Research advances on how metformin improves memory impairment in “chemobrain”
Ahmad Alhowail , Sridevi Chigurupati
Abstract Cognitive impairment caused by chemotherapy, referred to as “chemobrain,” is observed in approximately 70% of cancer survivors. However, it is not completely understood how chemotherapy induces cognitive dysfunction, and clinical treatment strategies for this problem are lacking. Metformin, used as a first-line treatment for type 2 diabetes mellitus,is reported to reduce the effects of chemobrain. Recently, several studies have examined the effect of metformin in rescuing chemobrain. This review discusses recent clinical/preclinical studies that addressed some mechanisms of chemobrain and evaluates the effect of metformin in rescuing chemobrain and its potential mechanisms of action.
Key Words: behavioral task; chemobrain; chemotherapy; cognitive impairments;inflammation; memory impairment; metformin; neuroprotective agent; review
Introduction
The term “chemobrain,” also known as “chemo fog,” describes a phenomenon in which cancer patients exhibit symptoms of cognitive impairment following chemotherapy. Cognitive dysfunction is observed in > 75% of patients with cancer subjected to chemotherapy (Schagen et al., 1999; Jenkins et al., 2006), which is found to be permanent in 17–34% of cancer survivors (Ahles and Saykin, 2007). Chemobrain has been reported since the 1970s, but surprisingly it did not gain significant attention until the mid-1990s (Ahles and Saykin, 2007). Studies in recent years have revealed causal relationships between chemotherapy and memory loss (Li et al., 2017), but the underlying mechanism remains largely undiscovered.
Chemotherapy has been used in cancer treatment since the early 20thcentury (DeVita and Chu, 2008), and aims in most cases to eliminate tumors by reducing the rate of cellular proliferation (Chabner and Roberts, 2005). Chemotherapeutic drugs are effective in treating different types of tumor;however, due to side effects like cognitive dysfunction, the optimum dose for clinical efficacy may be limited. Some chemotherapeutic agents can trigger cognitive impairment by their ability to access the brain by crossing the bloodbrain barrier (Janelsins et al., 2010). However, some agents that are unable to penetrate the blood-brain barrier can still induce chemobrain, for example, doxorubicin. Major domains of cognitive impairment include verbal learning and memory, visual learning and memory, speech and language,visuospatial processing, attention and concentration,executive function, sensory-perceptual functions, motor speed and strength, emotion and personality functioning, and academic skills (Pendergrass et al., 2018). The magnitude of cognitive impairment is observed in subjects with cancer and those in remission make cognitive function an exceptionally important target for pre-clinical trials and clinical practice in oncology. A few drugs, such as methylphenidate, modafinil and donepezil, are already used in the treatment of certain effects of cognitive impairment (Kohli et al., 2009; Gehring et al., 2012).
The drug metformin is used to treat type 2 diabetes mellitus(DM) because it has antihyperglycemic effects (Davidson and Peters, 1997). Unlike other classes of anti-DM drugs (e.g.,sulfonylureas), metformin does not cause hypoglycemia or hyperinsulinemia in healthy people or DM patients (Davidson and Peters, 1997; Dumitrescu et al., 2015). Several studies have shown that metformin also has beneficial effects for diseases other than DM. For example, metformin has an anticancer effect because it inhibits the expression of certain protein kinases, such as mammalian target of rapamycin, that are important for cellular proliferation, and it prolongs the lifespan of cancer sufferers (Song et al., 2019). Metformin has also been reported to reduce the cardiotoxicity,nephrotoxicity, and hepatotoxicity associated with some forms of chemotherapy (Kobashigawa et al., 2014; Li et al., 2016;Ling et al., 2017) by inhibiting nuclear factor kappa B (NF-κB),which activates proinflammatory markers, and by inhibiting mitochondrial complex I activity and thus reducing oxidative stress caused by chemotherapy (Deng et al., 2019). Metformin is also reported to make menstrual cycles more regular and increases fertility (Nasri and Rafieian-Kopaei, 2014).
Several studies have postulated metformin as a protective agent to counteract chemobrain (Li et al., 2016; Alhowail et al., 2019). The purpose of this review is to analyze existing literature to assess the effects of chemobrain on animal behavior, brain function, and biochemical parameters; to assess the ability of metformin to recover deficits in cognitive dysfunction caused by chemotherapy; and to illustrate the mechanisms of action of metformin in improving memory impairment.
Search Strategy
The articles used in this chemobrain review were retrieved by electronic search of the Medline database for literature describing human and animal models of chemobrain from 2009 to 2020 using the following conditions: (models, animal,human OR behavior, animal/physiology OR chemotherapy induced cognitive impairment/dysfunction). The results were further screened by title and abstract to only present rats,mice and human primates.
Pathophysiology of Chemobrain
It is well proven that chemotherapy for cancer can cause cognitive impairment, and there are many recent metaanalyses examining cognitive impairments caused by different cancer treatments. For example, patients in remission after oncological treatments had damage in the areas of memory,concentration, speed of executing an activity, executive function, and memory related to visual and verbal activities relative to people with no cancer (Boykoff et al., 2009; Lindner et al., 2014). In a study of 196 patients who were long-term fighters of breast cancer and undergoing treatment with cyclophosphamide, methotrexate, and fluorouracil (CMF), the chemotherapy group performed significantly worse in many cognitive tests, including those related to immediate and delayed verbal memory, speed in functional execution, and speed of psychomotor activities, compared with patients with no history of cancer (Koppelmans et al., 2012). In a separate examination of 189 survivors of breast cancer it was found that domain-specific memory and executive function disorders were present in 20% of the cohort (Pendergrass et al., 2018).
Although the exact etiology of chemobrain has yet to be elucidated, recent studies have provided some insight into potential causal mechanisms (Figure 1). These findings include disruption of hippocampal cell proliferation and neurogenesis (Briones and Woods, 2011; Christie et al.,2012), hormonal changes (Usuki et al., 1998), epigenetic alterations (Briones and Woods, 2011), increased oxidative stress and production of reactive oxygen species (Inagaki et al., 2007), chronic increases in inflammation (Inagaki et al.,2007), and reduction in long-term potentiation (Lee et al.,2006) during and after chemotherapy. Understanding the mechanisms underlying chemobrain can help in establishing preventive strategies and in reduction of the adverse effects of chemotherapy.
Additionally to the mechanism of chemobrain, proinflammatory cytokines levels such as tumor necrosis factor alpha (TNF-α), interleukin-6 and interleukin-1beta highly increased in cancer patients even without receiving chemotherapy treatment (Goldberg and Schwertfeger,2010; Sultani et al., 2012; Bradburn et al., 2017). Increasing levels of circulating interleukin-6 and TNF-α are associated with cognitive impairment (Krabbe et al., 2005). In addition,cytokines are also able to penetrate the blood-brain barrier and thus were found to promote the central inflammatory response, and thus increasing cytokine levels in the brain(Yarlagadda et al., 2009; Sultani et al., 2012). For example,preclinical and clinical studies have revealed that an increase,of peripheral cytokine enhances CNS cytokine release through the vagus nerve (Ahles and Saykin, 2007), which ultimately leads to cognitive deficits. Moreover, chemotherapy increases the levels of cytokines and that causes over-activation of HPA axis that also leads to impairment (Wilson et al., 2002; Yirmiya and Goshen, 2011). Therefore, the ability of metformin in reducing the inflammation could potentially protect these memory impairments caused by the elevated levels of cytokines.
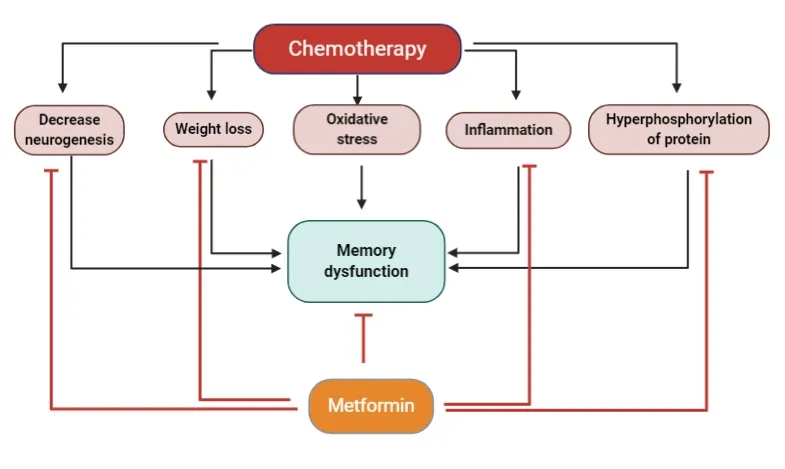
Figure 1|Mechanisms of chemotherapy-induced memory dysfunction and potential protective effects of metformin.Mechanisms of chemotherapy-induced memory dysfunction (black lines) and potential protective effects of metformin (red lines).
Effect of Cancer Treatment on Cognitive Impairments
It is well proven that cancer chemotherapy can cause cognitive impairment. It has been estimated that > 75% of patients receiving cancer chemotherapy have measurable cognitive impairment (Janelsins et al., 2014). There are many recent meta-analyses examining cognitive impairments caused by different cancer treatments for example:oncological treatments have shown that patients, those who are in remission have damage in the areas of memory,concentration, speed of executing an activity, executive function, memory related to visual and verbal activities relative to people with no cancer (Boykoff et al., 2009; Lindner et al., 2014). In other study involving 196 patients who are long-term fighters of breast cancer and going treatment with cyclophosphamide, methotrexate, and CMF, the researchers found that the chemotherapy group performed significantly worse on many of the cognitive tests, including immediate and delayed verbal memory, speed in functional execution,and speed of psychomotor activities compared to patients with no history of cancer (Koppelmans et al., 2012). In a separate examination of 189 survivors of breast cancer it was found that memory and executive function disorders were present in 20 percent of the cohort and exhibited a statistically significant connection with results of domain-specific cognitive examinations. This observation suggests that aged individuals are not more susceptible to the negative impacts on cognition of cancer treatments (Ganz et al., 2013).
Effect of Metformin on Memory Function in Chemobrain Models
It has been reported that in animal models of chemobrain,chemotherapy alters the expression of some protein kinases and protein phosphorylation, and damages mitochondrial function (Zhou et al., 2016). Metformin can improve memory function in some neurodegenerative diseases and DM(Pintana et al., 2012; Lin et al., 2018; Farr et al., 2019), and thus metformin could prevent chemobrain (Alhowail et al.,2019). Zhou et al. (2016) showed that cisplatin reduced the growth of dendritic spines in pyramidal neurons in the cingulate cortex and caused mitochondrial dysfunction.They then demonstrated that metformin treatment reversed the mitochondrial dysfunction and increased the growth of dendritic spines of the pyramidal neurons (Zhou et al., 2016).Metformin reported to have multiple mechanisms such as activation of adenosine monophosphate-activated protein kinase (AMPK) that inhibits NF-κB and glycogen synthase kinase-3 beta (GSK3β) as well as activation of Akt that leads to reduce neuroinflammation, facilitate glucose influx and finally improve memory impairment.
Mechanisms by Which Metformin Improves Chemobrain
Metformin potentially functions as a neuroprotective agent through reduction of protein hyperphosphorylation, oxidative stress, and neuroinflammation. Metformin decreases mitochondrial complex I activity, which ultimately leads to improved memory function by decreasing oxidative stress.There is a link between metformin and protection against neurodegeneration by decreasing insulin resistance, reducing adiposity, normalizing plasma glucose level, and reducing the formation of atherosclerotic plaques (Rotermund et al., 2018;Muri et al., 2019). Also, metformin has been proved to have anti-inflammatory and neuroprotective effects on the brain(?abuzek et al., 2010).
There is mechanistic data on how metformin improves memory function. For instance, Akt and GSK3β play a very important role in cognitive function (Shu et al., 2013). When the neurons are stimulated, Akt protein is activated by various receptors, including insulin receptor (IR) signaling.PI3K is activated by IR and consequently it activates Akt by phosphorylation of threonine-308 residue that in turn activatesphosphorylate serine-9 residue in GSK3β leading to deactivation of cognitive function (Matsuda et al., 2019).Therefore, Akt pathway activation deactivates GSK3β and thus reduces the glycogen synthesis allowing the cell to consume more glucose as energy that ultimately enhances memory formation and improves cognitive function (Venna et al.,2015). Alteration of the expression or phosphorylation of this protein plays an important role in cognitive impairment(Shonesy et al., 2012). GSK3β is also downstream of Akt, and phosphorylation of residue Ser-9 of GSK3 caused deactivation of this protein (Cross et al., 1995; Persad et al., 2000), leading to improvement of learning and memory processes. In addition, studies revealed that GSK3β negatively regulates insulin pathway and inhibition of GSK3β helps the induction of long-term potentiation in mice, which in turn increases GSK3B activity leading to Alzhiemer’s disease that attribute the inhibition of long-term potentiation and memory loss in Alzheimer’s disease models, and metformin treatment rescues this impairment (Farr et al., 2019).
IR substrate 1 (IRS-1) is a downstream protein associated with IR signaling cascades. When insulin binds to the IR, it increases tyrosine kinase activities of this receptor, and subsequently induces the tyrosine phosphorylation of substrates including IRS-1. Several lines of evidence have revealed that alteration in the phosphorylation of IRS-1 results in insulin resistance,which ultimately leads to altered signaling cascades. Insulin resistance reduces hippocampal neurogenesis and thus impairs memory function. Interestingly, a recent study by Tanokashira et al. (2018) revealed that metformin is able to increase the level of IRS-1 in the hippocampus, thus increasing hippocampal neurogenesis.
Acetyl-coenzyme A carboxylase-1 (ACC-1), a critical enzyme in the regulation of fatty acid synthesis and metabolism, has emerged as an attractive target for a plethora of diseases,such as DM, nonalcoholic fatty liver disease, cancer,bacterial infections, and others. ACC-1 inhibition was also revealed to enhance metabolism in diseases associated with hypometabolism and thus improve memory function (Chen and Zhong, 2013). AMPK is activated by metformin, and AMPK activation results in ACC-1 inhibition, ultimately leading to a decrease in fatty acid synthesis and an increase in fatty acid β-oxidation (Wakil and Abu-Elheiga, 2009). During fatty acid β-oxidation, carnitine is transported across the mitochondrial membrane and fatty acids are converted to acetyl-CoA and then to ketone bodies, which transport into the bloodstream(Daugherty et al., 2017). Ketone bodies are an essential energy source for the central nervous system. It is reported that ketone bodies are dysregulated in Alzheimer’s disease(Hertz et al., 2015). Thus, metformin treatment activates AMPK which inhibits ACC-1, and this inhibition contributes to mitochondrial homeostasis and improvement of memory function (Bredesen, 2014). A previous study has demonstrated that in addition to improving IR, metformin has physiological anti-inflammatory and antioxidant effects (Martin-Montalvo et al., 2013). The effect of metformin on non-dementia vascular cognitive impairment may result from several mechanisms,which include activation of AMPK, enhancing activity in the AMPK-Sirtuin 1-peroxisome coactivator 1α pathway (Qin et al., 2006), and the activation of peroxisome proliferatoractivated receptors gamma. In rats with cognitive dysfunction,this improves central cholinergic acetylation enzyme activity and insulin-like growth factor-1 expression, leading to the maintenance of cholinergic nerve function and mitigating cognitive failure. In addition, metformin can also activate longevity gene Sirtuin 1 to enhance plasticity of the central nervous system, and thus improve learning skills (Gao et al.,2010). Treatment that combines metformin with donepezil can improve IR and reduce fasting insulin levels. Meanwhile,metformin could also attenuate carotid artery lesions,contributing to improved cognitive function in patients with non-dementia vascular cognitive impairment (Lin et al., 2018).
Controversy about Effects of Metformin
As detailed above, metformin has been reported to improve memory in some brain disorders. However, long-term metformin administration to healthy animals has been shown to impair memory function (Alhowail et al., 2019;Alharbi et al., 2020). The effect of metformin on behavior and survival rates also needs special emphasis. One of the main mechanisms of action of metformin is activation of AMPK, and this protein activates and inhibits several other protein kinases. For example, AMPK inhibits mammalian target of rapamycin, which plays a major role in cell survival and prefiltration. It also inhibits NF-κB, which plays a vital role in increasing the expression of TNF-α by its ability to activate the promotor region for the gene of TNF-α thus induce inflammation, while metformin can inhibit the NF-κB and reduce its localization to the gene promotor region and ultimately reduce inflammation (Isoda et al., 2006).
Effect of metformin on behavior
Behavioral tasks are well-established methods to evaluate cognitive and memory function in animals. Such tasks include the Y-maze test, the novel object recognition (NOR) test, the Morris water maze, and the elevated plus maze test (Rodriguiz and Wetsel, 2006; Alharbi et al., 2020). Several studies have revealed that alterations in these behavioral tasks reveal memory impairment (Dinel et al., 2011). Some researchers have advocated metformin to rescue this impairment(Zhou et al., 2016; Alhowail et al., 2019), whereas other studies have shown failure of metformin to prevent these cognitive impairments. Such impairments may be induced by chemotherapy (Alharbi et al., 2020), depending on the mechanism of action of the chemotherapeutic agent. For instance, Zhou et al. (2016) used the NOR test in C57/BL6J mice to demonstrate that metformin could reduce memory impairment caused by cisplatin. However, Alhowail et al.(2019) showed using the NOR test in mice that metformin did not prevent memory impairment resulting from cyclophosphamide treatment, although the Y-maze test did indicate that metformin improved memory impairment.
The explanation for the different effects of chemotherapy and metformin on memory function probably lies in the pathways by which memory is formed in the hippocampus. During the formation of a memory and exposure of animals to a new object, the dentate gyrus pathway and the CA3 region of the hippocampus are engaged. However, when animals explore familiar objects, the lateral entorhinal cortex pathway to the CA1 region of the hippocampus takes precedence (Wilson et al., 2013). More electrophysiological studies are required to identify exact regions of the brain affected by chemotherapy.
Doxorubicin cause memory impairment (Salas-Ramirez et al.,2015; Keeney et al., 2018) as measured by the Y-maze, NOR,and elevated plus maze tests in rat models of chemobrain(Alharbi et al., 2020). Metformin administration failed to prevent memory impairment following doxorubicin treatment(Alharbi et al., 2020). This failure in memory improvement could result from the metformin dose used 30 mg/kg, whereas it has been reported that a metformin dose of around 200 mg/kg is required to affect protein kinase phosphorylation and attenuate proinflammatory markers related to memory dysfunction.
Toxicity of metformin when combined with chemotherapy
The survival rate can be used to evaluate drug efficacy and toxicity (DeWitt et al., 2005; Andri? et al., 2012; Cekanova and Rathore, 2014; Rossi et al., 2015). Evaluating the effect of metformin in combination with chemotherapy is difficult because studies differs based on the times and doses of treatment. Alhowail and Almogel (2020) reported that metformin in combination with acute doxorubicin treatment increased the survival rate in mice models of chemobrain.However, Alharbi et al. (2020) showed that co-administration of metformin with chronic doxorubicin treatment reduced the survival rate of rats in a chemobrain model. In addition, the co-administration of metformin with cyclophosphamide over a long period or CMF reduced the rodent survival rate (Alhowail et al., 2020). It remains essential to determine the toxicity and safety of metformin in combination with chemotherapy drugs as a treatment for chemobrain.
Conclusions
Long-term chemotherapy results in alterations in brain function that reduce the quality of life of cancer survivors.Several studies have postulated metformin as a protective agent to counteract chemobrain. Metformin may prevent chemobrain in treatment involving cyclophosphamide or cisplatin, but apparently not if the treatment involves doxorubicin. Metformin can increase the survival rate of animal models receiving acute doxorubicin treatment;however, the survival rate is reduced in the case of chronic doxorubicin, cyclophosphamide, and CMF treatment.
Author contributions:Both AA and SC contributed to data collection,manuscript writing and review. Both authors read and approved the final manuscript.
Conflicts of interest:Neither of the authors of this review has a financial interest related to this manuscript, and authors claim no conflicts of interest related to this work.
Financial support:This work was supported by the Deanship of Scientific Research, Qassim University.
Copyright license agreement:The Copyright License Agreement has been signed by both authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Alain Buisson, Université Grenoble Alpes, France;Christopher J Andrews, The University of Queensland, Australia; Sengal Bagci Taylan, Hakkari University, Turkey.
Additional file:Open peer review reports 1 and 2.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Genes for RNA-binding proteins involved in neuralspecific functions and diseases are downregulated in Rubinstein-Taybi iNeurons
- Dendritic spine density changes and homeostatic synaptic scaling: a meta-analysis of animal studies
- Optogenetic activation of intracellular signaling based on light-inducible protein-protein homo-interactions
- Presenilin mutations and their impact on neuronal differentiation in Alzheimer’s disease
- Growth differentiation factor 5: a neurotrophic factor with neuroprotective potential in Parkinson’s disease
- The promise of neuroprotection by dietary restriction in glaucoma

