Colorectal cancer carcinogenesis:From bench to bedside
lNTRODUCTlON
Colorectal cancer(CRC)is the third most frequently diagnosed cancer in the USA and Asia,and the second in Europe[1,2],being one of the leading causes of cancer death worldwide[3].Sporadic colorectal cancer represents about 70% of all cases and only 5% are related to known hereditary conditions such as Lynch syndrome(LS)and familiar adenomatous polyposis(FAP).The remaining cases have apparent familial predisposition with no identifiable single germline mutations[4].
Adenomas and serrated lesions are the precursors of the vast majority of CRCs and their number/characteristics at baseline screening colonoscopy allow the definition of adequate surveillance programs after polypectomy,with an impact on survival for over more than 10 years[5].It is reasonable to think that if we understand the molecular mechanisms underlying the appearance of these lesions,we can be even more effective in identifying grades and temporal windows of risk and in designing individualized strategies for preventing and treating CRC.
Colorectal cancer origin
Colonic stem cells(CSC)are now known to be located at the base of the crypt(cells initially identified in 1974 and called “crypt base columnar cells” - CBCC)[6].In the normal setting,the division of a stem cell does not generate a new stem cell and another cell committed to differentiation;on the contrary,each division usually generates two cells with the same destination,either stemness or differentiation.This is a random phenomenon of “neutral competition”[7],through which certain lineages are lost(when the two daughter cells progress to differentiation,as progenitors in the transit-amplifying zone)and crypts evolve to clonality,constitutionally.
Since 2007,CSC have also been known to be responsible for the generation of the entire CRC population[8,9].Certain mutations,namely,in the WNT pathway,may confer selective advantage to the stem cells,granting them a greater potential for subsequent clonal progression in the crypt.The WNT pathway is the main responsible for the proliferation and maintenance of stem cells in the colonic epithelium.However,its level of activity is modulated by several factors,such as NF-kB,KRAS,and the NOTCH signaling pathway[10,11].In the clonal competition process,either an APC loss(with WNT pathway activation)or a KRAS activation(which apparently leads to increased cell division)may confer a selective advantage.In the specific context of inflammatory bowel diseases,the loss of p53 function can also create a selection advantage for the mutated stem cell[10].
Aigoo, he said, which was like “Oh my!” in Korean. My mother said that word to me all the time. He waved his finger at me and said, “Korean important. Yes?”
Carcinogenesis pathways in colorectal cancer
In 1990,Fearon and Vogelstein published an important paper about colorectal carcinogenesis[12].The authors stated the need for the accumulation of several mutations in oncogenes and/or tumour suppressor genes for the development of a CRC.Although certain sequences of events are more frequent,it is the accumulation of mutations,more than its order,that leads to the biologic properties of the CRC[12,13].The authors also found that although most tumours have mutations in the same key genes,additional mutations in several other genes occur in highly variable frequencies,which may explain some of the heterogeneity in the biologic properties of tumours found in clinical practice[14].
He took the first path that presented itself and followed it at the top of his speed, often losing his way, or stumbling over some stone, or running up against a tree, and leaving behind him without regret the cottage which had been as little to his taste as the character of its possessor
In fact,the CRC molecular characterization done by the Cancer Genome Atlas Network found an altered WNT signaling pathway in 93% of tumours,but it also described two broadly distinct groups of tumours:The “hypermutated”(more than 12 mutations per 106 bases)and the “non-hypermutated”(less than 12 mutations per 106 bases)[15].
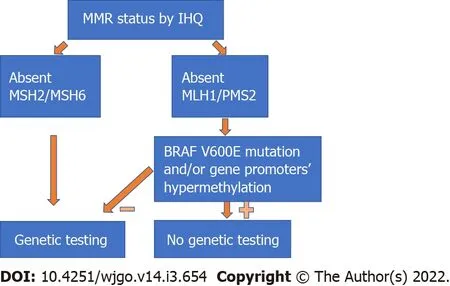
The MSI status can guide the clinician to better identify patients who will benefit from genetic testing.Studies have shown that almost 17% of all MSI-H tumours happen in LS patients[27],with several of them previously unidentified.Patients with LS may benefit from a more radical surgery due to a higher risk of metachronous cancers and require different surveillance protocols after CRC treatment[13,28,29].Moreover,the identification of an LS patient may potentially lead to screening and cancer prevention in several other family members.IHC has a significant role in the selection of patients for genetic testing,since the identification of tumours with absent MSH2/MSH6 protein expression should directly lead to referral for genetic testing,while MLH1/PMS2 loss should be followed by testing for
V600E mutation and/or gene promoter hypermethylation[21](Figure 3).Since most MSI-H tumours with absent MLH1/PMS2 protein expression are due to somatic changes,genetic testing can be omitted in most of these patients.
They came to the forest, which was bursting into bud, and out of it came a splendid horse which Gerda knew; it was the one which had drawn41 the gold coach ridden by a young girl with a red cap on and pistols in her belt
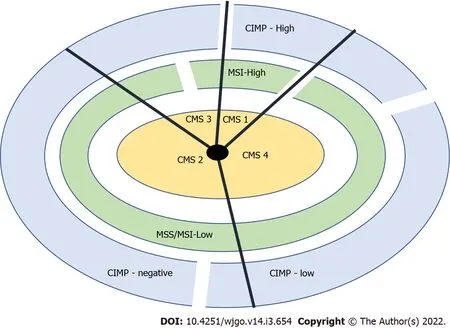
Based on gene expression profiles,a classification system comprising four consensus molecular subtypes(CMS 1-4)was created,each having typical histologic and clinical features[16-19].In Figure 1,we can see a classification system using the consensus molecular subtypes(CMS 1-4),CIMP(CpG island methylator phenotype),and microsatellite instability(MSI)status.The “non-hypermutated” tumours seem to correspond to group 4 and the “hypermutated” tumours to group 1 in the CRC classification proposed by Jass[20].
Multiple studies reported negative prognostic value of the
V600E mutation in patients with stage II,III,or IV CRC[40,41].Other
mutations,much less frequent,are associated with different clinical and histologic characteristics and have a better prognosis.The prognostic meaning of
mutations may,again,be modulated by other factors,such as tumour stage or MSI status[42].In fact,while MSS
V600E mutated tumours seem to have the worst prognosis,
V600E mutation in MSI tumours may have different meanings at distinct stages[43].
CIN is characterized by chromosome changes that include somatic copy number alterations caused by deletions,loss of aneuploidy,insertions,and amplifications.It was recently subdivided into three CMS:CMS 2,epithelial,with marked WNT and MYC activation;CMS 3,epithelial with important metabolic activation;CMS 4,mesenchymal,with TGF-β activation,stromal invasion,and angiogenesis[21].This pathway is observed in about 65-70% of colorectal tumours and usually associated with karyotypic abnormalities(loss of heterozygosity at chromosome arm 18q in 70% of CRC),with
(in 80% of CIN tumours)and
(in 60% of CIN tumours)mutations and with
activating mutations(in 40% of CRC)[13].
His foot hurt, but it was nothing compared with his hunger, which made him go on until darkness fell. His blanket was wet, but he knew only that he was hungry. Through his restless7 sleep he dreamed of banquets8() and of food. The man woke up cold and sick, and found himself lost. But the small sack was still with him. As he dragged himself along, the sack became heavier and heavier. The man opened the sack, which was full of small pieces of gold. He left half the gold on a rock.
In 1965, my world was suddenly uprooted7. I found myself alone with two young sons when my husband wanted a divorce. I was fortunate to receive a full scholarship to the University of Connecticut in the field of special education. I decided8 to sell the furniture and household items and return to my home state with just our clothes.
:MSI is characterized by a high frequency of genomic copy number variations,and corresponds to CMS 1 and the hypermutated tumours subgroup.It occurs in the presence of abnormal mismatch repair(MMR)proteins(MLH1,MSH2,MSH6,or PMS2),caused by sporadic epigenetic silencing(most commonly through gene promotor hypermethylation)or constitutional mutations(Lynch syndrome)[21].This pathway is observed in about 15% of sporadic tumours and in most CRC in LS.The most common cause of the MSI phenotype is somatic - the epigenetic silencing of
due to promoter hypermethylation(usually associated with
mutation and CIMP-high status,a clear example of pathways’ overlap).After the occurrence of MSI,the expression of an inability to correct DNA replication defects,colorectal carcinogenesis progresses more rapidly than through the CIN pathway(1 to 3 years in contrast to 10 years or more)[13].
This pathway is characterized by a phenotype of DNA hypermethylation at specific regulatory sites(CpG islands)in the promoter regions of genes - the CIMP[13].When this hypermethylation affects tumour suppressor genes,it leads to their silencing,promoting carcinogenesis.This pathway is responsible for nearly 15% of CRC and is commonly associated with
mutation(usually the first detected event),after which it can follow different routes.It can converge with the MSI pathway,through inactivation of
genes or it can overlap with the CIN pathway,through
mutations and WNT or TGFβ signalling pathway activation(resulting in MSS or MSI-L tumours)[13,22].
Although several other promising studies are available,liquid biopsy use in CRC still needs standardization of methods and validation in multicentric prospective trials.
With the increasing advances in technology,new subgroups of tumours are being identified - an example is the identification of DNA polymerase protein mutations(POLE and POLD1),that led to the description of a new molecular pathway,characterized by a hypermutated phenotype without MSI[13].
Molecular and genetic features in colorectal cancer screening
Currently,the predominant CRC screening tools are fecal occult blood testing(FOBT),endoscopic evaluation(colonoscopy or sigmoidoscopy),and CT colonography.CRC screening has proven to reduce the risk of CRC associated mortality[23];however,there are multiple limitations regarding test performances,and lack of access or compliance.
Multiple stool DNA-based markers have been evaluated but only the Cologuard multitarget stool DNA(MT-sDNA)test has been approved for clinical use - approved for CRC screening in asymptomatic individuals with ages between 50 and 84 years(United States Food and Drug Administration - US FDA).This test detects a combination of gene mutations(
),methylated DNA markers,and fecal immunochemical test(FIT)and has demonstrated the best clinical performance of CRC marker screening to date.In a recent study,MT-sDNA test proved to have an overall CRC detection rate similar to colonoscopy and a superior sensitivity(but lower specificity)when compared to FIT,for the detection of advanced adenomas and CRC.However,10% of patients with positive MT-sDNA have no polyps or CRC when they undergo colonoscopy[24,25].Overall,models using 3-year screening intervals predict a very high program sensitivity.
Several biomarkers have been investigated for their use in CRC screening,namely,DNA,proteins,and RNA(messenger or micro-RNA).These new non-invasive markers have the potential to improve screening by improving sensitivity,compliance,and accessibility.The detection of these biomarkers in blood,urine,and stool in people with colon polyps or CRC has been assessed and,to date,the most accurate tests are based on stool samples.This is explained by the abundant exfoliation of neoplastic cells from polyps and CRC into the mucocellular layer of the colonic lumen[24].From all the options,only DNA-based markers have been used in clinical testing so far.
An inviting place for a frolic, if it had not been for thenumber of poisonous adders of which the travellers spoke; they alsomentioned that the place had formerly been infested with wolves, andthat the district was still called Wolfsborg for this reason
Also consider the parallels between the spinner of flax and the spinner of tales, a storyteller. Storytellers spin stories with their words and imaginations. In times past, spinning and cloth production were places where storytelling took place and was in the domain of the women of the community.
Although CRC screening using stool based molecular markers is more and more a reality,there are also multiple promising assays in development regarding plasma molecular markers.For example,plasma detection of methylated
(a gene more frequently methylated in CRC
normal colon tissue)is currently available in China,USA,and Europe for CRC screening.However,these tests have suboptimal sensitivity for screening for colon polyps and early CRC compared to currently available screening tests[24].It is hypothesized that plasma and urine marker detection may depend on CRC vascular invasion,which would limit the detection of precursor and early lesions.
Circulating tumor cells,circulating tumor DNA,and serum,fecal,and salivary microRNAs and long non-coding RNAs are all potential biomarkers in this emerging area and their role in CRC screening remains to be established[26].
Diagnostic,therapeutic,and prognostic implications of CRC molecular and genetic features
Although highly correlated with dMMR status(MSI-H status),there is evidence that TILs are an independent predictor of outcome in CRC patients[56].Several lines of data support the fact that the host immunologic response(evaluated by histology)against the tumour is a good prognostic indicator.Elevated lymphocytic reaction to CRC is associated with better oncologic outcomes[57-60].Extensive lymphocytic infiltration is more common in MSI than MSS tumours.The relation between TIL and MSI status can help us even more to discriminate which CRC patients will have a better prognosis.Based on this premise,a TIL/MMR-based classification was created to distinguish the prognosis of CRC subtypes in patients with stage II and III tumours.TIL-low status identifies a clinically aggressive phenotype despite the MSI status[56].Although these data seem interesting,there is not enough evidence yet to support the utilization of TIL evaluation or TIL/MMR-status in clinical practice for prognostic stratification.
There are multiple implications for the evaluation of MSI status in CRC.
36 “You are not afraid of the sea, my dumb child,” said he, as they stood on the deck of the noble ship which was to carry them to the country of the neighboring king
Several studies indicate that MSI tumours may have a reduced response to 5-FU chemotherapy[13,30-32].Stage II MSI tumours have a better prognosis than MSS tumours and they probably do not benefit from adjuvant chemotherapy,namely,with 5-FU[13,33].However,data are more conflicting for stage III tumours,where MSI status does not seem to significantly influence response to 5-FU,especially when oxaliplatin is added to the regimen.Regarding irinotecan,data are scarce,but MSI tumours seem to be sensitive[21].
Tumours with MMR deficiency produce several abnormal proteins which seem to elicit antigen-driven immune responses.Perhaps as an adaptative mechanism,these tumours also show increased expression of several immune checkpoints.As a consequence,MSI CRC metastatic tumours have better response and survival patterns when immune checkpoint inhibitors(ICI)are used(as opposed with the disappointing results in non-MSI tumours).Despite a good response to ICI like pembrolizumab and nivolumab,almost 50% of the patients will progress during this therapy and there are no biomarkers available to predict this response.Tumour mutation burden(TMB)is a good predictor of response to ICI and,although they are rare,POLE/D1 mutations can lead to high TMB tumours that are MSS but may still show good response to ICI[22].
MSI tumours are generally considered to have a better prognosis,with less lymph node metastasis and synchronous liver metastasis.However,the prognostic meaning of MSI is modulated by several factors,and their interactions.Tumour stage is an example of this heterogeneity -while stage II MSI cancers have a better prognosis,metastatic MSI CRC globally have a worst prognosis than MSS ones[21,34-37].However,the grade of the tumour-infiltrating lymphocyte(TIL)response,the
mutation status,or the MSI origin(LS
sporadic)all interfere with the impact of MSI on prognosis[13,22].
BRAF
encodes a serine-threonine protein kinase that is a regulator of the MAPK pathway.It is an important oncogene that plays a central role in cancer initiation and progression.
mutations are strongly associated with MSI and hypermutated tumours in the CMS 1[38,39].
testing is almost mandatory in the metastatic CRC patient population,since it has both prognostic and therapeutic implications.
The still most widely used classification for CRC origin distinguishes three pathways that,in fact,have some overlapping features:Chromosomal instability(CIN),MSI,and serrated pathways[13].
In the fullness of time there was an eruption19 of the merry-makers to the sidewalk. The uninvited guests enveloped20 and permeated21 them, and upon the night air rose joyous22 cries, congratulations, laughter and unclassified noises born of McGary’s oblations(,) to the hymeneal() scene.
V600E mutation is known to be associated with intrinsic chemoresistance[43].Regarding targeted therapy,multiple studies also reported that
mutation is associated with cetuximab and panitumumab resistance.Although this association is not as strong as the known influence of the
status,and still controversial,it is thought that
mutated patients probably do not receive much benefit from being treated with these two drugs[44,45].Therefore,some authors advocate the triplet FOLFOXIRI in combination with bevacizumab as first-line therapy for stage IV
V600E mutated tumours.Due to the low numbers of these tumours in clinical trials,the clinical impact of this strategy remains yet to be demonstrated and the benefit of antiangiogenic drugs like bevacizumab in this subgroup of patients lacks positive results with statistical significance[43].
Finally,several BRAF inhibitors(iBRAF)are now available and have demonstrated important results in several other cancers,starting from melanoma.However,results in CRC were largely disappointing,due to the different carcinogenesis pathways involved.Strategies to overcome these limitations are being developed,mostly by using combinations with standard chemotherapy,targeted agents,and/or immunotherapy.
KRAS status
KRAS protein is a self-inactivating signal.When it binds to a tyrosine kinase receptor such as epidermal growth factor receptor(EGFR),it leads to the activation of the RAS-RAF-MEK kinase pathway.
activating mutations lead to oncogenesis.
mutations are frequently found in MSS tumours in the CMS 3 CIN subgroup[2].The assessment of the
status is also crucial because it may have prognostic and therapeutic implications.
Some studies associated the
mutations with a worse prognosis in the unresectable metastatic setting.However,conflicting results are yet not sufficient to recommend the evaluation of the
status for prognostication[46].
When her hunger was satisfied, the old witch, growing drowsy29, lay down on the stove and said: Listen to me well, and do what I bid thee. Tomorrow when I drive away, do thou clean the yard, sweep the floors and cook my supper. Then take a quarter of a measure of wheat from my store house and pick out of it all the black grains and the wild peas. Mind thou dost all that I have bade; if not, thou shalt be eaten for my supper.
The predictive value of the
status when choosing therapy in stage IV CRC is,however,undisputed.
exon 2 mutation is associated with intrinsic resistance to anti-EGFR antibodies.In
exon 2 wild type patients,
exons 3 and 4 mutations(as the less common
exons 2,3,and 4 mutations)have also been shown to be associated with intrinsic resistance to anti-EGFR antibodies(cetuximab and panitumumab),and CRC patients with these mutation have a worse overall survival when they receive anti-EGFR antibodies,either as monotherapy or combined with traditional chemotherapy[47,48]
Anti-EGFR antibodies have been used for the treatment of metastatic CRC since 2004.More recently,both cetuximab and panitumumab have been approved as first line treatments for
/
/
wild type patients,with a demonstrated increase in overall survival,response rate,and progression-free survival.However,there are still patients with the above tumour genotype that cannot obtain these benefits or who experience rapid drug resistance and disease progression[47,48].
In
wild-type patients,
mutant clones frequently emerge and lead to secondary resistance to anti-EGFR therapy[49].
Ongoing studies are investigating the use of targeted agent combinations to overcome both primary and acquired resistance to therapy due to
mutations.
Also at research stage,Adagrasib,an oral drug that selectively binds and irreversibly inhibits
with a specific mutation,has shown promising results in CRC patients in a phase I/II trial[50,51].
Finally,there is also recent evidence showing that metformin may be useful as an antitumor agent in
mutated CRC[52].
APC status
The WNT signaling pathway is an important mediator of stem cell activation and is the most commonly dysregulated oncogenic pathway in CRC.
,a crucial element in this pathway,is the most commonly mutated gene in sporadic CRC - this mutation is an early event in 80-85% of cases[53].So far,however,attempts to use WNT inhibitors as therapy for CRC have failed,mostly due to adverse effects[53].
Recent data has shown that different
mutations can lead to different prognoses in CRC patients[53,54].For instance,C-terminal
mutation led to a shorter survival,as opposed to N-terminal
mutations - it was suggested that these could be used as prognosis markers(with no therapeutic implications so far)[54].Research on molecules that target specific types of
mutations is also currently ongoing[55].
Are you crazy his eyes got funny and he said something like. The boat I want is the Supremo Numero-Uno blah-blah. Soon as I finish saving up 6,000 bucks2 for that baby I m going to order right from the manufacturer. Custom. In silver. Yesiree. This loser store wouldn t carry something like THAT. And I m sure not going near those sucker crowds.
TILs
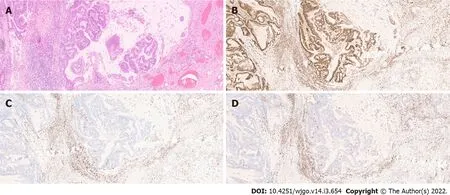
As previously stated,MSI is a major pathogenic pathway implicated in CRC development.The diagnosis of MSI is usually done by polymerase chain reaction(PCR)analysis of five microsatellite markers(the Bethesda panel)in both tumour and normal tissue - tumours with MSI in two or more of the markers are classified as MSI-High(MSI-H).However,immunohistochemistry(IHC)analysis of MMR protein expression has proven to identify around 95% of MSI-H tumours(in the remaining 5%,mutations that affect protein function but not its antigenicity may have happened).This technique is more widely available and does not require normal tissue samples[13](Figure 2).
Liquid biopsy in CRC
The term liquid biopsy refers to the isolation and analysis of tumour-derived material from blood or other bodily fluids[61].In CRC,potential applications range from diagnosis to therapeutic monitoring.The current limitations in its use for screening have already been discussed.
Regarding prognosis,Diehl
[62]found that cell-free DNA(cfDNA)analysis after surgery for CRC accurately predicted relapse,by identifying patients with otherwise undetectable residual disease.If validated,this information could also be used to select patients for adjuvant chemotherapy.
The utility in therapeutic monitoring has been exemplified in a study by Siravegna
[63],who found that clones with
mutations that lead to secondary resistance to anti-EGFR antibodies may lose dominance after therapy withdrawal and that this can be detected by cfDNA analysis,predicting a benefit of reinstitution of anti-EGFR therapy in these patients.
After the old man had bowed politely and taken farewell of them the eldest brother said to the rest, I will go in search of the water of life, and the talking bird, and the tree of beauty
CONCLUSlON
CRC is a heterogeneous entity and its molecular and genetic subtypes have significant implications,from familial risk assessment to therapeutic choices.
Regarding the most used classification for CRC origin,there are three important oncogenic pathways:CIN,MSI,and serrated pathways.They have different clinical and molecular/genetic characteristics.The MSI status,
,
and
mutation status,and the presence of TILs are the most studied tumour features and those more extensively correlated to clinical data.The combination of MSI status and
mutation status can be used to help identify patients with SL.However,tumour molecular and genetic analyses are now also known to predict response to chemotherapy or to immune checkpoint inhibitors and to affect prognosis.Finally,DNA-based markers have already undergone clinical testing in the field of CRC screening and were shown to be useful.
Clinicians should be aware of the major known carcinogenesis pathways and most commonly mutated genes,since some clinical implications are already proven and several others are currently under investigation.
FOOTNOTES
Rosa I and Currais P reviewed the literature and wrote the manuscript;Claro I critically reviewed the manuscript;all authors approved the final version of the manuscript.
The authors have no relevant conflicts of interest to declare.
This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial(CC BYNC 4.0)license,which permits others to distribute,remix,adapt,build upon this work non-commercially,and license their derivative works on different terms,provided the original work is properly cited and the use is noncommercial.See:https://creativecommons.org/Licenses/by-nc/4.0/
Portugal
Pedro Currais 0000-0003-1470-655X;Isadora Rosa 0000-0002-2953-5987;Isabel Claro 0000-0002-1860-6161.
American Gastroenterological Association,1248915.
Wang LL
But they soon found that they were left alone, and that their former friends even attributed their misfortunes to their own extravagance, and showed no intention of offering them any help
Wang TQ
Wang LL
1 Kasi A,Handa S,Bhatti S,Umar S,Bansal A,Sun W.Molecular Pathogenesis and Classification of Colorectal Carcinoma.
2020;16:97-106[PMID:32905465 DOI:10.1007/s11888-020-00458-z]
2 Ruffinelli JC,Santos Vivas C,Sanz-Pamplona R,Moreno V.New advances in the clinical management of RAS and BRAF mutant colorectal cancer patients.
2021;15:65-79[PMID:32946312 DOI:10.1080/17474124.2021.1826305]
3 Ferlay J,Steliarova-Foucher E,Lortet-Tieulent J,Rosso S,Coebergh JW,Comber H,Forman D,Bray F.Cancer incidence and mortality patterns in Europe:estimates for 40 countries in 2012.
2013;49:1374-1403[PMID:23485231 DOI:10.1016/j.ejca.2012.12.027]
4 Kastrinos F,Samadder NJ,Burt RW.Use of Family History and Genetic Testing to Determine Risk of Colorectal Cancer.
2020;158:389-403[PMID:31759928 DOI:10.1053/j.gastro.2019.11.029]
5 Ballegooijen Van,Hankey B,Shi W,Bond J.Colonoscopy polipectomy and long term prevention of colorectal death.
2012;366:687-696[PMID:22356322 DOI:10.1056/nejmoa1100370]
6 Cheng H.Origin,differentiation and renewal of the four main epithelial cell types in the mouse small intestine.IV.Paneth cells.
1974;141:521-535[PMID:4440634 DOI:10.1002/aja.1001410406]
7 Snippert HJ,van der Flier LG,Sato T,van Es JH,van den Born M,Kroon-Veenboer C,Barker N,Klein AM,van Rheenen J,Simons BD,Clevers H.Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells.
2010;143:134-144[PMID:20887898 DOI:10.1016/j.cell.2010.09.016]
8 Ricci-Vitiani L,Lombardi DG,Pilozzi E,Biffoni M,Todaro M,Peschle C,De Maria R.Identification and expansion of human colon-cancer-initiating cells.
2007;445:111-115[PMID:17122771 DOI:10.1038/nature05384]
9 O'Brien CA,Pollett A,Gallinger S,Dick JE.A human colon cancer cell capable of initiating tumour growth in immunodeficient mice.
2007;445:106-110[PMID:17122772 DOI:10.1038/nature05372]
10 Vermeulen L,Morrissey E,van der Heijden M,Nicholson AM,Sottoriva A,Buczacki S,Kemp R,Tavaré S,Winton DJ.Defining stem cell dynamics in models of intestinal tumor initiation.
2013;342:995-998[PMID:24264992 DOI:10.1126/science.1243148]
11 Snippert HJ,Schepers AG,van Es JH,Simons BD,Clevers H.Biased competition between Lgr5 intestinal stem cells driven by oncogenic mutation induces clonal expansion.
2014;15:62-69[PMID:24355609 DOI:10.1002/embr.201337799]
12 Knudson AG Jr.Hereditary cancer,oncogenes,and antioncogenes.
1985;45:1437-1443[PMID:2983882]
13 Nguyen LH,Goel A,Chung DC.Pathways of Colorectal Carcinogenesis.
2020;158:291-302[PMID:31622622 DOI:10.1053/j.gastro.2019.08.059]
14 Dwyer ML,Colombo A,Bozzi G.Retrieval technique of a PTCA guidewire.
1989;19:170-172[PMID:2527178]
15 Cancer Genome Atlas Network.Comprehensive molecular characterization of human colon and rectal cancer.
2012;487:330-337[PMID:22810696 DOI:10.1038/nature11252]
16 De Sousa E Melo F,Wang X,Jansen M,Fessler E,Trinh A,de Rooij LP,de Jong JH,de Boer OJ,van Leersum R,Bijlsma MF,Rodermond H,van der Heijden M,van Noesel CJ,Tuynman JB,Dekker E,Markowetz F,Medema JP,Vermeulen L.Poor-prognosis colon cancer is defined by a molecularly distinct subtype and develops from serrated precursor lesions.
2013;19:614-618[PMID:23584090 DOI:10.1038/nm.3174]
17 Sadanandam A,Lyssiotis CA,Homicsko K,Collisson EA,Gibb WJ,Wullschleger S,Ostos LC,Lannon WA,Grotzinger C,Del Rio M,Lhermitte B,Olshen AB,Wiedenmann B,Cantley LC,Gray JW,Hanahan D.A colorectal cancer classification system that associates cellular phenotype and responses to therapy.
2013;19:619-625[PMID:23584089 DOI:10.1038/nm.3175]
18 Guinney J,Dienstmann R,Wang X,de Reyniès A,Schlicker A,Soneson C,Marisa L,Roepman P,Nyamundanda G,Angelino P,Bot BM,Morris JS,Simon IM,Gerster S,Fessler E,De Sousa E Melo F,Missiaglia E,Ramay H,Barras D,Homicsko K,Maru D,Manyam GC,Broom B,Boige V,Perez-Villamil B,Laderas T,Salazar R,Gray JW,Hanahan D,Tabernero J,Bernards R,Friend SH,Laurent-Puig P,Medema JP,Sadanandam A,Wessels L,Delorenzi M,Kopetz S,Vermeulen L,Tejpar S.The consensus molecular subtypes of colorectal cancer.
2015;21:1350-1356[PMID:26457759 DOI:10.1038/nm.3967]
19 Marisa L,de Reyniès A,Duval A,Selves J,Gaub MP,Vescovo L,Etienne-Grimaldi MC,Schiappa R,Guenot D,Ayadi M,Kirzin S,Chazal M,Fléjou JF,Benchimol D,Berger A,Lagarde A,Pencreach E,Piard F,Elias D,Parc Y,Olschwang S,Milano G,Laurent-Puig P,Boige V.Gene expression classification of colon cancer into molecular subtypes:characterization,validation,and prognostic value.
2013;10:e1001453[PMID:23700391 DOI:10.1371/journal.pmed.1001453]
20 Jass JR.Classification of colorectal cancer based on correlation of clinical,morphological and molecular features.
2007;50:113-130[PMID:17204026 DOI:10.1111/j.1365-2559.2006.02549.x]
21 Ryan E,Sheahan K,Creavin B,Mohan HM,Winter DC.The current value of determining the mismatch repair status of colorectal cancer:A rationale for routine testing.
2017;116:38-57[PMID:28693799 DOI:10.1016/j.critrevonc.2017.05.006]
22 Cohen R,Rousseau B,Vidal J,Colle R,Diaz LA Jr,André T.Immune Checkpoint Inhibition in Colorectal Cancer:Microsatellite Instability and Beyond.
2020;15:11-24[PMID:31786718 DOI:10.1007/s11523-019-00690-0]
23 Ahlquist DA.Molecular detection of colorectal neoplasia.
2010;138:2127-2139[PMID:20420950 DOI:10.1053/j.gastro.2010.01.055]
24 Dickinson BT,Kisiel J,Ahlquist DA,Grady WM.Molecular markers for colorectal cancer screening.
2015;64:1485-1494[PMID:25994221 DOI:10.1136/gutjnl-2014-308075]
25 Imperiale TF,Ransohoff DF,Itzkowitz SH.Multitarget stool DNA testing for colorectal-cancer screening.
2014;371:187-188[PMID:25006736 DOI:10.1056/NEJMc1405215]
26 Vacante M,Ciuni R,Basile F,Biondi A.The Liquid Biopsy in the Management of Colorectal Cancer:An Overview.
2020;8[PMID:32858879 DOI:10.3390/biomedicines8090308]
27 Latham A,Srinivasan P,Kemel Y,Shia J,Bandlamudi C,Mandelker D,Middha S,Hechtman J,Zehir A,Dubard-Gault M,Tran C,Stewart C,Sheehan M,Penson A,DeLair D,Yaeger R,Vijai J,Mukherjee S,Galle J,Dickson MA,Janjigian Y,O'Reilly EM,Segal N,Saltz LB,Reidy-Lagunes D,Varghese AM,Bajorin D,Carlo MI,Cadoo K,Walsh MF,Weiser M,Aguilar JG,Klimstra DS,Diaz LA Jr,Baselga J,Zhang L,Ladanyi M,Hyman DM,Solit DB,Robson ME,Taylor BS,Offit K,Berger MF,Stadler ZK.Microsatellite Instability Is Associated With the Presence of Lynch Syndrome Pan-Cancer.
2019;37:286-295[PMID:30376427 DOI:10.1200/JCO.18.00283]
28 Heneghan HM,Martin ST,Winter DC.Segmental vs extended colectomy in the management of hereditary nonpolyposis colorectal cancer:a systematic review and meta-analysis.
2015;17:382-389[PMID:25510173 DOI:10.1111/codi.12868]
29 J?rvinen HJ,Aarnio M,Mustonen H,Aktan-Collan K,Aaltonen LA,Peltom?ki P,De La Chapelle A,Mecklin JP.Controlled 15-year trial on screening for colorectal cancer in families with hereditary nonpolyposis colorectal cancer.
2000;118:829-834[PMID:10784581 DOI:10.1016/s0016-5085(00)70168-5]
30 Sargent DJ,Marsoni S,Monges G,Thibodeau SN,Labianca R,Hamilton SR,French AJ,Kabat B,Foster NR,Torri V,Ribic C,Grothey A,Moore M,Zaniboni A,Seitz JF,Sinicrope F,Gallinger S.Defective mismatch repair as a predictive marker for lack of efficacy of fluorouracil-based adjuvant therapy in colon cancer.
2010;28:3219-3226[PMID:20498393 DOI:10.1200/JCO.2009.27.1825]
31 Hutchins G,Southward K,Handley K,Magill L,Beaumont C,Stahlschmidt J,Richman S,Chambers P,Seymour M,Kerr D,Gray R,Quirke P.Value of mismatch repair,KRAS,and BRAF mutations in predicting recurrence and benefits from chemotherapy in colorectal cancer.
2011;29:1261-1270[PMID:21383284 DOI:10.1200/JCO.2010.30.1366]
32 Ribic CM,Sargent DJ,Moore MJ,Thibodeau SN,French AJ,Goldberg RM,Hamilton SR,Laurent-Puig P,Gryfe R,Shepherd LE,Tu D,Redston M,Gallinger S.Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer.
2003;349:247-257[PMID:12867608 DOI:10.1056/NEJMoa022289]
33 Ota DM.Colon cancer.
1997;90:347-356[PMID:9367092 DOI:10.1007/978-1-4615-6165-1_18]
34 Sinicrope FA,Shi Q,Smyrk TC,Thibodeau SN,Dienstmann R,Guinney J,Bot BM,Tejpar S,Delorenzi M,Goldberg RM,Mahoney M,Sargent DJ,Alberts SR.Molecular markers identify subtypes of stage III colon cancer associated with patient outcomes.
2015;148:88-99[PMID:25305506 DOI:10.1053/j.gastro.2014.09.041]
35 Vasen HF,Blanco I,Aktan-Collan K,Gopie JP,Alonso A,Aretz S,Bernstein I,Bertario L,Burn J,Capella G,Colas C,Engel C,Frayling IM,Genuardi M,Heinimann K,Hes FJ,Hodgson SV,Karagiannis JA,Lalloo F,Lindblom A,Mecklin JP,M?ller P,Myrhoj T,Nagengast FM,Parc Y,Ponz de Leon M,Renkonen-Sinisalo L,Sampson JR,Stormorken A,Sijmons RH,Tejpar S,Thomas HJ,Rahner N,Wijnen JT,J?rvinen HJ,M?slein G;Mallorca group.Revised guidelines for the clinical management of Lynch syndrome(HNPCC):recommendations by a group of European experts.
2013;62:812-823[PMID:23408351 DOI:10.1136/gutjnl-2012-304356]
36 Miles A,McClements PL,Steele RJ,Redeker C,Sevdalis N,Wardle J.The Psychological Impact of a Colorectal Cancer Diagnosis Following a Negative Fecal Occult Blood Test Result.
2015;24:1032-1038[PMID:25924826 DOI:10.1158/1055-9965.EPI-15-0004]
37 Jennings BA,Loke YK,Skinner J,Keane M,Chu GS,Turner R,Epurescu D,Barrett A,Willis G.Evaluating predictive pharmacogenetic signatures of adverse events in colorectal cancer patients treated with fluoropyrimidines.
2013;8:e78053[PMID:24167597 DOI:10.1371/journal.pone.0078053]
38 Gonsalves WI,Mahoney MR,Sargent DJ,Nelson GD,Alberts SR,Sinicrope FA,Goldberg RM,Limburg PJ,Thibodeau SN,Grothey A,Hubbard JM,Chan E,Nair S,Berenberg JL,McWilliams RR;Alliance for Clinical Trials in Oncology.Patient and tumor characteristics and BRAF and KRAS mutations in colon cancer,NCCTG/Alliance N0147.
2014;106[PMID:24925349 DOI:10.1093/jnci/dju106]
39 Lipsyc M,Yaeger R.Impact of somatic mutations on patterns of metastasis in colorectal cancer.
2015;6:645-649[PMID:26697197 DOI:10.3978/j.issn.2078-6891.2015.045]
40 Fari?a-Sarasqueta A,van Lijnschoten G,Moerland E,Creemers GJ,Lemmens VEPP,Rutten HJT,van den Brule AJC.The BRAF V600E mutation is an independent prognostic factor for survival in stage II and stage III colon cancer patients.
2010;21:2396-2402[PMID:20501503 DOI:10.1093/annonc/mdq258]
41 Morris V,Overman MJ,Jiang ZQ,Garrett C,Agarwal S,Eng C,Kee B,Fogelman D,Dasari A,Wolff R,Maru D,Kopetz S.Progression-free survival remains poor over sequential lines of systemic therapy in patients with BRAF-mutated colorectal cancer.
2014;13:164-171[PMID:25069797 DOI:10.1016/j.clcc.2014.06.001]
42 Bl?ker H,Alwers E,Arnold A,Herpel E,Tagscherer KE,Roth W,Jansen L,Walter V,Kloor M,Chang-Claude J,Brenner H,Hoffmeister M.The Association Between Mutations in BRAF and Colorectal Cancer-Specific Survival Depends on Microsatellite Status and Tumor Stage.
2019;17:455-462.e6[PMID:29660527 DOI:10.1016/j.cgh.2018.04.015]
43 Caputo F,Santini C,Bardasi C,Cerma K,Casadei-Gardini A,Spallanzani A,Andrikou K,Cascinu S,Gelsomino F.BRAF-Mutated Colorectal Cancer:Clinical and Molecular Insights.
2019;20[PMID:31661924 DOI:10.3390/ijms20215369]
44 Rowland A,Dias MM,Wiese MD,Kichenadasse G,McKinnon RA,Karapetis CS,Sorich MJ.Meta-analysis of BRAF mutation as a predictive biomarker of benefit from anti-EGFR monoclonal antibody therapy for RAS wild-type metastatic colorectal cancer.
2015;112:1888-1894[PMID:25989278 DOI:10.1038/bjc.2015.173]
45 Pietrantonio F,Petrelli F,Coinu A,Di Bartolomeo M,Borgonovo K,Maggi C,Cabiddu M,Iacovelli R,Bossi I,Lonati V,Ghilardi M,de Braud F,Barni S.Predictive role of BRAF mutations in patients with advanced colorectal cancer receiving cetuximab and panitumumab:a meta-analysis.
2015;51:587-594[PMID:25673558 DOI:10.1016/j.ejca.2015.01.054]
46 Labianca R,Nordlinger B,Beretta GD,Mosconi S,Mandalà M,Cervantes A,Arnold D;ESMO Guidelines Working Group.Early colon cancer:ESMO Clinical Practice Guidelines for diagnosis,treatment and follow-up.
2013;24 Suppl 6:vi64-vi72[PMID:24078664 DOI:10.1093/annonc/mdt354]
47 Lièvre A,Bachet JB,Le Corre D,Boige V,Landi B,Emile JF,C?té JF,Tomasic G,Penna C,Ducreux M,Rougier P,Penault-Llorca F,Laurent-Puig P.KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer.
2006;66:3992-3995[PMID:16618717 DOI:10.1158/0008-5472.CAN-06-0191]
48 De Roock W,Piessevaux H,De Schutter J,Janssens M,De Hertogh G,Personeni N,Biesmans B,Van Laethem JL,Peeters M,Humblet Y,Van Cutsem E,Tejpar S.KRAS wild-type state predicts survival and is associated to early radiological response in metastatic colorectal cancer treated with cetuximab.
2008;19:508-515[PMID:17998284 DOI:10.1093/annonc/mdm496]
49 Misale S,Yaeger R,Hobor S,Scala E,Janakiraman M,Liska D,Valtorta E,Schiavo R,Buscarino M,Siravegna G,Bencardino K,Cercek A,Chen CT,Veronese S,Zanon C,Sartore-Bianchi A,Gambacorta M,Gallicchio M,Vakiani E,Boscaro V,Medico E,Weiser M,Siena S,Di Nicolantonio F,Solit D,Bardelli A.Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer.
2012;486:532-536[PMID:22722830 DOI:10.1038/nature11156]
50 J?nne P,Rybkin I,Spira A,Riely G,Papadopoulos K,Sabari J.KRYSTAL-1:Activity and Safety of Adagrasib(MRTX849)in Advanced/ Metastatic Non-Small-Cell Lung Cancer(NSCLC)Harboring KRAS G12C Mutation.
2020;138:S1-2[DOI:10.1016/s0959-8049(20)31076-5]
51 Riely G,Ou S,Rybkin I,Spira A,Papadopoulos K,Sabari J.KRYSTAL-1:Activity and preliminary pharmacodynamic(PD)analysis of adagrasib(MRTX849)in patients(Pts)with advanced non-small cell lung cancer(NSCLC)harboring KRASG12C mutation.
2021;16:S751-S752[DOI:10.1016/s1556-0864(21)01941-9]
52 Xie J,Xia L,Xiang W,He W,Yin H,Wang F,Gao T,Qi W,Yang Z,Yang X,Zhou T,Gao G.Metformin selectively inhibits metastatic colorectal cancer with the
mutation by intracellular accumulation through silencing MATE1.
2020;117:13012-13022[PMID:32444490 DOI:10.1073/pnas.1918845117]
53 Zhang L,Shay JW.Multiple Roles of APC and its Therapeutic Implications in Colorectal Cancer.
2017;109[PMID:28423402 DOI:10.1093/jnci/djw332]
54 Mondaca S,Walch H,Nandakumar S,Chatila WK,Schultz N,Yaeger R.Specific Mutations in APC,but Not Alterations in DNA Damage Response,Associate With Outcomes of Patients With Metastatic Colorectal Cancer.
2020;159:1975-1978.e4[PMID:32730818 DOI:10.1053/j.gastro.2020.07.041]
55 Wang W,Zhang L,Morlock L,Williams NS,Shay JW,De Brabander JK.Design and Synthesis of TASIN Analogues Specifically Targeting Colorectal Cancer Cell Lines with Mutant Adenomatous Polyposis Coli(APC).
2019;62:5217-5241[PMID:31070915 DOI:10.1021/acs.jmedchem.9b00532]
56 Williams DS,Mouradov D,Jorissen RN,Newman MR,Amini E,Nickless DK,Teague JA,Fang CG,Palmieri M,Parsons MJ,Sakthianandeswaren A,Li S,Ward RL,Hawkins NJ,Faragher I,Jones IT,Gibbs P,Sieber OM.Lymphocytic response to tumour and deficient DNA mismatch repair identify subtypes of stage II/III colorectal cancer associated with patient outcomes.
2019;68:465-474[PMID:29382774 DOI:10.1136/gutjnl-2017-315664]
57 Klintrup K,M?kinen JM,Kauppila S,V?re PO,Melkko J,Tuominen H,Tuppurainen K,M?kel? J,Karttunen TJ,M?kinen MJ.Inflammation and prognosis in colorectal cancer.
2005;41:2645-2654[PMID:16239109 DOI:10.1016/j.ejca.2005.07.017]
58 Ogino S,Nosho K,Irahara N,Meyerhardt JA,Baba Y,Shima K,Glickman JN,Ferrone CR,Mino-Kenudson M,Tanaka N,Dranoff G,Giovannucci EL,Fuchs CS.Lymphocytic reaction to colorectal cancer is associated with longer survival,independent of lymph node count,microsatellite instability,and CpG island methylator phenotype.
2009;15:6412-6420[PMID:19825961 DOI:10.1158/1078-0432.CCR-09-1438]
59 Mei Z,Liu Y,Liu C,Cui A,Liang Z,Wang G,Peng H,Cui L,Li C.Tumour-infiltrating inflammation and prognosis in colorectal cancer:systematic review and meta-analysis.
2014;110:1595-1605[PMID:24504370 DOI:10.1038/bjc.2014.46]
60 Ropponen KM,Eskelinen MJ,Lipponen PK,Alhava E,Kosma VM.Prognostic value of tumour-infiltrating lymphocytes(TILs)in colorectal cancer.
1997;182:318-324[PMID:9349235 DOI:10.1002/(SICI)1096-9896(199707)182:3<318::AID-PATH862>3.0.CO;2-6]
61 Corcoran RB,Chabner BA.Application of Cell-free DNA Analysis to Cancer Treatment.
2018;379:1754-1765[PMID:30380390 DOI:10.1056/NEJMra1706174]
62 Lindforss U,Zetterquist H,Papadogiannakis N,Olivecrona H.Persistence of K-ras mutations in plasma after colorectal tumor resection.
2005;25:657-661[PMID:15816642]
63 Siravegna G,Mussolin B,Buscarino M,Corti G,Cassingena A,Crisafulli G,Ponzetti A,Cremolini C,Amatu A,Lauricella C,Lamba S,Hobor S,Avallone A,Valtorta E,Rospo G,Medico E,Motta V,Antoniotti C,Tatangelo F,Bellosillo B,Veronese S,Budillon A,Montagut C,Racca P,Marsoni S,Falcone A,Corcoran RB,Di Nicolantonio F,Loupakis F,Siena S,Sartore-Bianchi A,Bardelli A.Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients.
2015;21:795-801[PMID:26030179 DOI:10.1038/nm.3870]
 World Journal of Gastrointestinal Oncology2022年3期
World Journal of Gastrointestinal Oncology2022年3期
- World Journal of Gastrointestinal Oncology的其它文章
- Re:Association between intestinal neoplasms and celiac disease -beyond celiac disease and more
- Association of Blastocystis hominis with colorectal cancer:A systematic review of in vitro and in vivo evidences
- Clinical efficacy and prognostic risk factors of endoscopic radiofrequency ablation for gastric low-grade intraepithelial neoplasia
- Pancreatic head vs pancreatic body/tail cancer:Are they different?
- Computed tomography-based radiomic to predict resectability in locally advanced pancreatic cancer treated with chemotherapy and radiotherapy
- Cost-effective low-coverage whole-genome sequencing assay for the risk stratification of gastric cancer
