumder S and Shivaji UN contributed literature search,data collection,data analysis,writing and editing manuscript,revision and final approval;Kasturi R,Sigamani A,Ghosh S and Iacucci M contributed
writing and editing manuscript,revision and final approval.
How was he to find his father and mother, and bring them back to life, if they were lying at the bottom of that horrible water? He turned away sadly and wandered back into the streets, hardly knowing where he was going; when a voice behind him cried: Stop, prince, I have caught you at last! It is a thousand years since I first began to seek you
The authors have none to declare.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See:https://creativecommons.org/Licenses/by-nc/4.0/
United Kingdom
Snehali Majumder 0000-0002-2745-493X;Uday Nagesh Shivaji 0000-0002-6800-584X;Rangarajan Kasturi 0000-0002-9421-7512;Alben Sigamani 0000-0002-6927-1947;Subrata Ghosh 0000-0002-1713-7797;Marietta Iacucci 0000-0003-2440-2592.
British Society of Gastroenterology,No.BSG61984.
Gao CC
A
Gao CC
1 Leong RW,Koo JH.Colorectal cancer in inflammatory bowel disease.
2009;24:503-505[PMID:19368629 DOI:10.1111/j.1440-1746.2009.05790.x]
2 Eaden JA,Abrams KR,Mayberry JF.The risk of colorectal cancer in ulcerative colitis:a meta-analysis.
2001;48:526-535[PMID:11247898 DOI:10.1136/gut.48.4.526]
3 Canavan C,Abrams KR,Mayberry J.Meta-analysis:colorectal and small bowel cancer risk in patients with Crohn's disease.
2006;23:1097-1104[PMID:16611269 DOI:10.1111/j.1365-2036.2006.02854.x]
4 Itzkowitz SH,Yio X.Inflammation and cancer IV.Colorectal cancer in inflammatory bowel disease:the role of inflammation.
2004;287:G7-17[PMID:15194558 DOI:10.1152/ajpgi.00079.2004]
5 Kim ER,Chang DK.Colorectal cancer in inflammatory bowel disease:the risk,pathogenesis,prevention and diagnosis.
2014;20:9872-9881[PMID:25110418 DOI:10.3748/wjg.v20.i29.9872]
6 Beaugerie L,Itzkowitz SH.Cancers Complicating Inflammatory Bowel Disease.
2015;373:195[PMID:26154801 DOI:10.1056/NEJMc1505689]
7 Choi CH,Rutter MD,Askari A,Lee GH,Warusavitarne J,Moorghen M,Thomas-Gibson S,Saunders BP,Graham TA,Hart AL.Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis:An Updated Overview.
2015;110:1022-1034[PMID:25823771 DOI:10.1038/ajg.2015.65]
8 Averboukh F,Ziv Y,Kariv Y,Zmora O,Dotan I,Klausner JM,Rabau M,Tulchinsky H.Colorectal carcinoma in inflammatory bowel disease:a comparison between Crohn's and ulcerative colitis.
2011;13:1230-1235[PMID:21689324 DOI:10.1111/j.1463-1318.2011.02639.x]
9 Kiran RP,Khoury W,Church JM,Lavery IC,Fazio VW,Remzi FH.Colorectal cancer complicating inflammatory bowel disease:similarities and differences between Crohn's and ulcerative colitis based on three decades of experience.
2010;252:330-335[PMID:20622662 DOI:10.1097/SLA.0b013e3181e61e69]
10 Zhou Q,Shen ZF,Wu BS,Xu CB,He ZQ,Chen T,Shang HT,Xie CF,Huang SY,Chen YG,Chen HB,Han ST.Risk of Colorectal Cancer in Ulcerative Colitis Patients:A Systematic Review and Meta-Analysis.
2019;2019:5363261[PMID:31781191 DOI:10.1155/2019/5363261]
11 S?derlund S,Granath F,Brostr?m O,Karlén P,L?fberg R,Ekbom A,Askling J.Inflammatory bowel disease confers a lower risk of colorectal cancer to females than to males.
2010;138:1697-1703[PMID:20167217 DOI:10.1053/j.gastro.2010.02.007]
12 Jess T,Rungoe C,Peyrin-Biroulet L.Risk of colorectal cancer in patients with ulcerative colitis:a meta-analysis of population-based cohort studies.
2012;10:639-645[PMID:22289873 DOI:10.1016/j.cgh.2012.01.010]
13 Gillen CD,Walmsley RS,Prior P,Andrews HA,Allan RN.Ulcerative colitis and Crohn's disease:a comparison of the colorectal cancer risk in extensive colitis.
1994;35:1590-1592[PMID:7828978 DOI:10.1136/gut.35.11.1590]
14 Stiehl A,Benz C,Sauer P.Primary sclerosing cholangitis.
2000;14:311-315[PMID:10799084 DOI:10.1155/2000/983681]
15 Ricciuto A,Kamath BM,Griffiths AM.The IBD and PSC Phenotypes of PSC-IBD.
2018;20:16[PMID:29594739 DOI:10.1007/s11894-018-0620-2]
16 Guerra I,Bujanda L,Castro J,Merino O,Tosca J,Camps B,Gutiérrez A,Gordillo ábalos J,de Castro L,Iborra M,Carbajo AY,Taxonera C,Rodríguez-Lago I,Mesonero F,de Francisco R,Gómez-Gómez GJ,Chaparro M,Tardillo CA,Rivero M,Algaba A,Martín Arranz E,Ca?ete F,Vicente R,Sicilia B,Antolín B,Prieto V,Márquez L,Benítez JM,Camo P,Piqueras M,Gargallo CJ,Hinojosa E,Huguet JM,Pérez Calle JL,Van Domselaar M,Rodriguez C,Calvet X,Mu?oz-Villafranca C,García-Sepulcre MF,Munoz-Garrido P,Fernández-Clotet A,Gómez Irwin L,Hernández S,Guardiola J,Sempere L,González Mu?oza C,Hernández V,Beltrán B,Barrio J,Alba C,Moraleja I,López-Sanromán A,Riestra S,Martínez Montiel P,Garre A,Arranz L,García MJ,Martín Arranz MD,Corsino P,Arias L,Fernández-Salazar L,Fernández-Pordomingo A,Andreu M,Iglesias E,Ber Y,Mena R,Arroyo Villarino MT,Mora M,Ruiz L,López-Serrano P,Blazquez I,Villoria A,Fernández M,Bermejo F,Banales JM,Domènech E,Gisbert JP;Spanish GETECCU group(ENEIDA Project).Clinical Characteristics,Associated Malignancies and Management of Primary Sclerosing Cholangitis in Inflammatory Bowel Disease Patients:A Multicentre Retrospective Cohort Study.
2019;13:1492-1500[PMID:31063540 DOI:10.1093/ecco-jcc/jjz094]
17 Mertz A,Nguyen NA,Katsanos KH,Kwok RM.Primary sclerosing cholangitis and inflammatory bowel disease comorbidity:an update of the evidence.
2019;32:124-133[PMID:30837784 DOI:10.20524/aog.2019.0344]
18 Lynch SV,Pedersen O.The Human Intestinal Microbiome in Health and Disease.
2016;375:2369-2379[PMID:27974040 DOI:10.1056/NEJMra1600266]
19 Ni J,Shen TD,Chen EZ,Bittinger K,Bailey A,Roggiani M,Sirota-Madi A,Friedman ES,Chau L,Lin A,Nissim I,Scott J,Lauder A,Hoffmann C,Rivas G,Albenberg L,Baldassano RN,Braun J,Xavier RJ,Clish CB,Yudkoff M,Li H,Goulian M,Bushman FD,Lewis JD,Wu GD.A role for bacterial urease in gut dysbiosis and Crohn's disease.
2017;9[PMID:29141885 DOI:10.1126/scitranslmed.aah6888]
20 Balzan S,de Almeida Quadros C,de Cleva R,Zilberstein B,Cecconello I.Bacterial translocation:overview of mechanisms and clinical impact.
2007;22:464-471[PMID:17376034 DOI:10.1111/j.1440-1746.2007.04933.x]
21 Yu LC.Microbiota dysbiosis and barrier dysfunction in inflammatory bowel disease and colorectal cancers:exploring a common ground hypothesis.
2018;25:79[PMID:30413188 DOI:10.1186/s12929-018-0483-8]
22 Rogala AR,Oka A,Sartor RB.Strategies to Dissect Host-Microbial Immune Interactions That Determine Mucosal Homeostasis vs.Intestinal Inflammation in Gnotobiotic Mice.
2020;11:214[PMID:32133003 DOI:10.3389/fimmu.2020.00214]
23 Matson V,Chervin CS,Gajewski TF.Cancer and the Microbiome-Influence of the Commensal Microbiota on Cancer,Immune Responses,and Immunotherapy.
2021;160:600-613[PMID:33253684 DOI:10.1053/j.gastro.2020.11.041]
24 K?hrstr?m CT.Host response:Phagocytosis runs like clockwork.
2012;10:162[PMID:22330881 DOI:10.1038/nrmicro2751]
25 Swidsinski A,G?ktas O,Bessler C,Loening-Baucke V,Hale LP,Andree H,Weizenegger M,H?lzl M,Scherer H,Lochs H.Spatial organisation of microbiota in quiescent adenoiditis and tonsillitis.
2007;60:253-260[PMID:16698947 DOI:10.1136/jcp.2006.037309]
26 Motta JP,Flannigan KL,Agbor TA,Beatty JK,Blackler RW,Workentine ML,Da Silva GJ,Wang R,Buret AG,Wallace JL.Hydrogen sulfide protects from colitis and restores intestinal microbiota biofilm and mucus production.
2015;21:1006-1017[PMID:25738373 DOI:10.1097/MIB.0000000000000345]
27 Díaz P,Valenzuela Valderrama M,Bravo J,Quest AFG.
and Gastric Cancer:Adaptive Cellular Mechanisms Involved in Disease Progression.
2018;9:5[PMID:29403459 DOI:10.3389/fmicb.2018.00005]
28 Flanagan L,Schmid J,Ebert M,Soucek P,Kunicka T,Liska V,Bruha J,Neary P,Dezeeuw N,Tommasino M,Jenab M,Prehn JH,Hughes DJ.Fusobacterium nucleatum associates with stages of colorectal neoplasia development,colorectal cancer and disease outcome.
2014;33:1381-1390[PMID:24599709 DOI:10.1007/s10096-014-2081-3]
29 Wu S,Rhee KJ,Albesiano E,Rabizadeh S,Wu X,Yen HR,Huso DL,Brancati FL,Wick E,McAllister F,Housseau F,Pardoll DM,Sears CL.A human colonic commensal promotes colon tumorigenesis
activation of T helper type 17 T cell responses.
2009;15:1016-1022[PMID:19701202 DOI:10.1038/nm.2015]
30 Wu N,Yang X,Zhang R,Li J,Xiao X,Hu Y,Chen Y,Yang F,Lu N,Wang Z,Luan C,Liu Y,Wang B,Xiang C,Wang Y,Zhao F,Gao GF,Wang S,Li L,Zhang H,Zhu B.Dysbiosis signature of fecal microbiota in colorectal cancer patients.
2013;66:462-470[PMID:23733170 DOI:10.1007/s00248-013-0245-9]
31 Gevers D,Kugathasan S,Denson LA,Vázquez-Baeza Y,Van Treuren W,Ren B,Schwager E,Knights D,Song SJ,Yassour M,Morgan XC,Kostic AD,Luo C,González A,McDonald D,Haberman Y,Walters T,Baker S,Rosh J,Stephens M,Heyman M,Markowitz J,Baldassano R,Griffiths A,Sylvester F,Mack D,Kim S,Crandall W,Hyams J,Huttenhower C,Knight R,Xavier RJ.The treatment-naive microbiome in new-onset Crohn's disease.
2014;15:382-392[PMID:24629344 DOI:10.1016/j.chom.2014.02.005]
32 Claessen MM,Schipper ME,Oldenburg B,Siersema PD,Offerhaus GJ,Vleggaar FP.WNT-pathway activation in IBDassociated colorectal carcinogenesis:potential biomarkers for colonic surveillance.
2010;32:303-310[PMID:20208143 DOI:10.3233/CLO-2009-0503]
33 Ma XT,Wang S,Ye YJ,Du RY,Cui ZR,Somsouk M.Constitutive activation of Stat3 signaling pathway in human colorectal carcinoma.
2004;10:1569-1573[PMID:15162527 DOI:10.3748/wjg.v10.i11.1569]
34 Lin L,Hron JD,Peng SL.Regulation of NF-kappaB,Th activation,and autoinflammation by the forkhead transcription factor Foxo3a.
2004;21:203-213[PMID:15308101 DOI:10.1016/j.immuni.2004.06.016]
35 Romano M,DE Francesco F,Zarantonello L,Ruffolo C,Ferraro GA,Zanus G,Giordano A,Bassi N,Cillo U.From Inflammation to Cancer in Inflammatory Bowel Disease:Molecular Perspectives.
2016;36:1447-1460[PMID:27069120]
36 Laurent C,Svrcek M,Flejou JF,Chenard MP,Duclos B,Freund JN,Reimund JM.Immunohistochemical expression of CDX2,β-catenin,and TP53 in inflammatory bowel disease-associated colorectal cancer.
2011;17:232-240[PMID:20815042 DOI:10.1002/ibd.21451]
37 Kanaan Z,Rai SN,Eichenberger MR,Barnes C,Dworkin AM,Weller C,Cohen E,Roberts H,Keskey B,Petras RE,Crawford NP,Galandiuk S.Differential microRNA expression tracks neoplastic progression in inflammatory bowel disease-associated colorectal cancer.
2012;33:551-560[PMID:22241525 DOI:10.1002/humu.22021]
38 Alpert L,Yassan L,Poon R,Kadri S,Niu N,Patil SA,Mujacic I,Montes D,Galbo F,Wurst MN,Zhen CJ,Cohen RD,Rubin DT,Pekow JR,Weber CR,Xiao SY,Hart J,Segal J,Setia N.Targeted mutational analysis of inflammatory bowel disease-associated colorectal cancers.
2019;89:44-50[PMID:31054900 DOI:10.1016/j.humpath.2019.04.013]
39 Watanabe S,Tsuchiya K,Nishimura R,Shirasaki T,Katsukura N,Hibiya S,Okamoto R,Nakamura T,Watanabe M.
Mutation by CRISPR System Enhances the Malignant Potential of Colon Cancer.
2019;17:1459-1467[PMID:30988165 DOI:10.1158/1541-7786.MCR-18-1195]
40 Lin L,Liu A,Peng Z,Lin HJ,Li PK,Li C,Lin J.STAT3 is necessary for proliferation and survival in colon cancerinitiating cells.
2011;71:7226-7237[PMID:21900397 DOI:10.1158/0008-5472.CAN-10-4660]
41 Corvinus FM,Orth C,Moriggl R,Tsareva SA,Wagner S,Pfitzner EB,Baus D,Kaufmann R,Huber LA,Zatloukal K,Beug H,Ohlschl?ger P,Schütz A,Halbhuber KJ,Friedrich K.Persistent STAT3 activation in colon cancer is associated with enhanced cell proliferation and tumor growth.
2005;7:545-555[PMID:16036105 DOI:10.1593/neo.04571]
42 Lin Q,Lai R,Chirieac LR,Li C,Thomazy VA,Grammatikakis I,Rassidakis GZ,Zhang W,Fujio Y,Kunisada K,Hamilton SR,Amin HM.Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and cell lines:inhibition of JAK3/STAT3 signaling induces apoptosis and cell cycle arrest of colon carcinoma cells.
2005;167:969-980[PMID:16192633 DOI:10.1016/s0002-9440(10)61187-x]
43 Gui X,Iacucci M,Ghosh S.Dysregulation of IL6/IL6R-STAT3-SOCS3 signaling pathway in IBD-associated colorectal dysplastic lesions as compared to sporadic colorectal adenomas in non-IBD patients.
2020;216:153211[PMID:32979687 DOI:10.1016/j.prp.2020.153211]
44 de Lau W,Barker N,Clevers H.WNT signaling in the normal intestine and colorectal cancer.
2007;12:471-491[PMID:17127311 DOI:10.2741/2076]
45 Pinto D,Clevers H.Wnt control of stem cells and differentiation in the intestinal epithelium.
2005;306:357-363[PMID:15925592 DOI:10.1016/j.yexcr.2005.02.022]
46 Aust DE,Terdiman JP,Willenbucher RF,Chang CG,Molinaro-Clark A,Baretton GB,Loehrs U,Waldman FM.The APC/beta-catenin pathway in ulcerative colitis-related colorectal carcinomas:a mutational analysis.
2002;94:1421-1427[PMID:11920497 DOI:10.1002/cncr.10334]
47 Nazemalhosseini Mojarad E,Kashfi SM,Mirtalebi H,Almasi S,Chaleshi V,Kishani Farahani R,Tarban P,Molaei M,Zali MR,J K Kuppen P.Prognostic Significance of Nuclear β-Catenin Expression in Patients with Colorectal Cancer from Iran.
2015;17:e22324[PMID:26421170 DOI:10.5812/ircmj.22324v2]
48 M?rtensson A,Oberg A,Jung A,Cederquist K,Stenling R,Palmqvist R.Beta-catenin expression in relation to genetic instability and prognosis in colorectal cancer.
2007;17:447-452[PMID:17203186]
49 Warren S,Sommers SC.Pathogenesis of ulcerative colitis.
1949;25:657-679[PMID:18152861]
50 Chiu K,Riddell RH,Schaeffer DF.DALM,rest in peace:a pathologist's perspective on dysplasia in inflammatory bowel disease in the post-DALM era.
2018;31:1180-1190[PMID:29789648 DOI:10.1038/s41379-018-0068-9]
51 Mark-Christensen A,Laurberg S,Haboubi N.Dysplasia in Inflammatory Bowel Disease:Historical Review,Critical Histopathological Analysis,and Clinical Implications.
2018;24:1895-1903[PMID:29668897 DOI:10.1093/ibd/izy075]
52 Iacucci M,Uraoka T,Fort Gasia M,Yahagi N.Novel diagnostic and therapeutic techniques for surveillance of dysplasia in patients with inflammatory bowel disease.
2014;28:361-370[PMID:25157526 DOI:10.1155/2014/825947]
53 Laine L,Kaltenbach T,Barkun A,McQuaid KR,Subramanian V,Soetikno R;SCENIC Guideline Development Panel.SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease.
2015;148:639-651.e28[PMID:25702852 DOI:10.1053/j.gastro.2015.01.031]
54 Wu XR,Zheng XB,Huang Y,Cao Q,Zhang HJ,Miao YL,Zou KF,Chen M,Zhang FM,Mei Q,Gonzalo D,Allende D,Hu PJ,Shen B,Liu XL,Lan P.Risk factors for colorectal neoplasia in patients with underlying inflammatory bowel disease:a multicenter study.
2019;7:67-73[PMID:30792868 DOI:10.1093/gastro/goy039]
55 de Jong ME,Kanne H,Nissen LHC,Drenth JPH,Derikx LAAP,Hoentjen F.Increased risk of high-grade dysplasia and colorectal cancer in inflammatory bowel disease patients with recurrent low-grade dysplasia.
2020;91:1334-1342.e1[PMID:31923409 DOI:10.1016/j.gie.2019.12.041]
56 Sonnenberg A,Genta RM.Epithelial Dysplasia and Cancer in IBD Strictures.
2015;9:769-775[PMID:26079724 DOI:10.1093/ecco-jcc/jjv108]
57 Rutter MD,Saunders BP,Wilkinson KH,Rumbles S,Schofield G,Kamm MA,Williams CB,Price AB,Talbot IC,Forbes A.Thirty-year analysis of a colonoscopic surveillance program for neoplasia in ulcerative colitis.
2006;130:1030-1038[PMID:16618396 DOI:10.1053/j.gastro.2005.12.035]
58 Cairns SR,Scholefield JH,Steele RJ,Dunlop MG,Thomas HJ,Evans GD,Eaden JA,Rutter MD,Atkin WP,Saunders BP,Lucassen A,Jenkins P,Fairclough PD,Woodhouse CR;British Society of Gastroenterology;Association of Coloproctology for Great Britain and Ireland.Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups(update from 2002).
2010;59:666-689[PMID:20427401 DOI:10.1136/gut.2009.179804]
59 Lutgens MW,Oldenburg B,Siersema PD,van Bodegraven AA,Dijkstra G,Hommes DW,de Jong DJ,Stokkers PC,van der Woude CJ,Vleggaar FP.Colonoscopic surveillance improves survival after colorectal cancer diagnosis in inflammatory bowel disease.
2009;101:1671-1675[PMID:19826420 DOI:10.1038/sj.bjc.6605359]
60 Lamb CA,Kennedy NA,Raine T,Hendy PA,Smith PJ,Limdi JK,Hayee B,Lomer MCE,Parkes GC,Selinger C,Barrett KJ,Davies RJ,Bennett C,Gittens S,Dunlop MG,Faiz O,Fraser A,Garrick V,Johnston PD,Parkes M,Sanderson J,Terry H;IBD guidelines eDelphi consensus group,Gaya DR,Iqbal TH,Taylor SA,Smith M,Brookes M,Hansen R,Hawthorne AB.British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults.
2019;68:s1-s106[PMID:31562236 DOI:10.1136/gutjnl-2019-318484]
61 Magro F,Gionchetti P,Eliakim R,Ardizzone S,Armuzzi A,Barreiro-de Acosta M,Burisch J,Gecse KB,Hart AL,Hindryckx P,Langner C,Limdi JK,Pellino G,Zagórowicz E,Raine T,Harbord M,Rieder F;European Crohn’s and Colitis Organisation[ECCO].Third European Evidence-based Consensus on Diagnosis and Management of Ulcerative Colitis.Part 1:Definitions,Diagnosis,Extra-intestinal Manifestations,Pregnancy,Cancer Surveillance,Surgery,and Ileoanal Pouch Disorders.
2017;11:649-670[PMID:28158501 DOI:10.1093/ecco-jcc/jjx008]
62 Iacucci M,McQuaid K,Gui XS,Iwao Y,Lethebe BC,Lowerison M,Matsumoto T,Shivaji UN,Smith SCL,Subramanian V,Uraoka T,Sanduleanu S,Ghosh S,Kiesslich R.A multimodal(FACILE)classification for optical diagnosis of inflammatory bowel disease associated neoplasia.
2019;51:133-141[PMID:30541154 DOI:10.1055/a-0757-7759]
63 Watanabe K,Nishishita M,Shimamoto F,Fukuchi T,Esaki M,Okamoto Y,Maehata Y,Oka S,Nishiyama S,Fujii S,Hirai F,Matsui T,Kakimoto K,Okada T,Inoue T,Hida N,Goto H,Nozaki R,Sakurai T,Kashida H,Takeuchi K,Ohmiya N,Saruta M,Saito S,Saito Y,Tanaka S,Fujiwara Y,Arakawa T,Suzuki Y,Ajioka Y,Tajiri H.722 Comparison Between Newly-Developed Narrow Band Imaging and Panchromoendoscopy for Surveillance Colonoscopy in Patients With Longstanding Ulcerative Colitis:A Prospective Multicenter Randomized Controlled Trial,Navigator Study.
2016;83:AB172[DOI:10.1016/j.gie.2016.03.147]
64 Bisschops R,Bessissow T,Dekker E,East JE,Para-Blanco A,Ragunath K,Bhandari P,Rutter M,Schoon E,Wilson A,John JM,Van Steen K,Baert F,Ferrante M.Pit pattern analysis with high-definition chromoendoscopy and narrow-band imaging for optical diagnosis of dysplasia in patients with ulcerative colitis.
2017;86:1100-1106.e1[PMID:28986266 DOI:10.1016/j.gie.2017.09.024]
65 Collins PD,Mpofu C,Watson AJ,Rhodes JM.Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease.
2006;CD000279[PMID:16625534 DOI:10.1002/14651858.CD000279.pub3]
66 Bye WA,Ma C,Nguyen TM,Parker CE,Jairath V,East JE.Strategies for Detecting Colorectal Cancer in Patients with Inflammatory Bowel Disease:A Cochrane Systematic Review and Meta-Analysis.
2018;113:1801-1809[PMID:30353058 DOI:10.1038/s41395-018-0354-7]
67 Watanabe T,Ajioka Y,Mitsuyama K,Watanabe K,Hanai H,Nakase H,Kunisaki R,Matsuda K,Iwakiri R,Hida N,Tanaka S,Takeuchi Y,Ohtsuka K,Murakami K,Kobayashi K,Iwao Y,Nagahori M,Iizuka B,Hata K,Igarashi M,Hirata I,Kudo SE,Matsumoto T,Ueno F,Watanabe G,Ikegami M,Ito Y,Oba K,Inoue E,Tomotsugu N,Takebayashi T,Sugihara K,Suzuki Y,Watanabe M,Hibi T.Comparison of Targeted
Random Biopsies for Surveillance of Ulcerative Colitis-Associated Colorectal Cancer.
2016;151:1122-1130[PMID:27523980 DOI:10.1053/j.gastro.2016.08.002]
68 Bisschops R,East JE,Hassan C,Hazewinkel Y,Kamiński MF,Neumann H,Pellisé M,Antonelli G,Bustamante Balen M,Coron E,Cortas G,Iacucci M,Yuichi M,Longcroft-Wheaton G,Mouzyka S,Pilonis N,Puig I,van Hooft JE,Dekker E.Advanced imaging for detection and differentiation of colorectal neoplasia:European Society of Gastrointestinal Endoscopy(ESGE)Guideline - Update 2019.
2019;51:1155-1179[PMID:31711241 DOI:10.1055/a-1031-7657]
69 Marion JF,Waye JD,Present DH,Israel Y,Bodian C,Harpaz N,Chapman M,Itzkowitz S,Steinlauf AF,Abreu MT,Ullman TA,Aisenberg J,Mayer L;Chromoendoscopy Study Group at Mount Sinai School of Medicine.Chromoendoscopy-targeted biopsies are superior to standard colonoscopic surveillance for detecting dysplasia in inflammatory bowel disease patients:a prospective endoscopic trial.
2008;103:2342-2349[PMID:18844620 DOI:10.1111/j.1572-0241.2008.01934.x]
70 Imperatore N,Castiglione F,Testa A,De Palma GD,Caporaso N,Cassese G,Rispo A.Augmented Endoscopy for Surveillance of Colonic Inflammatory Bowel Disease:Systematic Review With Network Meta-analysis.
2019;13:714-724[PMID:30597029 DOI:10.1093/ecco-jcc/jjy218]
71 Gasia MF,Ghosh S,Panaccione R,Ferraz JG,Kaplan GG,Leung Y,Novak KL,Seow CH,Iacucci M.Targeted Biopsies Identify Larger Proportions of Patients With Colonic Neoplasia Undergoing High-Definition Colonoscopy,Dye Chromoendoscopy,or Electronic Virtual Chromoendoscopy.
2016;14:704-12.e4[PMID:26804384 DOI:10.1016/j.cgh.2015.12.047]
72 Iacucci M,Kaplan GG,Panaccione R,Akinola O,Lethebe BC,Lowerison M,Leung Y,Novak KL,Seow CH,Urbanski S,Minoo P,Gui X,Ghosh S.A Randomized Trial Comparing High Definition Colonoscopy Alone With High Definition Dye Spraying and Electronic Virtual Chromoendoscopy for Detection of Colonic Neoplastic Lesions During IBD Surveillance Colonoscopy.
2018;113:225-234[PMID:29134964 DOI:10.1038/ajg.2017.417]
73 Lippman SM,Benner SE,Hong WK.Cancer chemoprevention.
1994;12:851-873[PMID:8151328 DOI:10.1200/jco.1994.12.4.851]
74 Lyakhovich A,Gasche C.Systematic review:molecular chemoprevention of colorectal malignancy by mesalazine.
2010;31:202-209[PMID:19891667 DOI:10.1111/j.1365-2036.2009.04195.x]
75 Andrews JM,Travis SP,Gibson PR,Gasche C.Systematic review:does concurrent therapy with 5-ASA and immunomodulators in inflammatory bowel disease improve outcomes?
2009;29:459-469[PMID:19077129 DOI:10.1111/j.1365-2036.2008.03915.x]
76 Qiu X,Ma J,Wang K,Zhang H.Chemopreventive effects of 5-aminosalicylic acid on inflammatory bowel diseaseassociated colorectal cancer and dysplasia:a systematic review with meta-analysis.
2017;8:1031-1045[PMID:27906680 DOI:10.18632/oncotarget.13715]
77 Velayos FS,Terdiman JP,Walsh JM.Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk:a systematic review and metaanalysis of observational studies.
2005;100:1345-1353[PMID:15929768 DOI:10.1111/j.1572-0241.2005.41442.x]
78 Bonovas S,Fiorino G,Lytras T,Nikolopoulos G,Peyrin-Biroulet L,Danese S.Systematic review with meta-analysis:use of 5-aminosalicylates and risk of colorectal neoplasia in patients with inflammatory bowel disease.
2017;45:1179-1192[PMID:28261835 DOI:10.1111/apt.14023]
79 Jess T,Lopez A,Andersson M,Beaugerie L,Peyrin-Biroulet L.Thiopurines and risk of colorectal neoplasia in patients with inflammatory bowel disease:a meta-analysis.
2014;12:1793-1800.e1[PMID:24907505 DOI:10.1016/j.cgh.2014.05.019]
80 Lu MJ,Qiu XY,Mao XQ,Li XT,Zhang HJ.Systematic review with meta-analysis:thiopurines decrease the risk of colorectal neoplasia in patients with inflammatory bowel disease.
2018;47:318-331[PMID:29205426 DOI:10.1111/apt.14436]
81 Wang D,Dubois RN.The role of COX-2 in intestinal inflammation and colorectal cancer.
2010;29:781-788[PMID:19946329 DOI:10.1038/onc.2009.421]
82 Rothwell PM,Wilson M,Price JF,Belch JF,Meade TW,Mehta Z.Effect of daily aspirin on risk of cancer metastasis:a study of incident cancers during randomised controlled trials.
2012;379:1591-1601[PMID:22440947 DOI:10.1016/S0140-6736(12)60209-8]
83 Burr NE,Hull MA,Subramanian V.Does aspirin or non-aspirin non-steroidal anti-inflammatory drug use prevent colorectal cancer in inflammatory bowel disease?
2016;22:3679-3686[PMID:27053860 DOI:10.3748/wjg.v22.i13.3679]
84 Leddin D,Tamim H,Levy AR.Is folate involved in the pathogenesis of inflammatory bowel disease?
2013;81:940-941[PMID:24045091 DOI:10.1016/j.mehy.2013.08.025]
85 Lashner BA,Heidenreich PA,Su GL,Kane SV,Hanauer SB.Effect of folate supplementation on the incidence of dysplasia and cancer in chronic ulcerative colitis.A case-control study.
1989;97:255-259[PMID:2568304 DOI:10.1016/0016-5085(89)90058-9]
86 Burr NE,Hull MA,Subramanian V.Folic Acid Supplementation May Reduce Colorectal Cancer Risk in Patients With Inflammatory Bowel Disease:A Systematic Review and Meta-Analysis.
2017;51:247-253[PMID:26905603 DOI:10.1097/MCG.0000000000000498]
87 Rutter MD.Importance of nonpolypoid(flat and depressed)colorectal neoplasms in screening for CRC in patients with IBD.
2014;24:327-335[PMID:24975524 DOI:10.1016/j.giec.2014.03.002]
88 Siegel CA,Schwartz LM,Woloshin S,Cole EB,Rubin DT,Vay T,Baars J,Sands BE.When should ulcerative colitis patients undergo colectomy for dysplasia?
2010;16:1658-1662[PMID:20186940 DOI:10.1002/ibd.21233]
89 Ten Hove JR,Mooiweer E,van der Meulen de Jong AE,Dekker E,Ponsioen CY,Siersema PD,Oldenburg B.Clinical implications of low grade dysplasia found during inflammatory bowel disease surveillance:a retrospective study comparing chromoendoscopy and white-light endoscopy.
2017;49:161-168[PMID:27951611 DOI:10.1055/s-0042-119394]
90 Kabir M,Fofaria R,Arebi N,Bassett P,Tozer PJ,Hart AL,Thomas-Gibson S,Humphries A,Suzuki N,Saunders B,Warusavitarne J,Faiz O,Wilson A.Systematic review with meta-analysis:IBD-associated colonic dysplasia prognosis in the videoendoscopic era(1990 to present).
2020;52:5-19[PMID:32432797 DOI:10.1111/apt.15778]
91 Fong W,Li Q,Yu J.Gut microbiota modulation:a novel strategy for prevention and treatment of colorectal cancer.
2020;39:4925-4943[PMID:32514151 DOI:10.1038/s41388-020-1341-1]
92 Zheng L,Wen XL.Gut microbiota and inflammatory bowel disease:The current status and perspectives.
2021;9:321-333[PMID:33521100 DOI:10.12998/wjcc.v9.i2.321]
93 Shaoul R,Day AS.Nutritional regulators of intestinal inflammation.
2019;35:486-490[PMID:31464809 DOI:10.1097/MOG.0000000000000585]
94 López-Alarcón M,Perichart-Perera O,Flores-Huerta S,Inda-Icaza P,Rodríguez-Cruz M,Armenta-álvarez A,Bram-Falcón MT,Mayorga-Ochoa M.Excessive refined carbohydrates and scarce micronutrients intakes increase inflammatory mediators and insulin resistance in prepubertal and pubertal obese children independently of obesity.
2014;2014:849031[PMID:25477716 DOI:10.1155/2014/849031]
95 De Almeida CV,de Camargo MR,Russo E,Amedei A.Role of diet and gut microbiota on colorectal cancer immunomodulation.
2019;25:151-162[PMID:30670906 DOI:10.3748/wjg.v25.i2.151]
96 Demeyer D,Honikel K,De Smet S.The World Cancer Research Fund report 2007:A challenge for the meat processing industry.
2008;80:953-959[PMID:22063824 DOI:10.1016/j.meatsci.2008.06.003]
97 Limketkai BN,Wolf A,Parian AM.Nutritional Interventions in the Patient with Inflammatory Bowel Disease.
2018;47:155-177[PMID:29413010 DOI:10.1016/j.gtc.2017.09.007]
98 Batista D,Raffals L.Role of intestinal bacteria in the pathogenesis of pouchitis.
2014;20:1481-1486[PMID:25046009 DOI:10.1097/MIB.0000000000000055]
99 Konstantinov SR,Kuipers EJ,Peppelenbosch MP.Functional genomic analyses of the gut microbiota for CRC screening.
2013;10:741-745[PMID:24042452 DOI:10.1038/nrgastro.2013.178]
100 Tong LC,Wang Y,Wang ZB,Liu WY,Sun S,Li L,Su DF,Zhang LC.Propionate Ameliorates Dextran Sodium Sulfate-Induced Colitis by Improving Intestinal Barrier Function and Reducing Inflammation and Oxidative Stress.
2016;7:253[PMID:27574508 DOI:10.3389/fphar.2016.00253]
101 Fukuda S,Toh H,Hase K,Oshima K,Nakanishi Y,Yoshimura K,Tobe T,Clarke JM,Topping DL,Suzuki T,Taylor TD,Itoh K,Kikuchi J,Morita H,Hattori M,Ohno H.Bifidobacteria can protect from enteropathogenic infection through production of acetate.
2011;469:543-547[PMID:21270894 DOI:10.1038/nature09646]
102 Costello SP,Hughes PA,Waters O,Bryant RV,Vincent AD,Blatchford P,Katsikeros R,Makanyanga J,Campaniello MA,Mavrangelos C,Rosewarne CP,Bickley C,Peters C,Schoeman MN,Conlon MA,Roberts-Thomson IC,Andrews JM.Effect of Fecal Microbiota Transplantation on 8-Week Remission in Patients With Ulcerative Colitis:A Randomized Clinical Trial.
2019;321:156-164[PMID:30644982 DOI:10.1001/jama.2018.20046]
103 Paramsothy S,Kamm MA,Kaakoush NO,Walsh AJ,van den Bogaerde J,Samuel D,Leong RWL,Connor S,Ng W,Paramsothy R,Xuan W,Lin E,Mitchell HM,Borody TJ.Multidonor intensive faecal microbiota transplantation for active ulcerative colitis:a randomised placebo-controlled trial.
2017;389:1218-1228[PMID:28214091 DOI:10.1016/S0140-6736(17)30182-4]
104 Lavoie S,Conway KL,Lassen KG,Jijon HB,Pan H,Chun E,Michaud M,Lang JK,Gallini Comeau CA,Dreyfuss JM,Glickman JN,Vlamakis H,Ananthakrishnan A,Kostic A,Garrett WS,Xavier RJ.The Crohn's disease polymorphism,
T300A,alters the gut microbiota and enhances the local Th1/Th17 response.
2019;8[PMID:30666959 DOI:10.7554/eLife.39982]
105 Liu JZ,van Sommeren S,Huang H,Ng SC,Alberts R,Takahashi A,Ripke S,Lee JC,Jostins L,Shah T,Abedian S,Cheon JH,Cho J,Dayani NE,Franke L,Fuyuno Y,Hart A,Juyal RC,Juyal G,Kim WH,Morris AP,Poustchi H,Newman WG,Midha V,Orchard TR,Vahedi H,Sood A,Sung JY,Malekzadeh R,Westra HJ,Yamazaki K,Yang SK;International Multiple Sclerosis Genetics Consortium;International IBD Genetics Consortium,Barrett JC,Alizadeh BZ,Parkes M,Bk T,Daly MJ,Kubo M,Anderson CA,Weersma RK.Association analyses identify 38 susceptibility loci for inflammatory bowel disease and highlight shared genetic risk across populations.
2015;47:979-986[PMID:26192919 DOI:10.1038/ng.3359]
106 Kudo T,Suzuki K,Mori Y,Misawa M,Ichimasa K,Takeda K,Nakamura H,Maeda Y,Ogawa Y,Hayashi T,Wakamura K,Ishida F,Inoue H,Kudo SE.Endocytoscopy for the differential diagnosis of colorectal low-grade adenoma:a novel possibility for the "resect and discard" strategy.
2020;91:676-683[PMID:31785276 DOI:10.1016/j.gie.2019.11.029]
107 Wang KK,Carr-Locke DL,Singh SK,Neumann H,Bertani H,Galmiche JP,Arsenescu RI,Caillol F,Chang KJ,Chaussade S,Coron E,Costamagna G,Dlugosz A,Ian Gan S,Giovannini M,Gress FG,Haluszka O,Ho KY,Kahaleh M,Konda VJ,Prat F,Shah RJ,Sharma P,Slivka A,Wolfsen HC,Zfass A.Use of probe-based confocal laser endomicroscopy(pCLE)in gastrointestinal applications.A consensus report based on clinical evidence.
2015;3:230-254[PMID:26137298 DOI:10.1177/2050640614566066]
108 Kudo T,Saito Y,Ikematsu H,Hotta K,Takeuchi Y,Shimatani M,Kawakami K,Tamai N,Mori Y,Maeda Y,Yamada M,Sakamoto T,Matsuda T,Imai K,Ito S,Hamada K,Fukata N,Inoue T,Tajiri H,Yoshimura K,Ishikawa H,Kudo SE.New-generation full-spectrum endoscopy
standard forward-viewing colonoscopy:a multicenter,randomized,tandem colonoscopy trial(J-FUSE Study).
2018;88:854-864[PMID:29908178 DOI:10.1016/j.gie.2018.06.011]
109 Stidham RW,Liu W,Bishu S,Rice MD,Higgins PDR,Zhu J,Nallamothu BK,Waljee AK.Performance of a Deep Learning Model
Human Reviewers in Grading Endoscopic Disease Severity of Patients With Ulcerative Colitis.
2019;2:e193963[PMID:31099869 DOI:10.1001/jamanetworkopen.2019.3963]
110 Maeda Y,Kudo SE,Ogata N,Misawa M,Mori Y,Mori K,Ohtsuka K.Can artificial intelligence help to detect dysplasia in patients with ulcerative colitis?
2021;53:E273-E274[PMID:33003217 DOI:10.1055/a-1261-2944]
111 Stewénius J,Adnerhill I,Anderson H,Ekelund GR,Florén CH,Fork FT,Janzon L,Lindstr?m C,Ogren M.Incidence of colorectal cancer and all cause mortality in non-selected patients with ulcerative colitis and indeterminate colitis in Malm?,Sweden.
1995;10:117-122[PMID:7636371 DOI:10.1007/bf00341210]
112 Casta?o-Milla C,Chaparro M,Gisbert JP.Systematic review with meta-analysis:the declining risk of colorectal cancer in ulcerative colitis.
2014;39:645-659[PMID:24612141 DOI:10.1111/apt.12651]
113 Olén O,Erichsen R,Sachs MC,Pedersen L,Halfvarson J,Askling J,Ekbom A,S?rensen HT,Ludvigsson JF.Colorectal cancer in Crohn's disease:a Scandinavian population-based cohort study.
2020;5:475-484[PMID:32066530 DOI:10.1016/S2468-1253(20)30005-4]
114 Laukoetter MG,Mennigen R,Hannig CM,Osada N,Rijcken E,Vowinkel T,Krieglstein CF,Senninger N,Anthoni C,Bruewer M.Intestinal cancer risk in Crohn's disease:a meta-analysis.
2011;15:576-583[PMID:21152994 DOI:10.1007/s11605-010-1402-9]
115 Fumery M,Dulai PS,Gupta S,Prokop LJ,Ramamoorthy S,Sandborn WJ,Singh S.Incidence,Risk Factors,and Outcomes of Colorectal Cancer in Patients With Ulcerative Colitis With Low-Grade Dysplasia:A Systematic Review and Meta-analysis.
2017;15:665-674.e5[PMID:27916678 DOI:10.1016/j.cgh.2016.11.025]
116 Friedman S,Rubin PH,Bodian C,Harpaz N,Present DH.Screening and surveillance colonoscopy in chronic Crohn's colitis:results of a surveillance program spanning 25 years.
2008;6:993-8;quiz 953[PMID:18585966 DOI:10.1016/j.cgh.2008.03.019]
117 Basseri RJ,Basseri B,Vassilaki ME,Melmed GY,Ippoliti A,Vasiliauskas EA,Fleshner PR,Lechago J,Hu B,Berel D,Targan SR,Papadakis KA.Colorectal cancer screening and surveillance in Crohn's colitis.
2012;6:824-829[PMID:22398087 DOI:10.1016/j.crohns.2012.01.005]
118 Keller DS,Windsor A,Cohen R,Chand M.Colorectal cancer in inflammatory bowel disease:review of the evidence.
2019;23:3-13[PMID:30701345 DOI:10.1007/s10151-019-1926-2]
119 Pache I,Rogler G,Felley C.TNF-alpha blockers in inflammatory bowel diseases:practical consensus recommendations and a user's guide.
2009;139:278-287[PMID:19452290]
120 Kobelt D,Dahlmann M,Dumbani M,Güllü N,Kortüm B,Vílchez MEA,Stein U,Walther W.Small Ones to Fight a Big Problem-Intervention of Cancer Metastasis by Small Molecules.
2020;12[PMID:32503267 DOI:10.3390/cancers12061454]
121 Atreya R,Neurath MF.Involvement of IL-6 in the pathogenesis of inflammatory bowel disease and colon cancer.
2005;28:187-196[PMID:16129903 DOI:10.1385/criai:28:3:187]
122 Allocca M,Jovani M,Fiorino G,Schreiber S,Danese S.Anti-IL-6 treatment for inflammatory bowel diseases:next cytokine,next target.
2013;14:1508-1521[PMID:24102406 DOI:10.2174/13894501113146660224]
123 Coskun M,Vermeire S,Nielsen OH.Novel Targeted Therapies for Inflammatory Bowel Disease.
2017;38:127-142[PMID:27916280 DOI:10.1016/j.tips.2016.10.014]
124 Danese S,Vermeire S,Hellstern P,Panaccione R,Rogler G,Fraser G,Kohn A,Desreumaux P,Leong RW,Comer GM,Cataldi F,Banerjee A,Maguire MK,Li C,Rath N,Beebe J,Schreiber S.Randomised trial and open-label extension study of an anti-interleukin-6 antibody in Crohn's disease(ANDANTE I and II).
2019;68:40-48[PMID:29247068 DOI:10.1136/gutjnl-2017-314562]
125 Murakami M,Kamimura D,Hirano T.Pleiotropy and Specificity:Insights from the Interleukin 6 Family of Cytokines.
2019;50:812-831[PMID:30995501 DOI:10.1016/j.immuni.2019.03.027]
126 Johnstone CN,Chand A,Putoczki TL,Ernst M.Emerging roles for IL-11 signaling in cancer development and progression:Focus on breast cancer.
2015;26:489-498[PMID:26209885 DOI:10.1016/j.cytogfr.2015.07.015]
127 Ren C,Chen Y,Han C,Fu D,Chen H.Plasma interleukin-11(IL-11)levels have diagnostic and prognostic roles in patients with pancreatic cancer.
2014;35:11467-11472[PMID:25123265 DOI:10.1007/s13277-014-2459-y]
128 Unver N,McAllister F.IL-6 family cytokines:Key inflammatory mediators as biomarkers and potential therapeutic targets.
2018;41:10-17[PMID:29699936 DOI:10.1016/j.cytogfr.2018.04.004]
129 Pastor MD,Nogal A,Molina-Pinelo S,Quintanal-Villalonga á,Meléndez R,Ferrer I,Romero-Romero B,De Miguel MJ,López-Campos JL,Corral J,García-Carboner R,Carnero A,Paz-Ares L.IL-11 and CCL-1:Novel Protein Diagnostic Biomarkers of Lung Adenocarcinoma in Bronchoalveolar Lavage Fluid(BALF).
2016;11:2183-2192[PMID:27524264 DOI:10.1016/j.jtho.2016.07.026]
130 Putoczki TL,Thiem S,Loving A,Busuttil RA,Wilson NJ,Ziegler PK,Nguyen PM,Preaudet A,Farid R,Edwards KM,Boglev Y,Luwor RB,Jarnicki A,Horst D,Boussioutas A,Heath JK,Sieber OM,Pleines I,Kile BT,Nash A,Greten FR,McKenzie BS,Ernst M.Interleukin-11 is the dominant IL-6 family cytokine during gastrointestinal tumorigenesis and can be targeted therapeutically.
2013;24:257-271[PMID:23948300 DOI:10.1016/j.ccr.2013.06.017]
131 Hohenberger M,Cardwell LA,Oussedik E,Feldman SR.Interleukin-17 inhibition:role in psoriasis and inflammatory bowel disease.
2018;29:13-18[PMID:28521565 DOI:10.1080/09546634.2017.1329511]
132 Moschen AR,Tilg H,Raine T.IL-12,IL-23 and IL-17 in IBD:immunobiology and therapeutic targeting.
2019;16:185-196[PMID:30478416 DOI:10.1038/s41575-018-0084-8]
133 Di Fusco D,Izzo R,Figliuzzi MM,Pallone F,Monteleone G.IL-21 as a therapeutic target in inflammatory disorders.
2014;18:1329-1338[PMID:25162763 DOI:10.1517/14728222.2014.945426]
134 Salzer E,Kansu A,Sic H,Májek P,Ikincio?ullari A,Dogu FE,Prengemann NK,Santos-Valente E,Pickl WF,Bilic I,Ban SA,Kulo?lu Z,Demir AM,Ensari A,Colinge J,Rizzi M,Eibel H,Boztug K.Early-onset inflammatory bowel disease and common variable immunodeficiency-like disease caused by IL-21 deficiency.
2014;133:1651-9.e12[PMID:24746753 DOI:10.1016/j.jaci.2014.02.034]
135 Neurath MF.IL-23 in inflammatory bowel diseases and colon cancer.
2019;45:1-8[PMID:30563755 DOI:10.1016/j.cytogfr.2018.12.002]
136 Rosenstock E,Farmer RG,Petras R,Sivak MV Jr,Rankin GB,Sullivan BH.Surveillance for colonic carcinoma in ulcerative colitis.
1985;89:1342-1346[PMID:4054527 DOI:10.1016/0016-5085(85)90653-5]
137 Lashner BA,Kane SV,Hanauer SB.Colon cancer surveillance in chronic ulcerative colitis:historical cohort study.
1990;85:1083-1087[PMID:2389720]
138 L?fberg R,Brostr?m O,Karlén P,Tribukait B,Ost A.Colonoscopic surveillance in long-standing total ulcerative colitis--a 15-year follow-up study.
1990;99:1021-1031[PMID:2394325 DOI:10.1016/0016-5085(90)90622-8]
139 Nugent FW,Haggitt RC,Gilpin PA.Cancer surveillance in ulcerative colitis.
1991;100:1241-1248[PMID:2013371]
140 Lynch DA,Lobo AJ,Sobala GM,Dixon MF,Axon AT.Failure of colonoscopic surveillance in ulcerative colitis.
1993;34:1075-1080[PMID:8174957 DOI:10.1136/gut.34.8.1075]
141 Jonsson B,Ahsgren L,Andersson LO,Stenling R,Ruteg?rd J.Colorectal cancer surveillance in patients with ulcerative colitis.
1994;81:689-691[PMID:8044548 DOI:10.1002/bjs.1800810520]
142 Karlén P,Kornfeld D,Brostr?m O,L?fberg R,Persson PG,Ekbom A.Is colonoscopic surveillance reducing colorectal cancer mortality in ulcerative colitis?
1998;42:711-714[PMID:9659169 DOI:10.1136/gut.42.5.711]
143 Friedman S,Rubin PH,Bodian C,Goldstein E,Harpaz N,Present DH.Screening and surveillance colonoscopy in chronic Crohn's colitis.
2001;120:820-826[PMID:11231935 DOI:10.1053/gast.2001.22449]
144 Biasco G,Rossini FP,Hakim R,Brandi G,Di Battista M,Di Febo G,Calabrese C,Santucci R,Miglioli M.Cancer surveillance in ulcerative colitis:critical analysis of long-term prospective programme.
2002;34:339-342[PMID:12118951 DOI:10.1016/s1590-8658(02)80127-x]
145 Hata K,Watanabe T,Kazama S,Suzuki K,Shinozaki M,Yokoyama T,Matsuda K,Muto T,Nagawa H.Earlier surveillance colonoscopy programme improves survival in patients with ulcerative colitis associated colorectal cancer:results of a 23-year surveillance programme in the Japanese population.
2003;89:1232-1236[PMID:14520452 DOI:10.1038/sj.bjc.6601247]
 World Journal of Gastrointestinal Oncology2022年3期
World Journal of Gastrointestinal Oncology2022年3期
- World Journal of Gastrointestinal Oncology的其它文章
- Re:Association between intestinal neoplasms and celiac disease -beyond celiac disease and more
- Association of Blastocystis hominis with colorectal cancer:A systematic review of in vitro and in vivo evidences
- Clinical efficacy and prognostic risk factors of endoscopic radiofrequency ablation for gastric low-grade intraepithelial neoplasia
- Pancreatic head vs pancreatic body/tail cancer:Are they different?
- Computed tomography-based radiomic to predict resectability in locally advanced pancreatic cancer treated with chemotherapy and radiotherapy
- Cost-effective low-coverage whole-genome sequencing assay for the risk stratification of gastric cancer
