Suppression of postprandial blood glucose elevation by buckwheat(Fagpopyrum esculentum) albumin hydrolysate and identification of the peptide responsible to the function
Kzumi Ninomiy, Yusuke Ymguchi, Fumie Shinmchi, Hitoshi Kumgi, Hitomi Kumgi,*
a Department of Food Science and Nutrition, Kyoritsu Women’s University, 2-2-1 Hitotsubashi, Chiyoda-ku, Tokyo 101-8347, Japan
b College of Bioresource Sciences, Nihon University, 1866 Kameino, Fujisawa-shi 252-0880, Japan
Keywords:
Buckwheat-albumin hydrolysate
Bioactive peptide
α-Amylase inhibitor
Postprandial hyperglycemia
Type 2 diabetes
A B S T R A C T
The objective of this study is to evaluate the suppressive effect of buckwheat-albumin hydrolysate on postprandial hyperglycemia and identify the peptide responsible to the function. Buckwheat-albumin hydrolysate was prepared by using digestive enzymes and was orally administered to rats together with soluble starch. The blood was taken from the tail vein up to 90 min after oral administration to measure blood-glucose and plasma-insulin levels. The peptide with α-amylase inhibitory activity was purified from the buckwheathydrolysate by gel- filtration chromatography, α-amylase affinity chromatography, and high performance liquid chromatography (HPLC). The amino-acid sequence of the peptide was identified by a protein sequencer and was compared with that in the buckwheat-genome database. Buckwheat-albumin hydrolysate significantly suppressed the elevation of blood glucose level 15 min after starch administration. The amino-acid sequence of the peptide with α-amylase inhibitory activity was YVEPDCGNLGCCYHC in the parental protein of molecular mass 17.8 kDa and theoretical pI 4.77. The amino-acid sequence, molecular weight, and pI of the parental protein in buckwheat albumin were different from those of α-amylase inhibitor in wheat albumin.This study suggests that the novel α-amylase inhibitor identified in buckwheat albumin is a potential candidate for a functional food material to suppress postprandial blood glucose elevation.
1. Introduction
Diabetes mellitus (DM) is one of the serious diseases because its symptom progresses without being recognized until various tissues are severely damaged. These damages include kidney failure, vision loss,foot ulcers, heart stroke, and so on, which strongly affect the quality of life. World Health Organization (WHO) estimates the number of DM patients to be 108 million in 1980 and 422 million in 2014 [1].The number of DM patients is still increasing. DM is classified into type 1 and 2 with different causes [2]. Type 1 DM results from pancreatic failure to produce insulin from β cells, while type 2 DM, which comprises the majority of DM patients, results from increased insulin resistance. In order to prevent or alleviate type 2 DM, suppression or retardation of the elevation of postprandial blood glucose level is effective.
Some food materials such as dietary fibers [3-6], polyphenols [7,8],proteins [9-14]and peptides [15,16]are reported to show suppressive effect on the increase in postprandial blood glucose level. Dietary fibers adsorb glucose and promote its excretion, while polyphenols,proteins, and peptides inhibit enzymes such asα-amylase andα-glycosidase that slow hydrolysis of glycosidic bonds and glucose production. Proteinaceousα-amylase inhibitor (α-AI) often exists in plants to protect themselves against bugs [17]. Wheat albumin,water-soluble protein fraction, is known to inhibitα-amylase and has already been used as a functional component to suppress the elevation of blood glucose level in Food for Specified Health Uses (FoSHU) in Japan [18-22].
Water-soluble protein can be used in a variety of food without impairing palatability of the food because it has no taste and smell.We have already found that rice albumin [23-25]and buckwheat albumin [26]also have a function to suppress postprandial hyperglycemia. Rice albumin suppresses postprandial hyperglycemia by adsorbing glucose and inhibiting Na+-D-glucose cotransporter SGLT1 expression [25]. On the other hand, buckwheat albumin suppresses the elevation of blood glucose level by inhibitingα-amylase and retains its inhibitory activity even afterin vitrohydrolysis by digestive enzymes [26]. In this study, we examined the suppressive effect of buckwheat albumin hydrolysates against postprandial hyperglycemiain vivoand analyzed the amino-acid sequence of the peptide responsible to this function after purification using gel-filtration chromatography, affinity chromatography, and high performance liquid chromatography (HPLC).
2. Materials and methods
2.1 Materials
Buckwheat flower (Tomizawa Shoten Co., Ltd., Tokyo, Japan)and wheat flower (Nisshin Flour Milling Inc., Tokyo, Japan) were purchased from a local market.α-Amylase from the porcine pancreas was obtained from Sigma-Aldrich (St. Louis, MO, USA), and 2-chloro-4-nitrophenyl-α-D-maltotrioside (G3-CNP), the substrate forα-amylase, was from Oriental Yeast (Tokyo, Japan). Pepsin from porcine stomach mucosa and trypsin from bovine pancreas were obtained from Wako Pure chemical Industries (Osaka, Japan). All other chemicals used were of reagent grade.
2.2 Preparation of buckwheat and wheat albumins
Buckwheat and wheat albumins were prepared according to our previous report [26]. Buckwheat flour was mixed with 5 times weight of 25 mmol/L 4-(2-hydroxyethyl) piperazine-1-ethanesulfonic acid(HEPES) buffer at pH 6.9 for 3 h at 4 °C followed by centrifugation at 15 000 ×gfor 15 min at 4 °C. As buckwheat and wheatα-AIs are heat stable, the supernatant was heated at 80 °C for 20 min to remove other proteins and centrifuged at 15 000 ×gfor 15 min at 4 °C.After the removal of precipitate, ammonium sulphate fractionation was conducted. The protein fraction precipitated with 40% saturated ammonium-sulfate solution was collected by centrifugation at 15 000 ×gfor 60 min at 4 °C and dialyzed against distilled water.After dialysis, insoluble fraction was removed by centrifugation at 15 000 ×gfor 15 min at 4 °C, and the supernatant was lyophilized.
2.3 Preparation of albumin hydrolysates of buckwheat and wheat
Albumin hydrolysates were prepared according to the method described in our previous paper [26]with some modifications.First,100 mg of albumin was suspended in 10 mL of trypsin solution and incubated at 37 °C. After 6 h of incubation, 10 mL of pepsin solution was added and incubated at 37 °C for 2 h. Enzymatic reaction was terminated by heating the sample at 100 °C for 5 min. After heating, high-molecular-weight compounds were removed using Amicon?Ultra-15 3000 NMML (Merck Millipore Co., Ltd., Tokyo,Japan) by centrifugation at 10 000 ×gfor 10 min at 4 °C. Obtained filtrate was lyophilized and the powder was designated as albumin hydrolysates. The degree of hydrolysis was evaluated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE).After electrophoresis, the gels were stained for protein with 0.025% coomassie Brilliant Blue R-250 solution.
2.4 Animals
Seven-week-old male Wistar rats were purchased from Japan SLC (Shizuoka, Japan). The rats were acclimatized for a period of 7 days. Throughout the acclimatization and subsequent study period,rats were maintained in controlled environment of (23 ± 1) °C, 55% humidity under a 12 h light/dark cycle with light from 08:00 to 20:00.All rat experiments were performed in accordance with the Guidelines for Animal Experiments of the college of Bioresource Science of Nihon University (Approval numbers: AP12B059).
2.5 Oral Starch Tolerance Test
Oral Starch Tolerance Test (OSTT) was conducted under non-anesthesia condition according to the method described in our previous paper [26]. After rats were divided into 3 groups of 7 each and fasted for overnight 14 h, the mixture containing 300 mg/kg body weight albumin hydrolysates of buckwheat or wheat and 1 g/kg body weight soluble starch in phosphate buffered saline was orally administered. Only 1 g/kg body weight soluble starch in phosphate buffered saline was administered in the control group.The blood was taken from the tail vein at 0, 15, 30, 45, and 90 min after oral administration and blood glucose level was measured with Dexter-ZⅡ and plasma insulin was measured by ELISA (Rat Insulin ELISA Kit (U-E type); Shibayagi, Gunma, Japan). The area under the curve (AUC) was calculated for blood glucose and plasma insulin according to the methods that were described by Wolever and Jenkins [27].
2.6 Measurement of α-amylase inhibitory activity
The inhibitory activity againstα-amylase was measured by using G3-CNP according to the method reported by Foo and Bais with some modifications [28]. This method measures the absorbance of 2-chloro-nitrophenol (CNP) at 405 nm produced from the cleavage of G3-CNP byα-amylase in the presence and absence ofα-AI. The standardα-amylase inhibition assay was carried out by preincubating 25 μL of 1.6 U/μLα-amylase solution with 25 μL of 1 μg/μL cerealα-AI solution for 30 min at 37 °C in 20 mmol/L HEPES buffer at pH 6.9 containing 50 mmol/L Nacl and 3 mmol/L CaCl2. The reaction was initiated by the addition of 50 μL of G3-CNP and incubated for 10 min at 37 °C. The enzyme reaction was terminated by the addition of 100 μL of 10% (V/V) Tris solution and the absorbance of CNP at 405 nm was measured. The control mixture was prepared by replacing theα-AI solution with 20 mmol/L HEPES buffer at pH 6.9 containing 50 mmol/L NaCl and 3 mmol/L CaCl2. The inhibitory activity ofα-AI againstα-amylase was expressed as percent inhibition relative to control using the following equation.
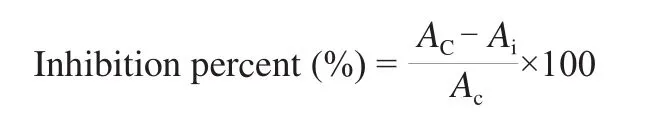
whereAiandAcstand for the absorbance at 405 nm in the presence and absence of an inhibitor, respectively.
2.7 Purification of buckwheat albumin with α-amylase inhibitory activity
Gel filtration chromatography was conducted to further purifyα-AI in buckwheat albumin. After equilibration with distilled water, about 15 mg of protein dissolved in 20 mL of distilled water was applied to a Sephadex G-50 column (2.5 × 100 cm) (GE Healthcare UK Ltd.,Buckinghamshire, UK) and eluted with distilled water at a flow rate of 0.2 mL/min. The absorbance of the eluate was monitored at 280 nm and each fraction of 5 mL was collected. The fractions showing more than 90% inhibitory activity againstα-amylase from porcine pancreas were lyophilized and stored at -20 °C until use.
2.8 Purification of buckwheat-albumin peptides with α-amylase inhibitory activity
In order to identify the peptide withα-amylase inhibitory activity,tryptic hydrolysates of buckwheat albumin were further purified by affinity chromatography and HPLC. First, 10 mg of buckwheat albumin was mixed with 1 mL of 1 mg/mL trypsin in 50 mmol/L Tricin buffer at pH 8.0 and incubated at 37 °C for 4 h. After incubation, the enzymatic hydrolysis was terminated by heating the solution at 100 °C for 5 min and the solution containing buckwheatalbumin peptides were lyophilized.
The affinity column was prepared by couplingα-amylase to a HiTrap NHS-activated HP column (0.7 cm × 2.5 cm, 17-0716-01,GE Healthcare Japan, Tokyo, Japan). After washing the column with 1 mmol/L HCl, 10 mg ofα-amylase from porcine pancreas(1 663 U/mg protein) in 1 mL of the coupling buffer containing 0.2 mol/L sodium hydrogen carbonate and 0.5 mol/L NaCl at pH 8.3 was applied to the column and stayed for 4 h at 4 °C. Then,3 mL of the coupling buffer was applied. In order to block the unreacted groups, 6 mL of the blocking buffer containing 0.5 mol/L monoethanolamine and 0.5 mol/L NaCl at pH 8.3 was applied and stayed for 1 h at room temperature. After 6 mL each of the washing buffer containing 0.1 mol/L sodium acetate and 0.5 mol/L NaCl at pH 4.0, the blocking buffer and the washing buffer were further applied in turn, 11 mL of the equilibration buffer containing 20 mmol/L HEPES, 50 mmol/L NaCl, and 3 mmol/L CaCl2at pH 6.9 was applied. Then, 20 mg of buckwheat-albumin peptides in 3 mL of the equilibration buffer was applied to the column and the equilibration buffer was kept applying until the absorbance of the eluate at 280 nm was almost zero. The buckwheat-albumin peptides bound to theα-amylase coupled in the column were eluted by the elution buffer containing 0.1 mol/L glycine and 0.02 mol/L HCl. The absorbance at 280 nm and theα-amylase inhibitory activity of each fraction (1 mL) were measured. The fractions showing more than 90%α-amylase inhibitory activity againstα-amylase from porcine pancreas were collected and lyophilized.
The HPLC of buckwheat-albumin peptides was conducted using a liquid chromatographic system (Shimadzu corporation.,Kyoto, Japan) equipped with COSMOSIL 5C18-AR-Ⅱ Column(4.6 mm ×150 mm, 38144-31, Nacalai Tesque, InC., Kyoto, Japan).The operating conditions were as follows: column oven temperature,40 °C: mobile phase, 100% solvent A (0.05% TFA in 5% acetonitrile solution) for 50 min, 80% solvent A/20% solvent B (0.05% TFA in acetonitrile) for 80 min, and 100% solvent B for 60 min: flow rate, 0.5 mL/min; injection volume, 20 μL; and detection, 215 nm.Buckwheat-albumin peptides were dissolved in solvent A and filtered through a 0.45 μm membrane-filter (Millex-LH, Merck Co., Ltd.,Tokyo, Japan). Each fraction of 0.5 mL was collected andα-amylase inhibitory activity was measured. The fraction having sufficient peptide content and high inhibitory activity againstα-amylase from porcine pancreas was lyophilized.
2.9 Analysis of buckwheat-albumin peptide with α-amylase inhibitory activity
Buckwheat-albuminα-AI peptide purified by HPLC was dissolved in 0.05% TFA and 5% acetonitrile aqueous solution and itsN-terminal protein sequence was analyzed using a protein sequencer (Applied Biosystems model 492, Thermo Fisher Scientific K.K., Tokyo, Japan).From the obtained amino acid sequence, the parental protein sequence was identified by searching buckwheat genome database established by Kazusa DNA Res. Inst. (http://www.kazusa.or.jp/e/resources/database.html) [29,30]. The molecular weight and the theoretical pIof the parental protein were estimated using ExPASy ProtParam tool(https://web.expasy.org/protparam/).
2.10 Two-dimensional electrophoresis
Two-dimensional electrophoresis was performed according to the method of Westermeier et al. [31]with some modifications.Buckwheat albumin or wheat albumin was loaded onto immobilized pH gradient (IPG) strips (pH 3-10 non-linear). Isoelectric focusing(IEF) was carried out under the following conditions: for the rehydration step the voltage was maintained for 20 min at 175 V and then the albumin was focused for 45 min at 175-2 000 V for 1 h at 2 000 V. After IEF, the strip gels were immersed in 50 mmol/L dithiothreitol (DTT) dissolved in SDS equilibration buffer(18.66 mmol/L Tris pH 8.8 containing 3 mol/L urea, 10% glycerol,1% SDS and 0.02% bromophenol blue) and 50 mmol/L acrylamide dissolved in SDS equilibration buffer for 15 min. Then the strip gels were loaded onto NuPAGE 4%-12% Bis-Tris ZOOM Gels, 1.0 mm, IPG well (NP0330BOX, Life Technologies Japan Ltd., Tokyo)and Xcell SureLock Mini-cell and SDS-PAGE was carried out for 45 min at 200 V. After electrophoresis, the gels were stained for protein with 0.025% coomassie Brilliant Blue R-250 solution (Wako Pure Chemical Industries, Osaka, Japan).
2.11 Statistical analysis
The data were represented as mean ± standard error (S.E.). The values were evaluated by repeated one-way ANOVA with post-hoc Turkey-Kramer multiple range test using Mac Toukeikaiseki ver.3.0(ESUMI co., Ltd., Tokyo, Japan). Values with different letters are significantly different atP< 0.05.
3. Results
3.1 Con firmation of enzymatic hydrolysis of buckwheat and wheat albumins
SDS-PAGE of buckwheat and wheat albumins before and after hydrolysis by pepsin and trypsin shows that all bands larger than 10 kDa observed before digestion disappeared by enzymatic hydrolysis(Fig. 1). Both buckwheat and wheat albumins were hydrolyzed to peptides smaller than 10 kDa by digestive enzymes.

Fig. 1 SDS-polyacrylamide gel electrophoresis patterns of (A) wheat albumin and (B) buckwheat albumin digested by pepsin and trypsin.(M) Marker; (1) undigested; (2) digested by pepsin for 2 h and trypsin for 6 h.
3.2 Effect of buckwheat-albumin and wheat-albumin hydrolysates on oral starch tolerance test
The oral administration of buckwheat-albumin hydrolysates significantly suppressed the postprandial blood glucose elevation at 15 min after starch loading, the glucose concentration being 87% compared with that of the control (Fig. 2). The blood glucose level at 15 min after the oral administration of wheat-albumin hydrolysate together with starch was about 93% of that of the control (P= 0.25).Although there was no significant difference, the plasma insulin level of the buckwheat-albumin and wheat-albumin hydrolysate groups were lower than that of the control group at 15 min (Fig. 3,P= 0.33 and 0.35, respectively).
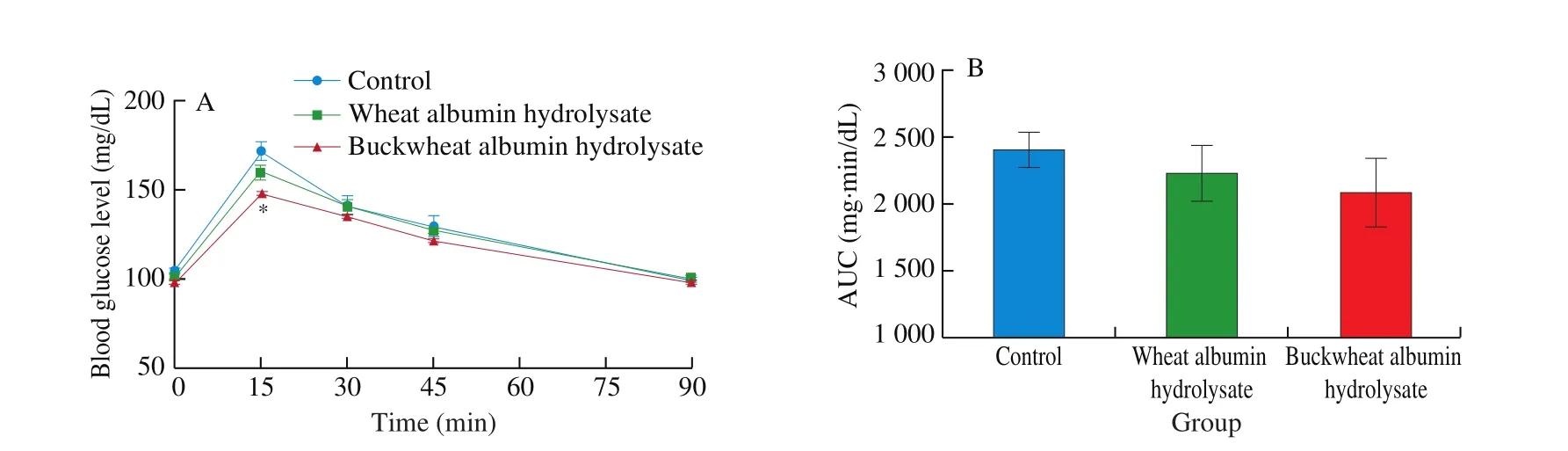
Fig. 2 Effect of buckwheat and wheat α-AIs hydrolysates on (A) blood glucose level and (B) glucose AUC after oral starch tolerance test (OSTT) using Wister rats. Each value is the mean of 7 experiments with S.E. shown as a vertical bar. Values with different letters are significantly different at P < 0.05.
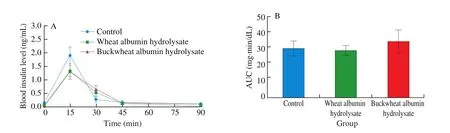
Fig. 3 Effect of buckwheat and wheat α-AIs hydrolysates on (A) plasma insulin level and (B) insulin AUC after oral starch tolerance test (OSTT) using Wister rats. Each value is the mean of 7 experiments with S.E. shown as a vertical bar. Values with different letters are significantly different at P < 0.05.
3.3 Purification of buckwheat-albumin α-AI peptide
Buckwheat albumin was separated by gel filtration chromatography (Fig. 4). As fractions number 27-51 showed highα-amylase inhibitory activity, they were collected and hydrolyzed by trypsin. Afterα-amylase affinity chromatography of buckwheatalbumin peptides, the fractions number 8 and 9 eluted by the elution buffer containing 0.1 mol/L glycine and 0.02 mol/L HCl showed highα-amylase inhibitory activity (Fig. 5). These fractions were further purified by HPLC and a single fraction with high peptide concentration andα-amylase inhibitory activity at 74 min was obtained (Fig. 6). There were some other fractions with highα-amylase inhibitory activity, but the peptide concentration was not sufficient enough for the analysis of amino-acid sequence. The purification procedure was repeated 3 times and almost identical chromatograms were obtained.
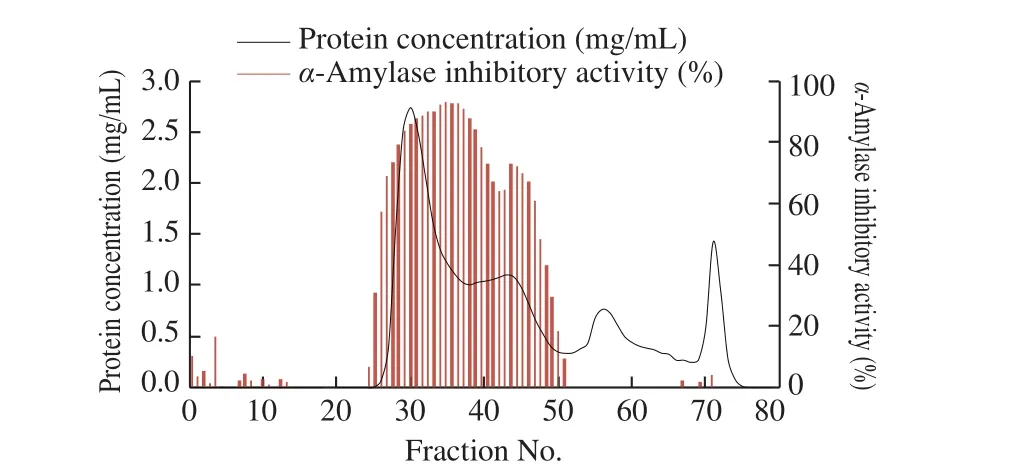
Fig. 4 Sephadex G-50 gel filtration chromatogram of buckwheat albumin and α-amylase inhibitory activity of each fraction.

Fig. 5 α-Amylase affinity chromatogram of buckwheat peptides and α-amylase inhibitory activity of each fraction.

Fig. 6 Peptide concentration (solid line) and α-amylase inhibitory activity (bars) of buckwheat albumin hydrolysates separated by HPLC chromatography.
3.4 Analysis of buckwheat α-AI peptide
N-Terminal amino-acid sequence of the fraction at 74 min by HPLc separation was analyzed using a protein sequencer. As the purification procedure was repeated 3 times, three peptides were obtained from three separate purifications. However, they were almost the same as shown in Table 1, and some of the amino-acid residues in the peptide were not identified.
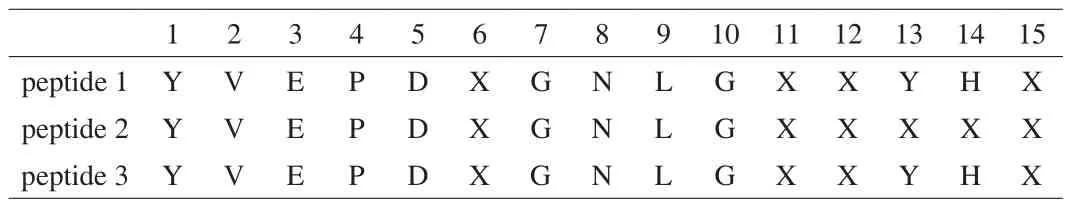
Table 1Amino-acid sequence of buckwheat α-AI peptide measured by protein sequencer.
According to the buckwheat genome database, this peptide was in a parental protein of molecular weight 17.8 kDa and theoretical pI4.77 and unidentified amino acids were found to be a cysteine residue(Fig. 7).

Fig. 7 Homology of amino-acid sequences between buckwheat-albumin α-AI and wheat-albumin α-AI.
3.5 Comparison of buckwheat-albumin α-AI with wheatalbumin α-AI
Amino-acid sequence of buckwheat-albuminα-AI was quite different from that of wheat-albuminα-AI (Fig. 7). Two-dimensional electrophoresis of buckwheat albumin showed 4 spots covering the pIrange 4.1-4.8 and having the molecular weight 10-17 kDa. The spot shown at around 17 kDa would be the identified buckwheat-albuminα-AI (Fig. 8). On the other hand, the spots of wheat albumin had the pIrange 4.5-5.7 and molecular weight 10 kDa.
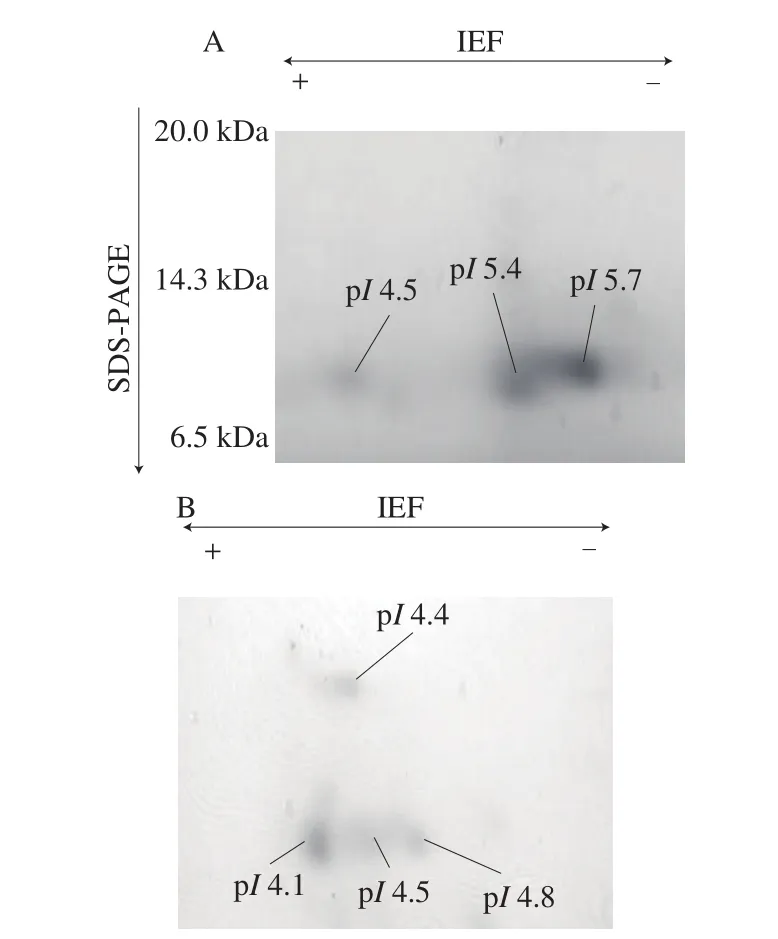
Fig. 8 Two-dimensional-electrophoresis of (A) wheat-albumin α-AI and(B) buckwheat-albumin α-AI.
4. Discussion
Wheat albumin is known to inhibitα-amylase activity and has been commercially used as a functional component in Food for Specified Health Uses (FoSHU) to suppress blood glucose elevation.We have previously reported that oral administration of buckwheat albumin suppressed postprandial rapid blood glucose elevation by inhibitingα-amylase activity [26]. In addition, different from wheat albumin, buckwheat albumin retained highα-amylase inhibitory activity even after digestion by pepsin and trypsinin vitro. This study demonstrated that buckwheat-albumin hydrolysates significantly suppressed the rapid increase in blood glucose level 15 min after oral administration of starch although proteins were hydrolyzed to small peptides by digestive enzymes. This result indicates starch hydrolysis to glucose is suppressed by some small peptides in buckwheatalbumin hydrolysate and the amount of glucose absorbed from the small intestine decreased.
The plasma insulin level of the buckwheat-albumin hydrolysate group did not increase and rather tended to decrease at 15 min, which indicates that the suppressive effect of buckwheat-albumin would not be attributed to the promotion of glucose incorporation into cells by insulin, but would be attributed toα-amylase inhibition in the gut. The suppressive effect of buckwheat-albumin hydrolysates on hyperglycemia was somewhat higher than that of wheat-albumin hydrolysates probably because wheat albumin lowers the inhibitory effect againstα-amylase during digestion [26].
In order to identify the peptide responsible for the suppression of postprandial hyperglycemia, the buckwheat-albumin peptide was purified byα-amylase affinity chromatography and HPLC. This procedure was repeated 3 times and almost identical chromatograms were obtained every time. The fraction having highα-amylase inhibitory activity and sufficient peptide concentration was used for the measurement ofN-terminal amino-acid sequence by a protein sequencer. Although some of the residues could not be identified,the amino-acid sequences of three peptides obtained from three separate purifications were almost identical. Then, this sequence was compared with that in the buckwheat genome database, and the unreadable amino-acid residues were found to be cysteine residues.The identified amino-acid sequence, YVEPDCGNLGCCYHC, was in the parental protein of molecular weight 17.8 kDa and theoretical pI4.77. The homology of amino-acid sequence between buckwheatalbuminα-AI and wheat-albuminα-AI was quite low. In addition, the two-dimensional electrophoresis pattern of buckwheat-albuminα-AI was different from that of wheat-albuminα-AI, indicating that aminoacid sequence withα-amylase inhibitory activity in buckwheatα-AI is different from that in wheat-albuminα-AI [32,33]. According to the Lineweaver-Burk plots in our previous report [26], buckwheatalbuminα-AI inhibitsα-amylase in a competitive manner, while wheat-albuminα-AI inhibits it in a non-competitive manner. The different inhibition manners of buckwheat-albuminα-AI and wheatalbuminα-AI also support the difference in amino-acid sequences between them. Some researchers reported glycoproteins obtained from plants inhibitα-amylase in a competitive manner [34-36].From inhibition manner of buckwheat-albuminα-AI, as it is unlikely that peptide itself fit the active site ofα-amylase, theα-AI peptide identified in this study is speculated to be glycosylated.
We have shown that rice albumin suppresses postprandial hyperglycemia not only by oral starch loading but also by oral glucose loading [23]. Rice albumin inhibits the activity ofα-amylase from insects but does not inhibit that from mammals. Therefore, the mechanism of action to suppress postprandial blood glucose level is different from that of buckwheat albumin. Rice albumin is indigestible by pepsin and trypsin probably because its tight structure formed by disulfide bonds. However, the number of cysteine residues is 15 in buckwheat albuminα-AI, which is more than that in rice albumin, but buckwheat-albumin was easily hydrolyzed by digestive enzymes. This indicates that cysteine residues in buckwheat albuminα-AI would not contribute to the formation of tight structure by disul fide bonds.
This study showed that buckwheat-albumin hydrolysate has suppressive effect on postprandial blood glucose elevationin vivoby inhibitingα-amylase activity in the gut and identified the peptide responsible to this function. However, as there were several fractions having highα-amylase inhibitory activity with low peptide concentration, there would be some otherα-AI peptides in buckwheat hydrolysate. Further study is necessary to elucidate the aminoacid sequences of these peptides and prove their function byin vivoexperiments.
5. Conclusion
We demonstrated buckwheat-albumin hydrolysate suppressed postprandial rapid blood glucose elevation after oral starch loading by inhibitingα-amylase activity. The suppressive effect was somewhat higher than that of wheat-albumin hydrolysate which is known to inhibitα-amylase activity and suppress postprandial hyperglycemia.The amino-acid sequence and molecular weight of buckwheat albumin were different from those of wheat albumin. Therefore,buckwheat albumin has potential to be used as a dietary supplement or a nutraceutical product to suppress the postprandial glucose elevation,which helps to slow the onset and progression of insulin resistance,but more experiments are warranted to further determine the effects and mechanisms of action.
Declaration of competing interest
The authors declare that they have no competing interests.
- 食品科學與人類健康(英文)的其它文章
- Dietary bioactives and essential oils of lemon and lime fruits
- Green tea, epigallocatechin gallate and the prevention of Alzheimer’s disease: clinical evidence
- Simultaneous quantification of 18 bioactive constituents in Ziziphus jujuba fruits by HPLC coupled with a chemometric method
- A systematic study on mycochemical profiles, antioxidant, and anti-inflammatory activities of 30 varieties of Jew’s ear (Auricularia auricula-judae)
- GPP (composition of Ganoderma lucidum polysaccharides and Polyporus umbellatus polysaccharides) protects against DSS-induced murine colitis by enhancing immune function and regulating intestinal flora
- Immunoregulatory polysaccharides from Apocynum venetum L.flowers stimulate phagocytosis and cytokine expression via activating the NF-κB/MAPK signaling pathways in RAW264.7 cells

