Dense copper azide synthesized by in-situ reaction of assembled nanoporous copper microspheres and its initiation performance
Xing-yu Wu,Ming-yu Li,Qing-xuan Zeng,Qing-xia Yu
State Key Laboratory of Explosion and Technology,Beijing Institute of Technology,Beijing,100081,PR China
Keywords:Nanoporous copper Electroless plating In-situ reaction Copper azide
ABSTRACT Copper azide with high density was successfully synthesized by in-situ reaction of nanoporous copper(NPC)precursor with HN3 gaseous.NPC with pore size of about 529 nm has been prepared by electroless plating using polystyrene (PS) as templates.The copper shells thickness of NPC was controlled by adjusting the PS loading amount.The effects of copper shell on the morphology,structure and density of copper azide were investigated.The conversion increased from 87.12% to 95.31% when copper shell thickness decrease from 100 to 50 nm.Meanwhile,the density of copper azide prepared by 529 nm NPC for 24 h was up to 2.38 g/cm3.The hollow structure of this NPC was filled by swelling of copper azide which guaranteed enough filling volume for keeping the same shape as well as improving the charge density.Moreover,HNS-IV explosive was successfully initiated by copper azide with minimum charge thickness of 0.55 mm,showing that copper azide prepared has excellent initiation performance,which has more advantages in the application of miniaturized explosive systems.
1.Introduction
With the miniaturization of weapons systems,the traditional primary explosive,such as lead azide,can hardly meet the limiting dosage and assembly requirements very well [1-3].Copper azide(Cu(N)or CuN) possesses excellent initiation performance,which is considered as a desirable replacement,but its high sensitivity limits application in miniaturized explosive systems[4,5].For production and application safety,one of the promising processes is in-situ synthesis of copper with hydrazoic acid (HN)[6-8].For this conversion process,copper as a precursor reacts with hydrazoic acid(HN)to transform to copper azide.This in-situ approach can achieve the direct production of copper azide and enhance the safety in the fabrication process.More importantly,it is compatible with automatic assembly technology,which has expensive application prospect in large-scale production of miniaturized explosive systems.
Up to now,some pioneering works were set to prepare copper azides and can be classified into two general approaches.One is to encapsulating copper azide with organic materials to reduce its electrostatic sensitivity,such as carbon nanotubes [9] and metalorganic frameworks (MOFs) [10].The other is to adopt nanoporous copper(NPC)precursors to in-situ prepare copper azide on a target substrate to improve its safety.Various approaches have been exploited to synthesize NPC precursors,such as hydrogen bubble template method [11-13],anodic aluminum oxide (AAO)[14],decomposition and sintering of copper oxalate[15].Moreover,employing porous Cu and CuO nanorods as precursors,porous CuN[16]and nest-like Cu(N)[17]film were fabricated by in-situ liquid-solid method,respectively.These works laid a good foundation for the safe and efficient preparation of copper azide,and some of them have been applied to synthesize other sensitive explosives,such as AgN[18].However,these methods disregarded the effect of carbon additive and porous structure,which cause copper azide prepared often difficult to achieve high density.
The polystyrene (PS) sphere,as a typical template of NPC synthesis,has been successfully used to synthesize copper azide in our previous work [19,20].It technically demonstrated advantages in controllability of copper azide density through adjusting loading amount and particle size of PS.However,we found that the size of PS microspheres (2-4 μm) was relatively large that copper azide formed could not fill with reserved cavity completely and resulted in the low charge density (Fig.1).Besides,the copper shell of 100 nm could not convert to copper azide completely.Therefore,a small-sized PS template with proper structure is needed to increase utilization of hollow structure and improve charge density of copper azide.
In this paper,PS/Cu composites with core-shell structures were prepared by electroless copper plating on the surface of PS templates.Then NPC blocks with different shell thickness were prepared by sintering PS/Cu composites and then in-situ reacted with HNgaseous to prepare copper azide.The effects of NPC shell thickness on structure,density and conversion of copper azide were investigated.The initiation performance of the copper azide with different structures was also studied.
2.Experimental
2.1.Materials
Copper sulfate pentahydrate (CuSO·5HO),ethylene diamine tetraacetic acid disodium (NaEDTA),sodium hydroxide (NaOH),Hydrochloric acid (HCl),Potassium hydroxide (KOH) and formaldehyde (HCHO) were purchased from Xilong Chemical Co.,Ltd(China).Stearic acid(Tianjin Fuchun Chemical Reagents Factory,99%) and sodium azide (MYM Biological Technology Co.,Ltd,99%)were used as received.All chemicals used in this investigation were of analytic grade.All solutions were prepared with deionized water.
2.2.Methods
2.2.1.Synthesis of NPC block
Monodisperse PS microspheres were synthesized by soap-free emulsion polymerization and preprocessed,including roughening,sensitization and activation,to decorate catalytic center on surface.The detailed experiment processes has been given in our recent report[19].The PS/Cu core-shell composites were prepared by electroless copper plating using PS microspheres templates.The preprocessed PS (0.5-1.5 g) was uniformly dispersed into 1.0 L alkaline bath (0.1 mol/L CuSO·5HO and 0.12 mol/L NaEDTA,pH=12.05),followed by adding 15.0 mL HCHO to induce depositing reaction.The deposition was performed at 40C water bath for 15 min under ultrasonic.Then,the PS/Cu core-shell microspheres were obtained by centrifuging the mixed solution at 4000 rpm for 5 min,washed by deionized water for three times and dried in a vacuum at 50C for 24 h.The PS/Cu powders were pressed by a powder pressing machine to form round block.Finally,NPC block was produced by sintering PS/Cu block in Natmosphere at 400C for 1 h to remove PS templates.
2.2.2.Fabrication of copper azide
The available NPC block was pressed into the pores in polycarbonate confinements which was custom-made for fabrication of copper azide micro-charge.The NPC block with polycarbonate confinements was then in situ reacted with the gaseous HN.The polycarbonate confinements were indispensable in azide reactions,which could avoid direct contact and handling with copper azide,thus reducing potential hazards of primary explosives.A mixture of sodium azide(2.0 g)and stearic acid(10.0 g)was heated to generate gaseous HN.The NPC with polycarbonate confinements were separately placed on the sand core filter for maximum exposure to HN.Before reaction,a flow of Nwas running into the reaction device for 10 min to drive away the air and then the flask was heated by oil bath to 120C.The gaseous HNand the azide formation occurred according to the reactions [21,22]:
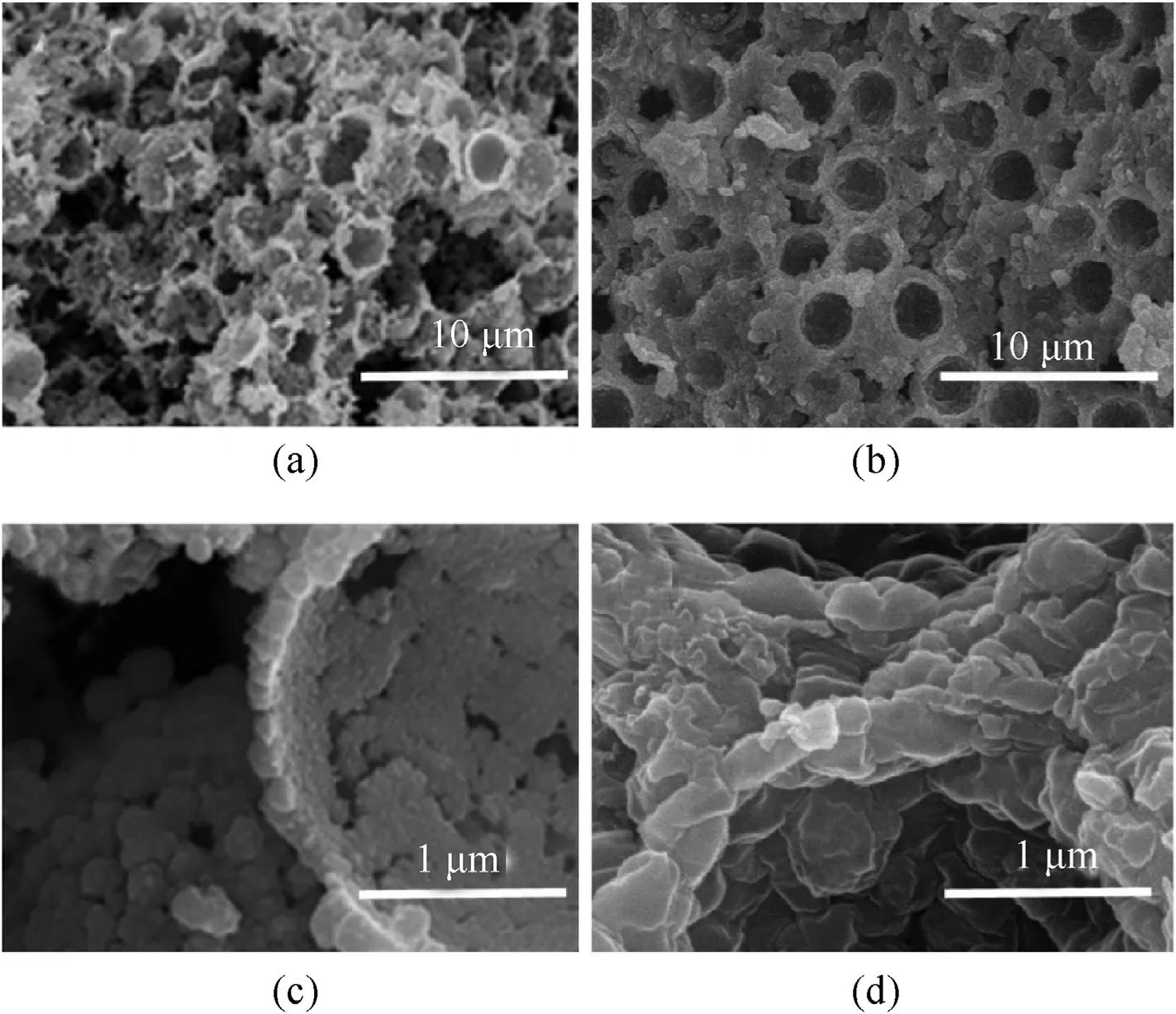
Fig.1.Cross-section images of (a,b) [19] NPC prepared by 3.257 μm PS template and (c,d) copper azide prepared for 24 h.
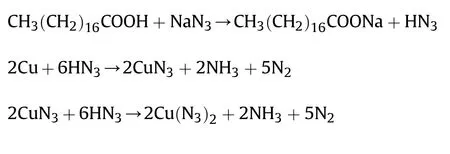
2.2.3.Initiation test of copper azide
A miniature initiation device was shown in Fig.12,which was usually composed of a base plate with Ni-Cr bridge-wire,the available copper azide with polycarbonate confinements,titanium flyer layer and barrel.The copper azides were initiated by applying a voltage of 5 V to the Ni-Cr bridge-wire with a resistance of 2.1 Ω,which was integrated on a base plate.The thickness of the titanium flyer and barrel were 0.025 and 0.68 mm,respectively,and the barrel had the same diameter with copper azide micro-charge.The bare HNS-IV explosive was initiated by copper azide driven flyer,and the test result was analyzed by observing the dents caused by the explosive on the steel witness board,which was made of No.20 cold drawn steel with a thickness of 15 mm.
2.3.Materials characterization
Particle size and the particle size distribution of PS microspheres are performed by laser particle size analyzer (Mastersizer 2000,UK).The specific BET (Brunauer-Emmentt-Teller) surface area of nanoporous copper block was measured through the nitrogen(N)adsorption-desorption process using surface area and porosimetry system (ASAP 2460,Micromeritics,USA) at 77 K.X-ray diffraction(XRD) analysis is performed on a diffractometer (Rigaku,Japan)with Cu Kα radiation in the range from 5to 80.The morphology and microstructure of samples was observed by scanning electron microscopy (SEM,S4800,Japan) and transmission electron microscopy (TEM,TECNAI G2 F20,USA).The IR spectra of copper azides were collected by infrared spectrometer(TENSOR 27,Germany).To analyze the conversion of copper,copper azide prepared was dissolved in 0.5 mol/L HCl solution at 70C for 2 h.The Cuconcentration was measured by inductively coupled plasma optical emission spectrometer(ICP-OES,PE OPTIMA 7000DV,USA).
3.Results and discussion
3.1.PS microsphere template
Fig.1 shows the cross-section images of NPC prepared by 3.257 μm PS template and copper azide prepared for 24 h.It is clearly that copper azide(Fig.1 a,b)prepared for 24 h keep the same hollow structure as NPC (Fig.1 c,d) which is prepared by 3.257 μm PS template,and the shell thickness increases from 100 nm to 500 nm.The structure diagram of copper core-shell microsphere is shown in Fig.2.Assuming that copper azide expands to the inside of Cu sphere completely after reaction,the actual volume expanding multiple Kcould be calculated by the following equation:

where r is the radius of PS microsphere;h and H are the thickness of copper shell and copper aizde shell,respectively.
It is calculated that the actual volume of copper expands 3.9 times,which does not meet the expected 8 times in theory when transforming copper azide.The SEM images imply that the size of PS microspheres is relatively large that copper azide formed could not fill with reserved cavity completely.Besides,the density of copper azide obtained was 2.108 g/cmfor 24 h,which is less than the theoretical density.These results suggest that copper shell of 100 nm could not convert to copper azide completely.This may be due to the thick shell,which makes the copper particle over accumulation,and thus was not conducive to copper azide expansion.Therefore,the conversion rate may be increased by decreasing the shell thickness.The shell thickness increased 4 times after reaction.Assuming the copper shell is around 50 nm,the shell thickness of copper azide is around 250 nm after reaction.Thus,the average diameter of PS used to prepare porous copper should be 500 nm to improve utilization of hollow structure.
The particle size distribution of PS is presented in Fig.3.The PS size ranges from 100 nm to 1 μm,and the average diameter is 529 nm.The gentle peak distributed from 2 μm to 11 μm is caused by the agglomeration of PS due to its sub-micrometer diameter.Fig.4 shows the morphologies of PS microspheres.It can be seen that PS with 529 nm in diameter is monodisperse and the surface of the microsphere is very smooth.
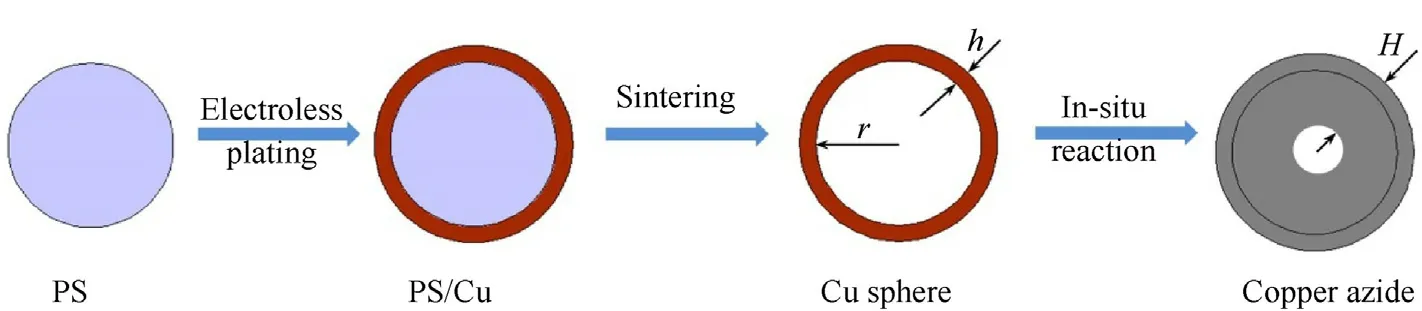
Fig.2.The structure diagram of copper core-shell microsphere.
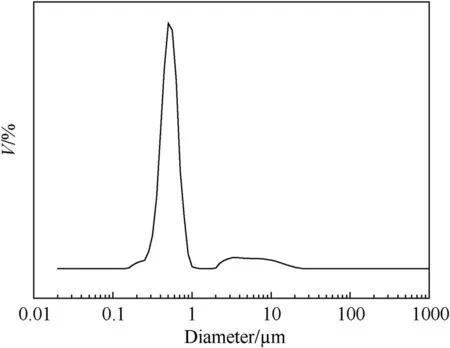
Fig.3.Particle size distribution of PS microsphere.
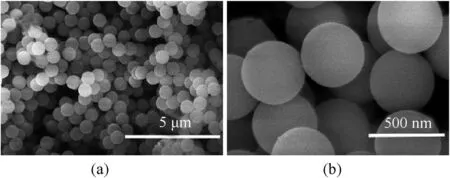
Fig.4.SEM images of PS microspheres.
3.2.PS/Cu core-structure
In recent study,we reported that the thickness of the copper shell is related to PS loading amount.Fig.5 shows the morphologies of PS/Cu core-shell structure at different loading amounts (0.5 g/L,1.0 g/L and 1.5 g/L).As shown in Fig.5,copper particles are deposited on the surface of the PS microsphere and form a coreshell structure.When the loading amount is 0.5 g/L,most PS/Cu microspheres with a core-shell structure have good integrity,but a part of copper particle are not deposited on the surface of the PS microsphere and scattered sporadically around PS microspheres because of the insufficient PS surface area.When the loading amount increases to 1.0 g/L,the copper particles distributed uniformly on the surface of each PS microspheres and form a core-shell structure have a good integrity.When the loading amount is further increased to 1.5 g/L,some part of PS surface is not covered completely by copper due to copper particle deficiency.It can be seen clearly from the SEM images of NPC.
3.3.NPC block
Fig.6a is the XRD pattern of pure NPC.All the diffraction peaks can be well indexed to in face-centered cubic Cu (JCPDS No.04-0836) without obvious crystal face preferred orientation.The peaks at 43.31,50.46and 74.13correspond to crystalline planes of(111),(200) and (220),respectively.No other peaks for impurities are observed,indicating that the sintering after-treatment process could eliminate PS templates and form porous copper microspheres with high purity.The nitrogen(N)adsorption-desorption process was showed in Fig.6b.The Brunauer-Emmett-Teller surface area of NPC is calculated to be 6.064 m/g with the pore diameter in the range of 0-180 nm,which indicated that the pores in the NPC block are open and interconnected.
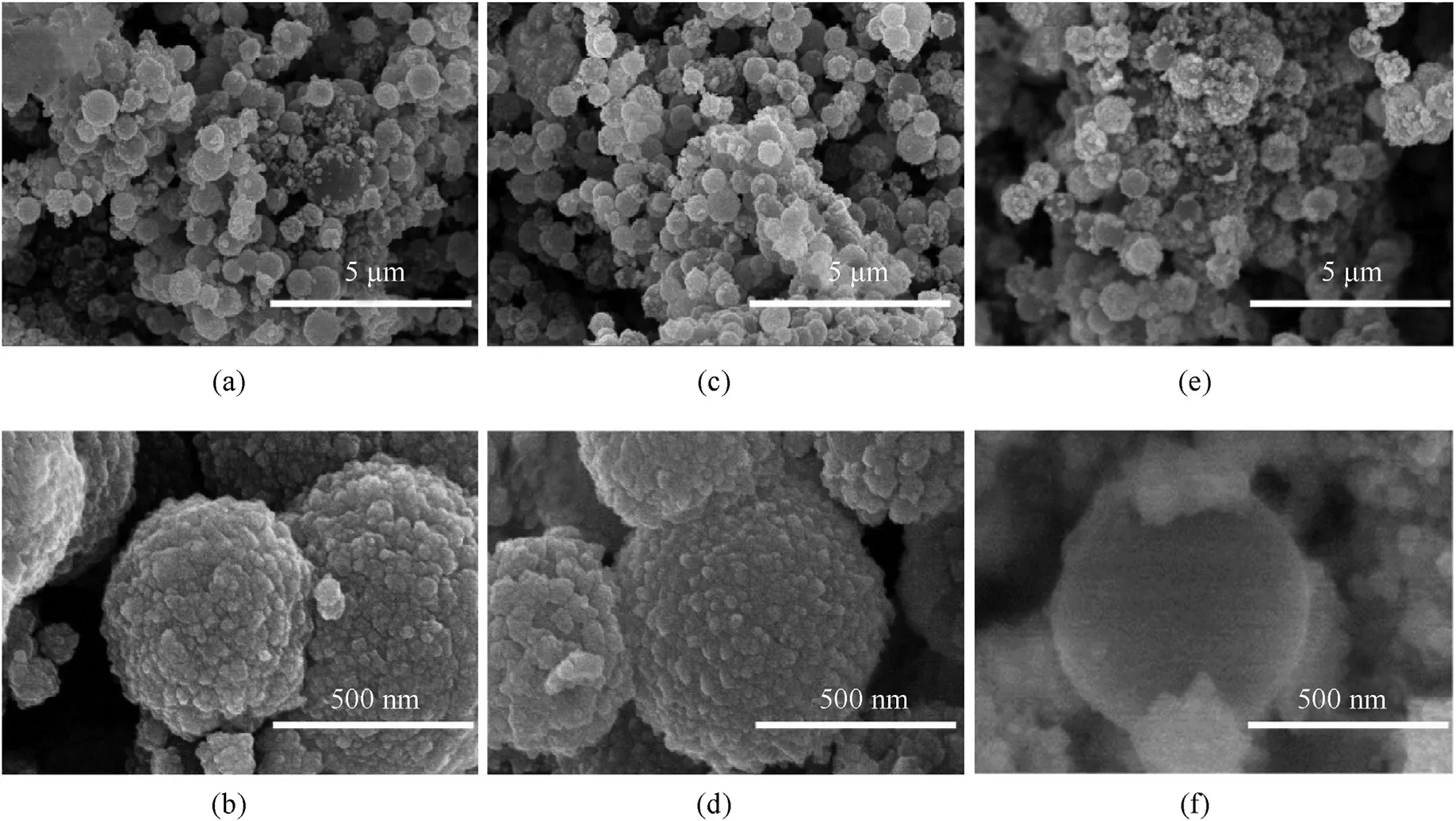
Fig.5.PS/Cu core-shell structure at different PS loading amount.(a,b) 0.5 g/L (c,d) 1.0 g g/L(e,f) 1.5 g/L.
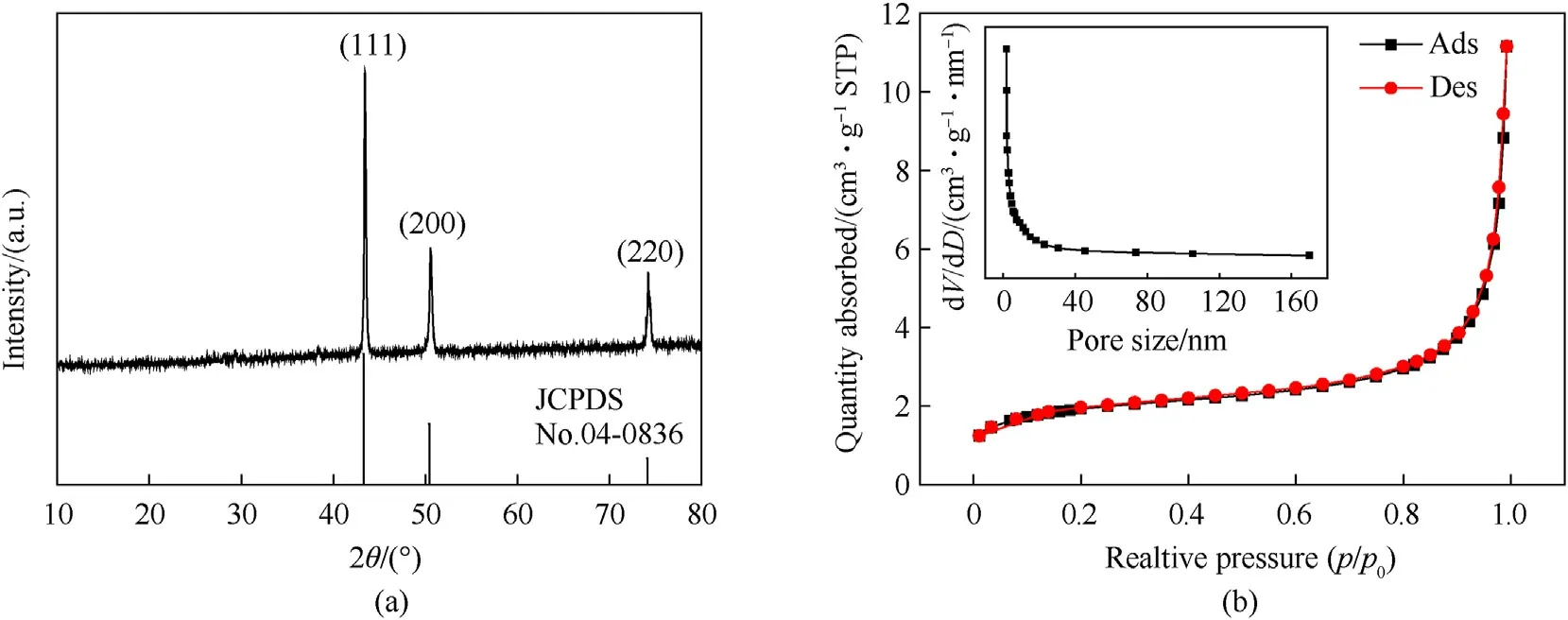
Fig.6.(a) XRD patterns of NPC,(b) N2 adsorption-desorption isotherm of NPC.
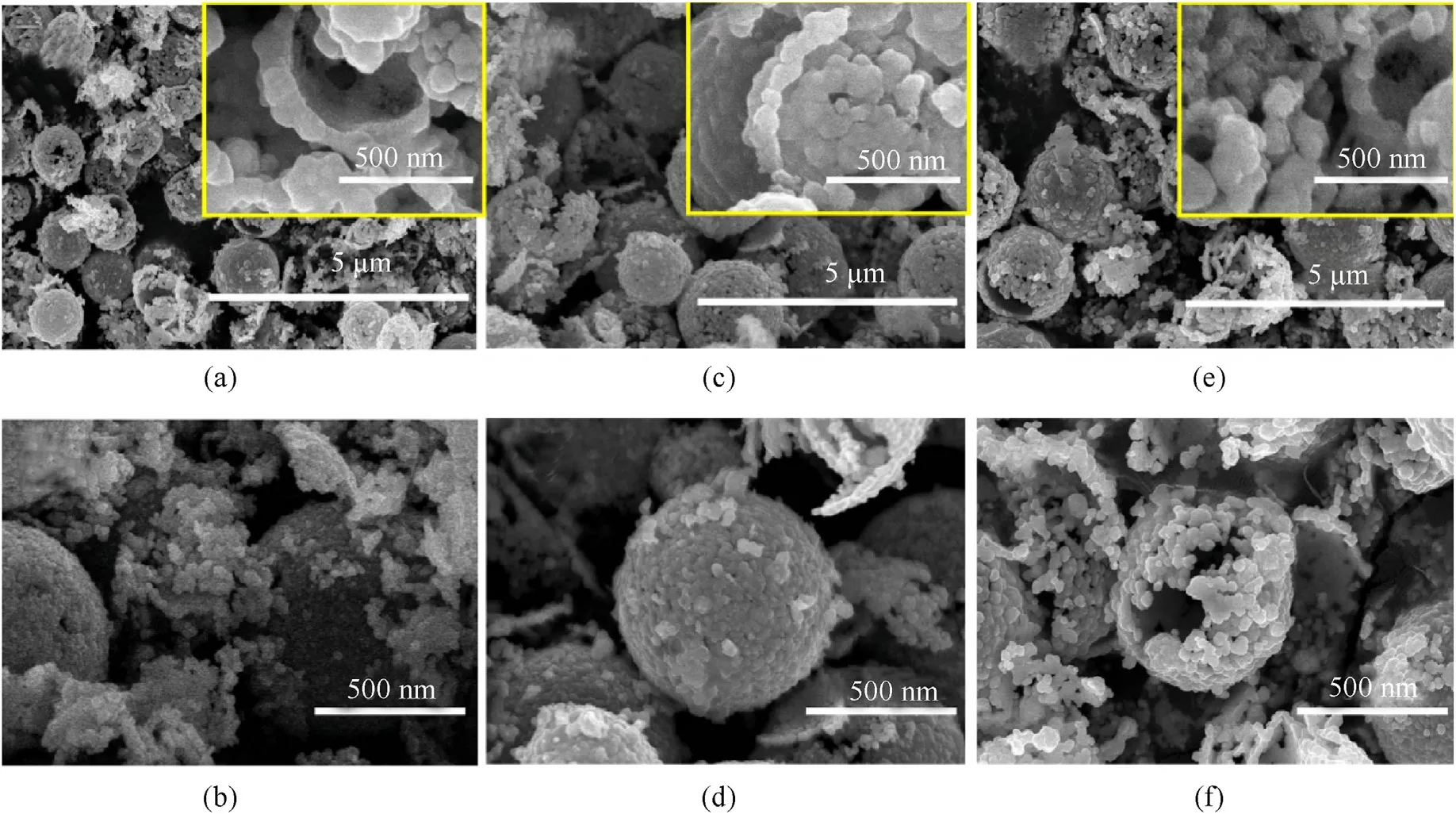
Fig.7.NPC at different PS loading amount:(a,b) 0.5 g/L;(c,d) 1.0 g/·L;(e,f) 1.5 g/L on the density of the NPC block.
Fig.7 shows the SEM images of NPC block prepared at different loading amounts (0.5 g/L,1.0 g/L and 1.5 g/L).As shown in Fig.7,most of the copper shells still retain a stable spherical shape after sintering despite that a part of copper particle are not deposited on the surface of the PS microsphere and scattered sporadically.While removing of PS skeleton,the copper shells synthesized with a loading amount of 1.5 g/L collapsed because of the uneven coating of copper particles(Fig.7e and f).When the loading amount is 0.5,1.0 and 1.5 g/L,the thickness of the copper shell is about 100,50,less than 50 nm,respectively.The above discussion strongly indicated that loading amount around 1.0 g/L is the optimal value to achieving higher copper utilization rate as well as a better microstructure of copper sphere(Fig.7c and d).The density and porosity of NPC with different PS loading amounts are given in Table 1.When the loading of plating solution increased from 0.5 g/L to 1.5 g/L,the density of NPC decreased from 2.17 g/cmto 1.83 g/cm,and the porosity increased from 75.78% to 79.57%.These results demonstrate that the copper shell of NPC is controlled by adjusting the loading amount.
3.4.Copper azide
The formation of the copper azide is apparent from the XRD and FT-IR analysis.In the XRD pattern of copper azide (Fig.8 a),the peaks at 18.91,29.16and 38.44corresponding to the reflections of(101),(211)and(202)crystalline planes of tetragonal phase CuN(JCPDS No.04-0622),respectively,indicates that CuNis generated in the azide reaction between Cu and HNgaseous.Meanwhile,the diffraction peaks at 11.76,27.95and 31.94corresponded to thereflections of (120),(230) and (021) crystalline planes of orthorhombic Cu(N)(JCPDS No.21-0281),which means Cu(N)is also produced during the azide reaction.This is due to part of CuNtransforms into Cu(N)further in the presence of HN.The existence of Cu implies that copper is not converted to copper azide completely.The FT-IR spectrum also confirms the formation of copper azides.As shown in Fig.8b,the typical asymmetric azide peaks are observed at 2084 and 2125 cm,and the symmetric vibrational frequencies for azide ion are observed at 1262 and 1296 cm.The broad band at 3441 cmis the characteristic O-H bending vibration.

Table 1 Effect of PS loading amount on the density of NPC block.

Fig.8.(a)XRD patterns and (b) FT-IR spectrum of the prepared copper azide.
To investigate the effect of NPC precursor on copper azide morphology and structure,three groups of NPC blocks with different shell thickness were used.Fig.9 shows the SEM picture of copper azide prepared with different shell thickness.As shown in Fig.9 (a,b),the microstructure still remains sphere structure,without any swelling out of confinement,when the shell thickness of NPC above 50 nm.When the shell thickness of NPC less than 50 nm,the original hollow structure basically disappears and copper azide swell out of confinement due to limited space,and thus the morphology could not be maintained.This result indicates that increasing the shell thickness can improve the morphology and structure of copper azide.
The conversion of copper azide prepared by NPC block with different shell thickness is given in Table 2,where NPC blocks are reacted with HNfor 24 h under the same condition.The conversion decreases from 95.31% to 87.12% when copper shell thickness increases from 50 to 100 nm,suggesting that the conversion degree of copper can be improved by decreasing the shell thickness.This may be ascribed to plane formed on the surface,which prevent the HNgas from getting inside and make the reaction stop.Furthermore,the conversion of azide reaction is exceeded 94.56%when the shell thickness is 50 nm.The density increases from 1.94 to 2.38 g cmobviously when PS diameter decrease from 3257 to 529 nm.These results show that the hollow structure of 529 nm PS microsphere with shell of 50 nm is fully utilized and the ultimate goal of promoting copper azide density and conversion efficiency of copper can be realized.
Fig.10 is the cross-section images of the prepared copper azide with different azide reaction time (6 h,15 h and 24 h).As the reaction time increases,the pores in NPC block wan filled gradually by copper azide.When the reaction is carried out for 6 h,it can be seen in Fig.10a,b that copper azide with short columnar appears on the inner surface of the shell,indicating that Cu has reacted with HN.After 15 h,copper azide continues to grow and interconnect(Fig.10 c,d).The copper azide bonded together at the surface and thus grew to a plane after 24 h (Fig.10 e,f).The original hollow structure of NPC was filled with expanded copper azide after reaction,compared with Fig.7e,f.Fig.11 show the TEM images of copper spheres and copper azide(reacted for 24 h).The interplanar distances of d=0.21 nm and d=0.18 correspond to(111)and(200)planes of copper crystal,respectively.The interplanar distances of d=0.54 nm and d=0.47 nm correspond to(120)planes of Cu(N)crystal and (101) planes of CuNcrystal (Fig.11d),respectively.It can be seen from Fig.11a that the hollow structure of the copper sphere.Fig.11 c further confirms dense structure of copper azide.Besides,the dense structure of copper azide prevents the HNgas from getting inside and makes the reaction stop,which is considered as the reason why copper could not convert to copper azide completely.Decreasing copper shell thickness can reduce HNgaseous diffusion resistance;as a result,it is in favor of improving the conversion degree.
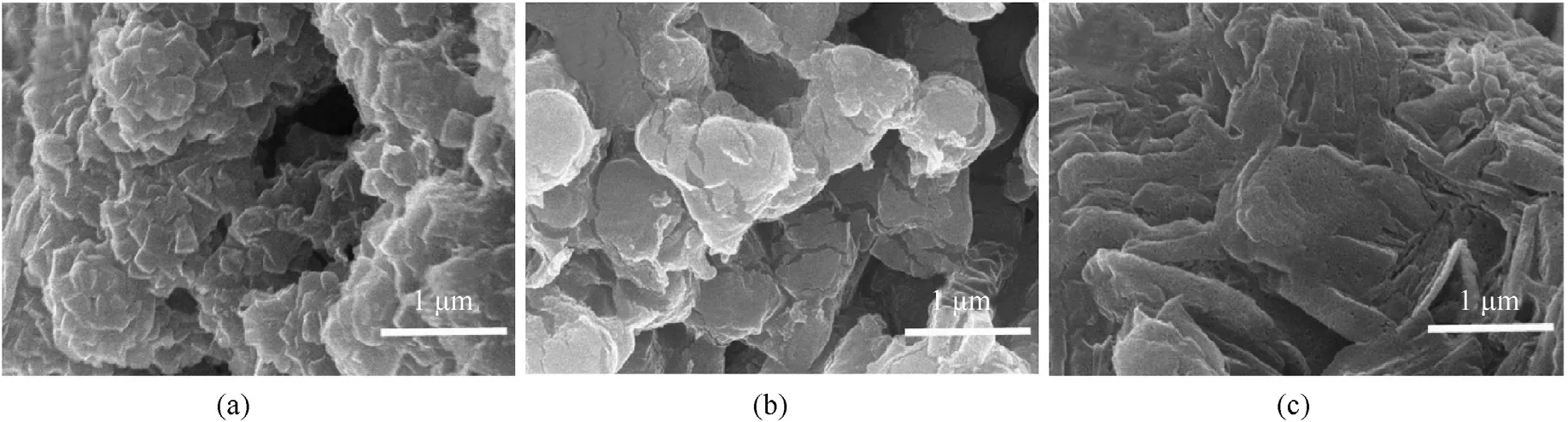
Fig.9.Copper azide prepared for 24 h at different shell thickness.(a) 100 nm,(b) 50 nm,(c) less than 50 nm.
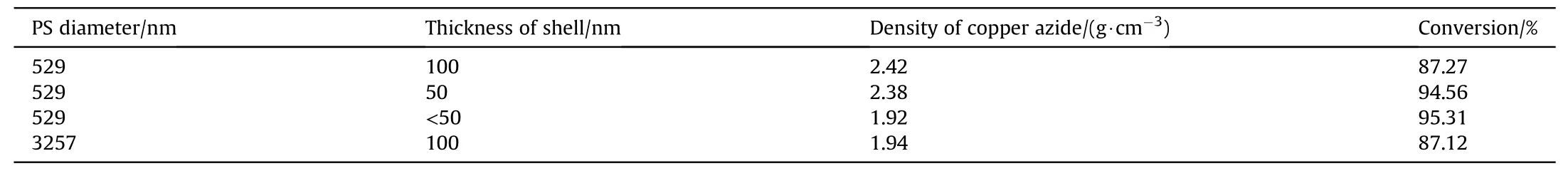
Table 2 Conversion of copper azide prepared by NPC block with different shell thickness.
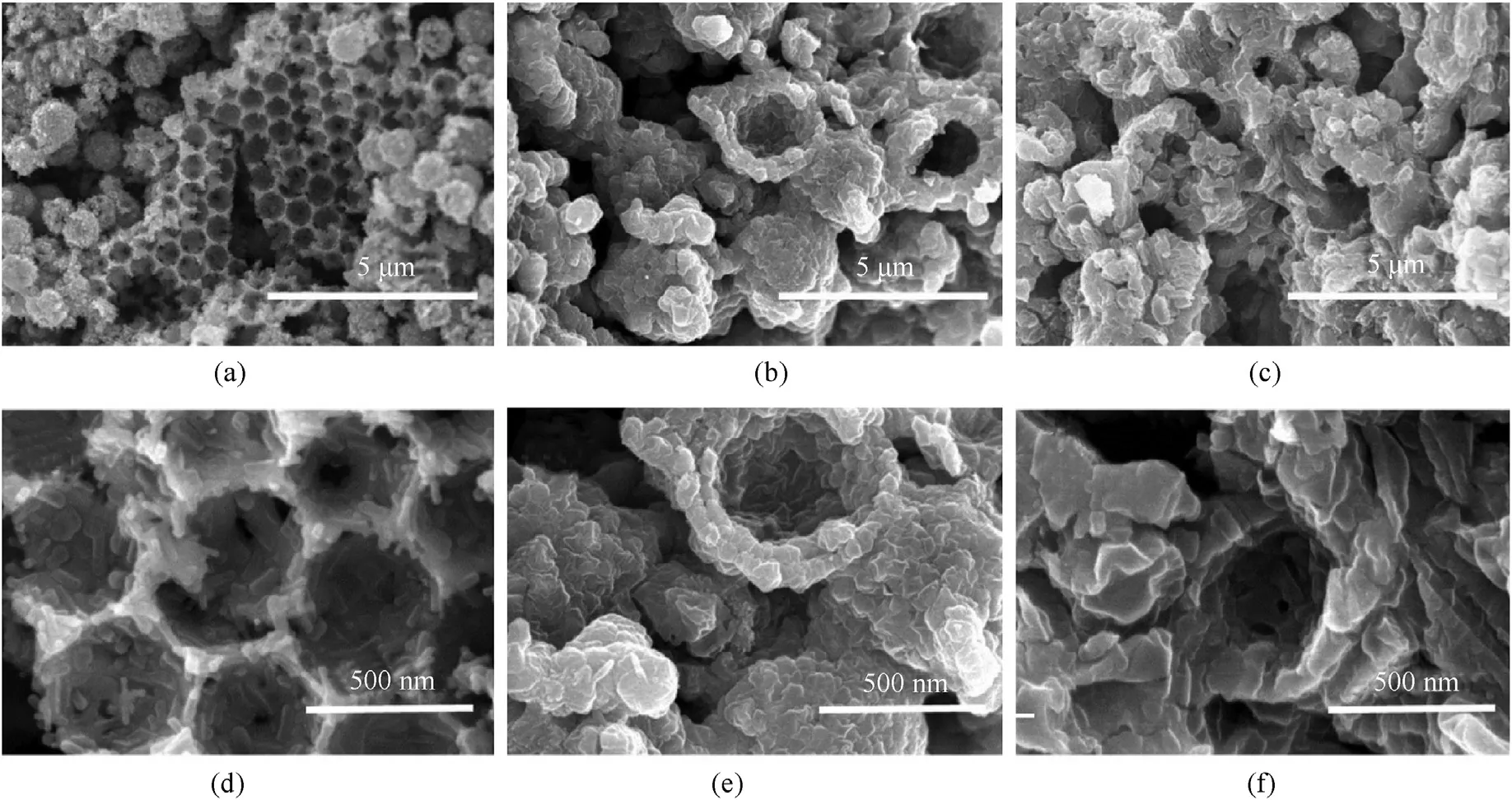
Fig.10.Cross-section images of copper azide prepared with different reaction time:(a,b) 6 h;(c,d) 15 h;(e,f) 24 h.
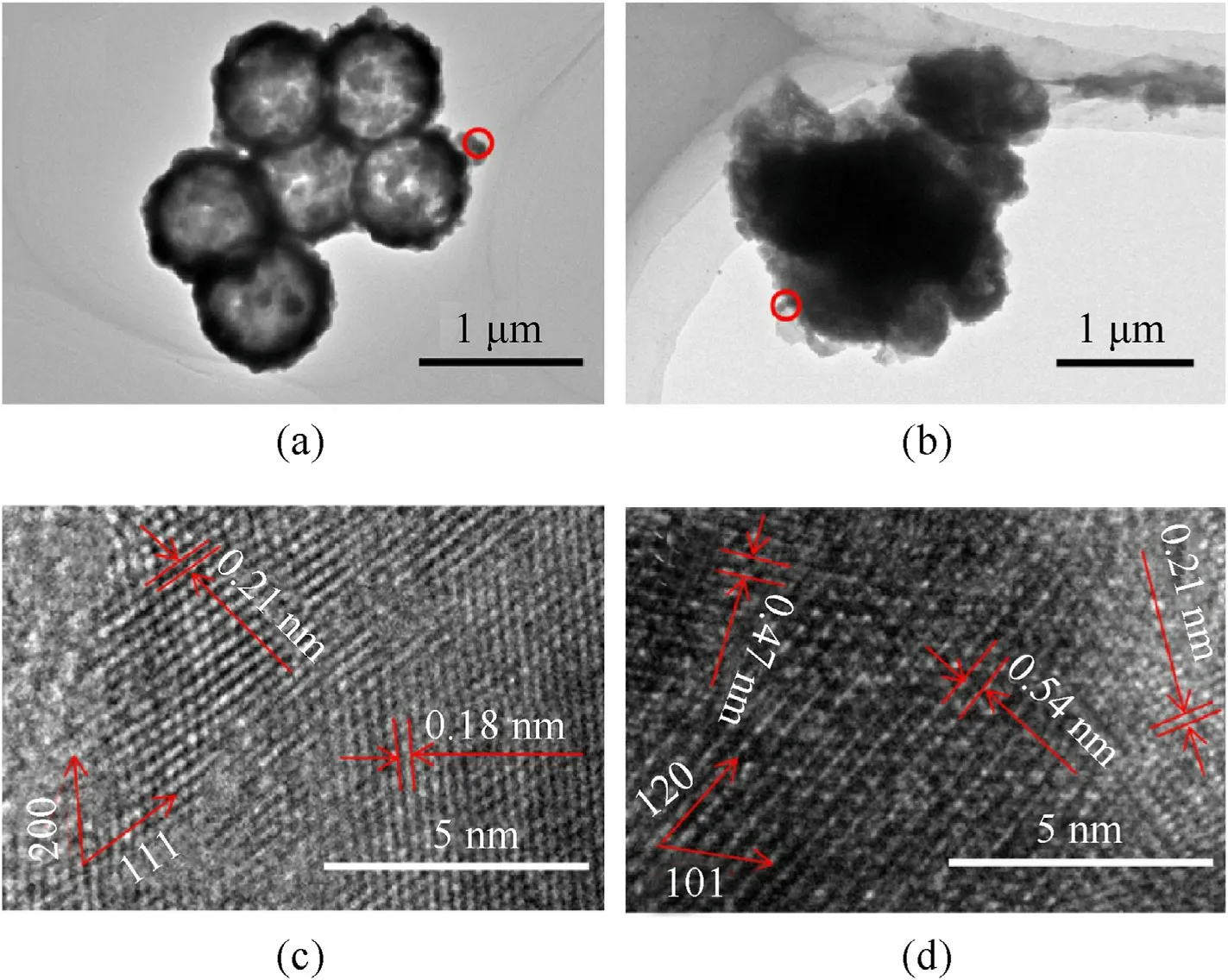
Fig.11.TEM (a) and HRTEM (b) images of the NPC;TEM (c) and HRTEM (d) images of copper azide.
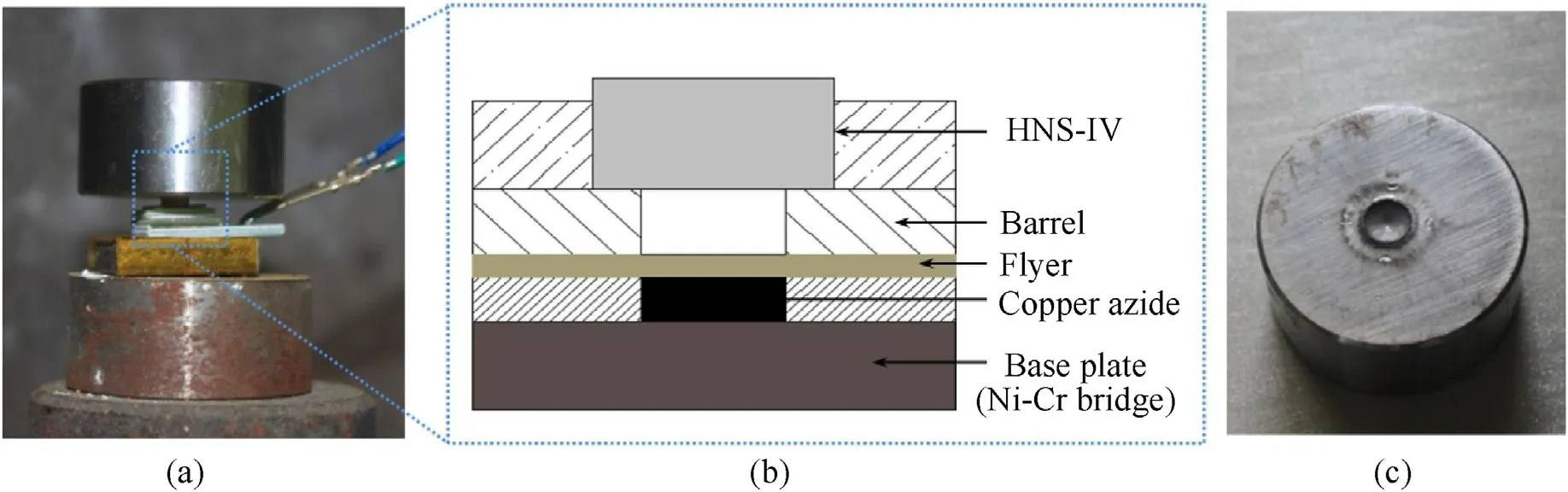
Fig.12.Initiation test of copper azide (a,b) and steel dent experiment results (c).
3.3.Initiation test of copper azide
In order to verify the initiation ability,copper azide prepared with polycarbonate confinements is fabricated into a miniature device[3].The experiment of initiating HNS-IV explosive using the miniature device driven by prepared copper azide is carried out,which can be seen from Fig.12(a and b).The bare HNS-IV explosive used in experiments has a column shape of φ 4.0 × 4.0 mm with density of 1.57 g/cm.The barrel size is φ 1.0×0.68 mm,and flyer is made of titanium with thickness of 25 μm.From Fig.12(c),we can see that the dent depth caused by HNS-IV explosive of steel board is about 0.30 mm,the diameter is about 4 mm,which is consistent with the explosive diameter,indicating that HNS-IV explosive are successfully initiated.Under the same conditions,the minimum size of copper azide prepared by 3257 and 529 nm PS template initiated HNS-IV explosive was φ 1.0×0.8 mm and φ 1.0×0.55 mm,respectively.This is attributed to high charge density of copper azide,which is responsible for the improvement in initiation performance.It also suggests that submicro PS template can enhances utilization of hollow structure and improve initiation performance of copper azide.
4.Conclusions
In summary,we have demonstrated that copper azide synthesized by NPC with submicron PS possesses high charge density and has an increased initiation performance.The hollow structure of NPC was filled with copper azide completely,and copper azide particles increased continuously and connected to form a dense solid.The density of copper azide prepared by 1.97 g/cmNPC precursor for 24 h was up to 2.38 g/cm.The conversion of azide reaction is exceeded 94% when the shell thickness decreased to 50 nm.These results show that the hollow structure of 529 nm PS microsphere with shell of 50 nm is fully utilized and the ultimate goal of promoting copper azide density and conversion efficiency of copper is realized.Furthermore,the HNS-IV explosive with density of 1.57 g/cmwas initiated by copper azide with the size of φ 1.0 × 0.55 mm,which showed that the copper azide prepared has excellent initiation performance.
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
The authors appreciate the financial support provided by the National Natural Science Foundation of China (No.11872013).
- Defence Technology的其它文章
- Ultra-lightweight CNN design based on neural architecture search and knowledge distillation:A novel method to build the automatic recognition model of space target ISAR images
- Ballistic impact response of resistance-spot-welded (RSW) doublelayered plates for Q&P980 steel
- Calculating detonation performance of explosives by VLWR thermodynamics code introduced with universal VINET equation of state
- Design and motion analysis of reconfigurable wheel-legged mobile robot
- A Multi-UCAV cooperative occupation method based on weapon engagement zones for beyond-visual-range air combat
- Experimental study on anti-penetration mechanism of bolted composite protective structure with limited span under impact of lowvelocity projectile

