Growth differentiation factor 5: a neurotrophic factor with neuroprotective potential in Parkinson’s disease
Susan R. Goulding, Jayanth Anantha, Louise M. Collins, , Aideen M. Sullivan, ,Gerard W. O’Keeffe,
Abstract Parkinson’s disease is the most common movement disorder worldwide, affecting over 6 million people. It is an age-related disease, occurring in 1% of people over the age of 60,and 3% of the population over 80 years. The disease is characterized by the progressive loss of midbrain dopaminergic neurons from the substantia nigra, and their axons, which innervate the striatum, resulting in the characteristic motor and non-motor symptoms of Parkinson’s disease. This is paralleled by the intracellular accumulation of α-synuclein in several regions of the nervous system. Current therapies are solely symptomatic and do not stop or slow disease progression. One promising disease-modifying strategy to arrest the loss of dopaminergic neurons is the targeted delivery of neurotrophic factors to the substantia nigra or striatum, to protect the remaining dopaminergic neurons of the nigrostriatal pathway. However, clinical trials of two well-established neurotrophic factors, glial cell line-derived neurotrophic factor and neurturin, have failed to meet their primary end-points. This failure is thought to be at least partly due to the downregulation by α-synuclein of Ret, the common co-receptor of glial cell line-derived neurorophic factor and neurturin. Growth/differentiation factor 5 is a member of the bone morphogenetic protein family of neurotrophic factors, that signals through the Ret-independent canonical Smad signaling pathway. Here, we review the evidence for the neurotrophic potential of growth/differentiation factor 5 in in vitro and in vivo models of Parkinson’s disease. We discuss new work on growth/differentiation factor 5’s mechanisms of action, as well as data showing that viral delivery of growth/differentiation factor 5 to the substantia nigra is neuroprotective in the α-synuclein rat model of Parkinson’s disease. These data highlight the potential for growth/differentiation factor 5 as a disease-modifying therapy for Parkinson’s disease.
Key Words: adeno-associated virus; bone morphogenetic protein; dopaminergic neurons;growth/differentiation factor 5; neurodegeneration; neuroprotection; neurotrophic factor;Parkinson’s disease; Smad signaling; α-synuclein
Introduction
Over 200 years have passed since the manifestations of PD were first documented in 1817 (Parkinson, 1817,2002). Although many advances have been made in the development of therapies that provide symptomatic relief,there is no therapy that can halt the unrelenting progression of this disease. PD is characterized by the progressive loss of dopaminergic (DA) neurons from the substantia nigra(SN) and their axons projecting to the striatum, as well as accumulation of aggregated α-synuclein within neurons,all of which lead to debilitating motor symptoms and nonmotor symptoms of the disease (Poewe et al., 2017; O’Keeffe and Sullivan, 2018). Midbrain DA neurons are categorized into three subpopulations – A8 in the retrorubral field, A9 in the substantia nigra and A10 in the ventral tegmental area(German and Manaye, 1993). Since PD is characterized by the loss of the A9 population, research efforts have focused on approaches to protect this specific neuronal population.One such approach is the use of neurotrophic factor therapy.Within the developing SN, neurotrophic factors for DA neurons play critical roles in the generation of DA neurons,the proliferation of DA progenitors, and the projection of their axons to the striatum and subsequent innervation of that region (Krieglstein et al., 1995; Hegarty et al., 2013a).Neurotrophic support is therefore crucially involved in the development, growth, proliferation, maturation and survival of DA neurons. The goal of neurotrophic factor therapy in PD is to harness these properties and apply them to protect DA neurons from further degeneration, as well as to promote reinnervation of the striatum.
Many neurotrophic factors for DA neurons have already been identified, the most notable being glial cell line-derived neurotrophic factor (GDNF) and neurturin, both of which belong to the transforming growth factor (TGF-β) superfamily.Due to their potent neurotrophic effects in animal models of PD, both GDNF and neurturin were subsequently tested in clinical trials (Nutt et al., 2003; Slevin et al., 2005; Patel et al.,2005; Lang et al., 2006; Decressac et al., 2011; Warren Olanow et al., 2015; Whone et al., 2019a, b). However, despite initial beneficial effects in open-label trials, GDNF and neurturin have so far failed to reach their primary endpoints in randomized placebo-controlled studies (Paul and Sullivan, 2019; Barker et al., 2020). The bone morphogenetic proteins (BMPs),members of the largest subgroup of the TGF-β superfamily which comprises the BMPs and growth/differentiation factors(GDFs), are secreted growth factors which are known to play important roles in embryogenesis, skeletal development and neurogenesis, among other crucial processes (Bragdon et al., 2011). Several members of the BMP family have been identified as neurotrophic factors for DA neurons and have been shown to have neuroprotective effects inin vitromodels of PD (Krieglstein et al., 1995; Jordan et al., 1997; Goulding et al., 2019). Therefore, in the pursuit of neurotrophic factors that may slow or stop disease progression, the BMP family merits investigation. To date, GDF5 has been one of the most extensively studied members of the BMP family, and the one which has shown neuroprotective effectsin vivo; therefore,this review will focus on the evidence for GDF5 as a DA neurotrophic factor and its potential role in PD therapy.
Search Strategy and Selection Criteria
For this review, we searched NCBI and Science Direct for literature published from inception to January 8, 2021 for articles referencing one or more of the following: GDF5,BMP, neurotrophic factor, dopaminergic neurons, Parkinson’s disease, α-synuclein, neuroprotection.
GDF5 Signaling
GDF5, like most of the BMP family, is known to signal via the canonical Smad signaling pathway (Hegarty et al., 2013b;Goulding et al., 2020a) (Figure 1). To activate this pathway,BMP ligands signal through a receptor complex consisting of two type I BMP receptors (BMPRs) and two type II BMPRs.Upon ligand binding to the extracellular part of the receptor complex, intracellular Smad proteins become activated and can form a complex which translocates to the nucleus and binds to DNA to alter gene transcription (Wrana and Attisano, 2000). Each BMP ligand has a varying affinity for each receptor subtype and GDF5 is known to preferentially bind to the BMPR1b type I receptor and BMPR2 type II receptor (Nishitoh et al., 1996; Nickel et al., 2005). Like that of GDF5, these receptors are expressed in the developing rat ventral mesencephalon (VM) at embryonic day (E)11 and their expression continues throughout development until adulthood, at least up to postnatal day (P)90 (Hegarty et al.,2014c). This pattern of expression coincides with the critical period in the development of the nigrostriatal pathway. In addition, both BMPR1b and BMPR2 are expressed on DA neurons in the adult rat SN (Hegarty et al., 2014c). Thus, it is possible that GDF5 and the BMP-Smad signaling pathway play an active role in regulating the development of nigrostriatal DA neuronal projections towards their target, the dorsal striatum; however, evidence is needed to support this proposal. The sustained expression of GDF5 and its receptors through to adulthood also suggests a role in the maintenance of DA neurons. In strong support of the role of the BMPRs in DA maintenance, BMPR2 dominant negative (BMPR2DN) adult mice showed a 20% reduction in the number of DA neurons in the SN and almost 90% loss of striatal innervation. These mice also displayed lower locomotor ability when compared to wild-type controls (Chou et al., 2008). This adds to the evidence for a role for endogenous BMP-Smad signaling,possibly facilitated by GDF5, in the development, maintenance and function of nigrostriatal DA neurons.
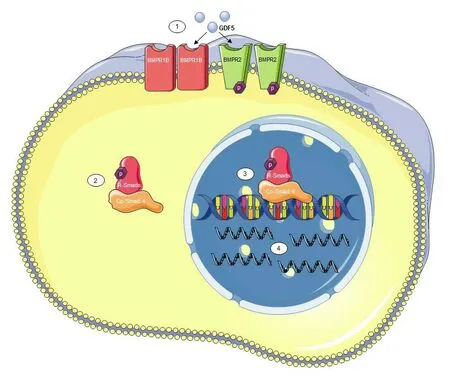
Figure 1|Growth/differentiation factor 5 (GDF5) signals via the canonical Smad signaling pathway.
Evidence for Neurotrophic Effects of Growth/Differentiation Factor 5 In Vitro
The evidence for the strong expression of GDF5 in the adult rat SN (O’Keeffe et al., 2004b), and the fact that the GDF5 receptors, BMPR1b and BMPR2, are expressed on DA neurons in the adult human SN (Hegarty et al., 2014c; Goulding et al., 2019), suggest that GDF5 may provide trophic support to these mature DA neurons to promote their survival and growth. Many studies have investigated this premise, the first of which found that treatment of E14 rat VM cultures with 20 ng/mL of GDF5, in its active dimeric form, for up to 8 daysin vitro(DIV), resulted in a 1.6-fold increase in the survival of tyrosine hydroxylase-positive (TH+) DA neurons (Krieglstein et al., 1995). Consistent with this, another study applied 1 ng/mL or 10 ng/mL GDF5 and reported 3.1- and 2.6-fold increases,respectively, in the number of TH+neurons in primary cultures of E14 rat VM (O’Keeffe et al., 2004a); this study also reported significant effects of GDF5 to increase axon length and branching of DA neurons. Furthermore, both concentrations of GDF5 led to significant increases in the number of astrocytes in these cultures (O’Keeffe et al., 2004a), suggesting a possible association between astrocytes and GDF5-induced signaling,as also documented in previous studies (Krieglstein et al.,1995; Wood et al., 2005). However, the beneficial effects of GDF5 on TH+neurons were also seen in glial-depleted E14 VM cultures, indicating that GDF5 acts directly on DA neurons,independently of astrocytes (Wood et al., 2005). In that study,the neurotrophic effects of GDF5 on DA neurons in E14 VM cultures were compared with those of the ‘gold standard’DA neurotrophic factor, GDNF. Doses of 1, 10 or 100 ng/mL GDNF or GDF5 were added to normal cultures and to glialdepleted cultures every 48 hours for a period of 8 DIV. GDF5 increased the survival of TH+neurons at all doses up to 6 DIV, while 100 ng/mL was also effective at 8 DIV; the same effects were seen in VM cultures treated with GDNF (Wood et al., 2005). Furthermore, there were no differences between the numbers of TH+neurons in glial-depleted and in normal cultures following treatment with 1 ng/mL GDF5. These results illustrate that while GDF5 treatment can stimulate astroglial proliferation, indicating a potential role for GDF5 in the regulation of neural progenitor cell differentiation during development, this role is independent of its direct trophic effects on DA neurons. The study also found that co-treatment of E14 VM cultures with 1 ng/mL GDNF and 1 ng/mL GDF5 resulted in an increase of 11.6% in TH+cell number, compared to an increase of 5.1% in cultures treated with GDF5 alone and of 4.9% in those treated with GDNF alone (Wood et al.,2005). The reason for this additive effect was postulated to be due to the differential expression patterns of the receptors required by these neurotrophic factors to elicit their effects,suggesting that a combination therapy of neurotrophic factors may warrant investigation.
Given that GDF5 is capable of promoting the survival of TH+neurons in mesencephalic cultures, the question arose as to whether this neurotrophic factor could also protect these neurons from neurotoxin-induced degeneration. To answer this question, one study treated E14 VM cultures with 20 ng/mL GDF5 for 24 hours prior to the administration of 0.5 mM of the DA toxin, 1-methyl-4-phenylpyridinium ion (MPP+), for 4 DIV (Krieglstein et al., 1995). MPP+treatment was found to reduce TH+neuronal survival by 67%, while pre-treatment with GDF5 significantly reduced this toxic effect of MPP+to 30%,indicating a partial neuroprotective effect (Krieglstein et al.,1995). A subsequent study investigated the potential of GDF5 to protect against free radical damage, since this plays a role in neurodegenerative disorders (Lingor et al., 1999). E14 VM cultures were treated with 10 ng/mL GDF5 for one week prior to addition of 30 μM FeCl2or 10 μM sodium-nitroprusside for 24 hours. Co-treatment with GDF5 significantly protected against FeCl2-induced decreases in TH+neuronal survival, but not against sodium-nitroprusside toxicity (Lingor et al., 1999).This study was the first to highlight the potential of GDF5 in rescuing DA neurons from free radical-induced injury and death, which is important given the evidence of elevated iron deposits in the brains of PD patients (Hirsch et al., 1991; Sofic et al., 1991).
In contrast to the positive findings described above, other research groups have reported unfavorable outcomes. One study treated E14 VM cultures with 30 μM 6-hydoxydopamine(6-OHDA, a DA neurotoxin) after 6 DIV, prior to the addition of GDF5, or of other BMP family members, on day 7 (Brederlau et al., 2002). GDF5 was found to have no protective or survivalpromoting effects on DA cells. However, in this study, cultures were not exposed to GDF5 until after 6 DIV making the cultures effectively E20 in nature, a time when the expression of GDF5 is downregulatedin vivo; thus developing DA neurons may be unresponsive to the effects of exogenous GDF5 at certain stages of development (O’Keeffe et al., 2004b). In addition, the authors noted that caution is needed when interpreting data from studies on mixed cell cultures, as glial cells, which offer potent neuroprotective effects, can also be affected by 6-OHDA (Brederlau et al., 2002). This is in contrast to thein vivosituation, where this compound is known to act more selectively on DA neurons. A more recent study used cultures of postnatal day (P)0 rat SN to study the abilities of five different neurotrophic factors to protect against MPP+(Jaumotte et al., 2016). Cultures were treated with 100 ng/mL of either GDNF, brain-derived neurotrophic factor, TGF-β,fibroblast growth factor-2 or GDF5, at either 1 hour prior to,during, or 48 hours after, exposure to 10–500 μM MPP+. No survival-enhancing effects on DA neurons were found for any of the neurotrophic factors when tested alone. However,when all five factors were given in combination, a significant protective effect against MPP+-induced loss in survival of TH+cells was seen (Jaumotte et al., 2016). These apparent synergistic effects of the neurotrophic factors could be due to the factors acting on several subtypes of neurotrophic factor receptor on DA cells, and/or by altered signaling cascades. It is also important to note each neurotrophic factor acts not only on DA neurons, but also on a variety of non-DA cells whose paracrine signaling may exert critical influences on DA neuron survival (Jaumotte et al., 2016). The fact that no neurotrophic factor alone was found to promote survival of DA neurons in this study may be due to oversaturation of the relevant receptors above certain doses of each neurotrophic factor,rendering high concentrations of these factors ineffective(Jaumotte et al., 2016). Nevertheless, these results reiterate the potential of a combination therapy for PD, which would apply a cocktail of neurotrophic factors. However, since most studies on the neurotrophic effects of GDF5 and GDNF have been conducted on embryonic DA neuronsin vitro, the Jaumotte study was interesting as it used P0 SN cultures. At this age of development, the SN and ventral tegmental area are easily distinguished and so this may provide a more robust model for studying potential PD therapies.
Having reviewed the availablein vitroevidence on the neurotrophic effects of GDF5, and considering certain discrepancies in the results between studies, it is clear that there is a need for standardized testing parameters when investigating potential therapies in cell models of PD. Despite this, it remains clear that GDF5 possesses neurotrophic capacity, as it promotes DA cell survival and morphological differentiation, all of which warranted its further studyin vivo.
Evidence for Neurotrophic Effects of Growth/Differentiation Factor 5 In Vivo
Complementing the reported neurotrophic effects of GDF5in vitro, several studies have shown potent actions of GDF5in vivo. The firstin vivostudy investigated the neuroprotective potential of intracerebral administration of GDF5 protein in the 6-OHDA lesion rat model of PD (Sullivan et al., 1997).Here, 50 μg of either the dimeric (active) or monomeric(inactive) forms of GDF5 was injected into the left SN and left lateral ventricle prior to a 6-OHDA injection into the leftmedian forebrain bundle (MFB) of adult rats. At one week post-surgery, animals underwent behavioral testing in the form of amphetamine-induced rotations. Animals which had received the 6-OHDA lesion, as well as those that received 6-OHDA combined with monomeric GDF5, displayed rotation rates indicative of a 95% depletion of the nigrostriatal pathway. In contrast, animals who had been administered dimeric GDF5 with 6-OHDA did not rotate at all, indicating preservation of nigrostriatal function (Sullivan et al., 1997). A subset of each experimental group also underwent positron emission topography scanning using a tracer for the DA transporter, a measure of DA uptake by neurons. This showed that all 6-OHDA-lesioned animals, and those that had received monomeric GDF5 as well as 6-OHDA, had a complete loss of DA uptake within the ipsilateral striatum. In contrast, animals that had received dimeric GDF5 along with 6-OHDA exhibited intact striatal DA uptake (Sullivan et al., 1997). Post-mortem HPLC analysis found similar levels of DA and its metabolites,DOPAC and HVA, in the GDF5 dimer-treated group and in control animals, while animals that had received 6-OHDA alone or in combination with the GDF5 monomer exhibited marked decreases in DA and its metabolites. Furthermore,TH immunostaining confirmed extensive loss of DA neurons within the SN and ventral tegmental area of animals that had received 6-OHDA alone or with the GDF5 monomer,whereas TH+neurons were largely spared in the GDF5 dimer plus 6-OHDA group (Sullivan et al., 1997). These results demonstrated that GDF5 protected against 6-OHDA-induced degeneration of nigral DA neuronal cell bodies and their striatal terminalsin vivo, as well as striatal DA turnover and function. These findings established GDF5 as a candidate for neurotrophic factor therapy for PD. In a follow-up study, three different injection sites were compared and it was found that GDF5 injection into either the striatum or SN, but not into the lateral ventricle, produced optimal neuroprotective and functional effects on the nigrostriatal DA pathway (Sullivan et al., 1999).
Another study by this group investigated the efficacy of GDF5 to enhance the survival and function of foetal mesencephalic grafts in the 6-OHDA lesion rat model (Sullivan et al., 1998).Adult rats received a 6-OHDA lesion of the MFB, then 4 weeks later, received intrastriatal injection of untreated VM grafts,or those which had been pre-treated with 500 μg of GDF5 or GDNF. Amphetamine-induced rotational testing showed that animals that had received GDF5-treated or GDNF-treated grafts displayed no rotations, indicating restoration of striatal DA levels. In contrast, all animals that had received a lesion only showed high contralateral rotation rates, indicating substantial depletion of striatal DA neurotransmission(Sullivan et al., 1998). Positron emission topography scans indicated that pre-treatment with either GDF5 or GDNF enhanced striatal DA uptake by the transplanted cells, and post mortem immunohistochemistry showed that grafts pretreated with GDF5 or GDNF displayed significant increases in the number and density of TH+neurons compared with untreated grafts (Sullivan et al., 1998). This work was the first to show the neurotrophic and neuroprotective effects of GDF5 on foetal VM grafts. This study also showed that GDF5 was as efficacious as GDNF, which is notable in the pursuit of new neurotrophic therapies in light of the failure of GDNF in clinical trials to date.
Following the studies showing GDF5 to be effective in protecting TH+neurons from 6-OHDA lesioning when administered concurrently, it was important to investigate whether GDF5 also had restorative effects on the nigrostriatal pathway, when administered post-lesion, as a more accurate model of the pathological degeneration that occurs in human PD. One study administered 25 μg of GDF5 into the left striatum or left SN, one or two weeks after intrastriatal 6-OHDA lesion, which results in a more protracted degeneration of the nigrostriatal pathway than lesioning of the MFB, which induces acute nigrostriatal degeneration (Hurley et al., 2004).Amphetamine-induced testing showed that all 6-OHDAlesioned animals rotated at a rate of at least 5 turns/minute,indicative of a partial striatal lesion. Animals that had received GDF5 into the striatum at one week, but not at two weeks,post-lesion showed significant reductions in rotational behavior. Animals that received GDF5 into the SN at one or two weeks post-lesion also had significantly decreased rotation rates compared to those that received 6-OHDA alone(Hurley et al., 2004). Post-mortem immunohistochemistry showed 60% loss of TH+neurons in the ipsilateral SN in the 6-OHDA group in comparison to the contralateral side, while animals injected with GDF5 at one week post-lesion into the SN or striatum had only 30–35% loss of TH+neurons from the SN. However, injection of GDF5 into either SN or striatum at two weeks post 6-OHDA did not confer significant protection to nigral DA neurons (Hurley et al., 2004). This is likely to be due to the fact that significant degeneration of striatal fibers had already occurred by the time of GDF5 administration(Rosenblad et al., 1999). Therefore, administration of GDF5 protein at two weeks post-lesion is likely to be too late to achieve axonal protection in this model of PD, a result also found with intranigral delivery of GDNF (Kirik et al., 2001).
While these initial experiments demonstrated important proof-of-principle of DA neuroprotective effects of GDF5,one issue with the therapeutic use of neurotrophic factors is they are rapidly metabolized in the brain by endogenous enzymes and cannot be given systemically due to their inability to cross the blood brain barrier. To circumvent this,studies have applied gene therapy using viral vectors or lipidmediated transfection to overexpress GDF5 in cells which can be transplanted into the PD brain. One study overexpressed GDF5 in E13 rat VM grafts using cationic lipid-mediated gene delivery (O’Sullivan et al., 2010). Initialin vitrostudies showed that cells transfected with GDF5 displayed significantly lower levels of TH+cell death than controls after treatment with 50 μM 6-OHDA (O’Sullivan et al., 2010). GDF5-transfected E13 VM cells, or non-transfected E13 VM cells, were then transplanted into the striata of adult rats at 2 weeks after 6-OHDA intrastriatal lesion. At 2 weeks post-graft, animals that had received GDF5-transfected grafts displayed significantly lower amphetamine-induced rotation rates than those that had received mock-transfected or untransfected grafts. The somal area and total neurite length within the grafts were significantly higher in GDF5-transfected grafts compared to mock-transfected or untransfected grafts (O’Sullivan et al.,2010). This study demonstrated the feasibility of transfection with GDF5 prior to transplantation of foetal tissue, to enhance the survival and function of these grafts. This approach could be applied to other cell types for transplantation, such as neural progenitors or stem cells.
In a further study, a CHO cell line was stably transfected with the human GDF5 gene before transplantation into the 6-OHDA-lesioned rat striatum (Costello et al., 2012). Firstly,in vitrostudies showed that the transfected CHO cells could secrete both GDF5 precursors and mature GDF5 protein for at least 15 days, and as a consequence, could increase by 2-fold the survival of TH+cells in E14 VM co-cultures (Costello et al., 2012).In vivostudies found that GDF5-transfected CHO cells secreted GDF5 protein for at least 1 week following transplantation in rats with 6-OHDA lesions and induced significant reductions in amphetamine-induced rotations and significant protection of the nigrostriatal pathway. These neuroprotective effects of GDF5-transfected CHO cells were evident when they were injected into either striatum or SN at one week after an intrastriatal lesion, or into the striatum at the same time as a MFB lesion (Costello et al., 2012). This data once again highlights the protective and restorative effects of GDF5 on DA neuronsin vivo, and shows that sustained,targeted and long-term delivery of GDF5 can be achieved.
Evidence for Neurotrophic Effects of Growth/Differentiation Factor 5 against α-Synuclein
α-Synuclein (SNCA) is intrinsically linked to PD, since mutations (Kruger et al., 1998; Zarranz et al., 2004; Appel-Cresswell et al., 2013; Lesage et al., 2013; Pasanen et al.,2014; Yoshino et al., 2017), duplications (Chartier-Harlin et al., 2004) and triplications (Singleton et al., 2003) of theSNCAgene contribute to autosomal dominant forms of familial PD. Linkage and genome-wide association studies have also identified polymeric variants in theSNCAgene in patients with idiopathic PD (Billingsley et al., 2018). α-Synuclein is further linked to PD as aggregates of misfolded α-synuclein comprise the major component of abnormal protein inclusions found throughout the central nervous system and peripheral nervous system (Spillantini et al., 1997). These atypical aggregates, which are pathological hallmarks of PD, are found both in neuronal cells and their presynaptic terminals, and are known respectively as Lewy bodies and Lewy neurites(Spillantini et al., 1997; Meade et al., 2019). The function of these aggregates, and whether they are protective (Tanaka et al., 2004; Bodner et al., 2006; Outeiro et al., 2019) or pathological (Wakabayashi et al., 2013; Wong and Krainc,2017; Visanji et al., 2019), currently remains unclear. However,given the inherent association of α-synuclein with the clinical onset and progression of PD, we recently sought to investigate the neuroprotective effect of GDF5 against α-synuclein overexpressionin vivo. In this study, adult female Sprague Dawley rats were given a unilateral intranigral injection of an AAV2/6-α-synuclein (wild-type) vector in combination with an empty AAV2/5-Null control vector or an AAV2/5-GDF5 vector(Goulding et al., 2020b) (Figure 2). After 20 weeks, animals were sacrificed and analyzed for α-synuclein pathology and nigrostriatal integrity, or for gene expression in the SN. We showed that AAV-α-synuclein caused a significant loss of DA neurons from the SN, reduced DA striatal innervation and loss of striatal dopamine, all of which were prevented by AAVmediated delivery of GDF5 (Goulding et al., 2020b).
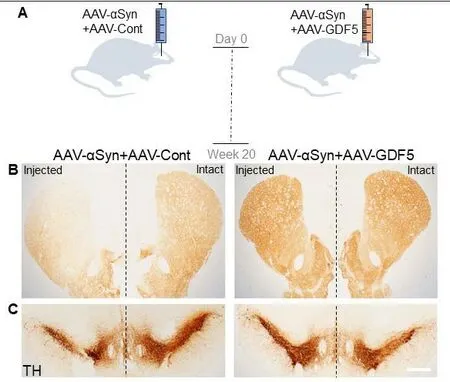
Figure 2|Viral overexpression of growth/differentiation factor 5 (GDF5)in the substantia nigra protects dopaminergic neurons and their striatal terminals against α-synuclein-induced degeneration.
The disappointing outcomes of GDNF and neurturin in clinical trials and the failure of GDNF to exert neuroprotection in the AAV-α-synuclein rat modelin vivohave been postulated to be due to downregulation of the GDNF receptor Ret by α-synuclein (Decressac et al., 2011, 2012b; Drinkut et al.,2016). Therefore, in our study, we examined the effect of nigral α-synuclein overexpression on the transcripts forRet,as well as transcripts for the GDF5 signaling mediators. We found that AAV-α-synuclein led to significant reductions in the expression of transcripts forTh,DatandRet, but did not affect the expression of the GDF5 signaling mediators,Bmpr2,Bmpr1bandSmad 1, in the SN at the 20-week timepoint tested. The results of our study highlight the fact that while the selective DA neurotoxins that are commonly employed in pre-clinical models of PD are certainly useful, they do not mimic the α-synuclein pathology that is central to PD progression. Investigating potential neurotrophic factors using α-synuclein models of PD in pre-clinical studies is therefore crucial to highlight potential issues, early in the discovery phase. Our results show that viral-mediated delivery of GDF5 is well tolerated in rats and that GDF5 is the first neurotrophic factor to exert neuroprotection against α-synucleinin vivo, thus paving the way for further development of this therapeutic approach.
However, while this α-synuclein pre-clinical model has certain advantages over DA neurotoxins in terms of clinical relevance and a progressive degenerative pathology, there are certain disadvantages that need to be considered. These include the slow development of neurological changes,the lack of a consistent behavioral phenotype and the level of α-synuclein burden required within the brain to induce neurodegeneration, which exceeds that seen in the human disease by almost 5-fold (Decressac et al., 2012a;Su et al., 2017; Duffy et al., 2018). Nevertheless, this model demonstrates that excessive α-synuclein within the nigrostriatal system is detrimental to DA neurons and their axons and is therefore particularly useful to evaluate the neuroprotective potential of neurotrophic factors against α-synuclein-induced degenerationin vivo. This is a critically important testing element prior to clinical evaluation, given the failure of GDNF to exert protection in the same α-synuclein animal model and the unsuccessful clinical trials to date using GDNF and neurturin.
Molecular Mechanisms Mediating the Effects of Growth Differentiation Factor 5
Given the potent neurotrophic effects of GDF5 (O’Keeffe et al.,2017), there is much interest in understanding the molecular mechanisms underlying its effects, to enable the development of novel therapeutics. Since GDF5 promotes DA neurite growth, previous studies have used neurite growth as a single cell phenotypic readout of GDF5’s neurotrophic action, in order to characterize the mechanisms involved (O’Keeffe et al.,2017). For example, knockdown of key parts of the canonical BMP-Smad pathway, including BMPR1B (Hegarty et al., 2013b,2014c), or of the transcription factors Smad1 and Smad4(Hegarty et al., 2017, 2018), prevented the neuritotrophic effects of GDF5in vitro. However, the downstream mediators and mechanism(s) of action are not fully understood.
Recently, Anantha et al. (2020) carried out an untargeted proteomics analysis of SH-SY5Y cells treated with recombinant human GDF5 and found that GDF5 led to the upregulation of two proteins, serine-threonine kinase receptor protein (STRAP;UniProtKB Q9Y3F4) and nucleoside diphosphate kinase A(NME1; UniProtKB: P15531). NME1 was found to be expressed by DA neurons in the rat SN, while stereotaxic injection of AAV-GDF5 into the rat SN resulted in up-regulation of NME1 in DA neuronsin vivo(Anantha et al., 2020). To determine the contributions of STRAP and NME1 to the effects of GDF5,this study used the neurite growth assay as a single cell readout of neuritotrophic action. Transfection with siRNAs targetingSTRAPandNME1led to a significant reduction in basal levels of neurite growth compared to controls. As expected, treatment with GDF5 led to a significant increase in neurite growth, however, this was completely prevented by knockdown ofSTRAPorNME1(Anantha et al., 2020). In support of these findings, the overexpression of STRAP or NME1 alone in SH-SY5Y cells promoted neurite growth to the same extent as did GDF5 treatment, while recombinant human NME1 increased neurite growth in SH-SY5Y cells and in cultured E14 rat VM DA neurons (Anantha et al., 2020).Collectively, these studies show that NME1 and STRAP are key mediators of GDF5’s neurotrophic effects, at leastin vitro.
In a wider context, a recent study has also identified NME1 as a genetic modifier of mutant huntingtin toxicityin vivo(Wertz et al., 2020). Specifically, they showed thatNme1acts to suppress mutant huntingtin toxicity, and its aggregation (Wertz et al., 2020). When considered in light of our recent work showing that GDF5 can protect against α-synuclein-induced DA neuron degenerationin vivo(Goulding et al., 2020), and that GDF5 up-regulates NME1 in DA neuronsin vivo(Anantha et al., 2020), whether or not NME1 can protect against α-synuclein-induced degeneration is an important question for future research.
Conclusions and Future Perspectives
In conclusion, GDF5 is expressed in the rat brain from early development through to adulthood, coinciding with the generation of DA neurons and the maturation and maintenance of the nigrostriatal pathway. Co-administration of GDF5 with DA grafts in preclinical models of PD has been shown to be significantly beneficial to the survival and growth of the grafted DA neurons. Treatment of animal models of PD with recombinant human GDF5 protein, GDF5-overexpressing cells, or AAV-induced GDF5 overexpression has been shown to exert potent protective effects on the integrity of the nigrostriatal DA pathway, and to ameliorate behavioral deficits. This review highlights the significant potential of GDF5 to be used in a novel therapeutic approach in PD. In the future, it will important to evaluate whether there are neurorestorative effects of GDF5 in the α-synuclein animal model after degeneration of the nigrostriatal system has already developed, as this more closely mimics the pathology of human PD.
Author contributions:SRG reviewed and analyzed the literature, cowrote and edited the manuscript and prepared the figures. JA, LMC, AMS,GWOK co-wrote and edited the manuscript. All authors approved the final version of the manuscript.
Conflicts of interest:The authors declare no conflicts of interest.
Financial support:None.
Copyright license agreement:The Copyright License Agreement has been signed by all authors before publication.
Plagiarism check:Checked twice by iThenticate.
Peer review:Externally peer reviewed.
Open access statement:This is an open access journal, and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License, which allows others to remix,tweak, and build upon the work non-commercially, as long as appropriate credit is given and the new creations are licensed under the identical terms.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Genes for RNA-binding proteins involved in neuralspecific functions and diseases are downregulated in Rubinstein-Taybi iNeurons
- Research advances on how metformin improves memory impairment in “chemobrain”
- Dendritic spine density changes and homeostatic synaptic scaling: a meta-analysis of animal studies
- Optogenetic activation of intracellular signaling based on light-inducible protein-protein homo-interactions
- Presenilin mutations and their impact on neuronal differentiation in Alzheimer’s disease
- The promise of neuroprotection by dietary restriction in glaucoma

