Impact of rotavirus vaccine implementation on Israeli children: a comparison between pre- and post-vaccination era
Hussein Zaitoon.Shaden Hanna.Ellen Bamberger
Abstract Background Worldwide rotavirus vaccination has resulted in a substantial decrease in rotavirus-induced severe gastroenteritis and related hospitalizations among children. Still, the characterization of patients warranting hospitalization needs to be further elucidated. The purpose of the study is to compare the clinical and laboratory features of children hospitalized with acute rotavirus infection before and after the introduction of routine vaccination.Methods This is a retrospective observational study. Participants were pediatric patients who presented to the Bnai Zion Medical Center pediatric emergency department and were diagnosed with rotavirus acute gastroenteritis between 2017 and 2019.Results During the pre-vaccination period (2007–2009), 114 infants and young children (median age: 14 months, range: 1–72 months; 59 male, 55 female) were hospitalized for rotavirus-induced acute gastroenteritis with a rate of 11.71 positive rotavirus tests per 1000 emergency room visits. In the post-vaccination period (2012–2019), 168 infants and young children (median age: 17 months, range: 0–84 months; 90 male, 78 female) were hospitalized with a rate of 4.18 positive rotavirus tests per 1000 emergency room visits. There were no statistical differences between the two groups in gender, breast-feeding rates and sibling(s). The proportion of cases with moderate-to-severe dehydration was higher in the post-vaccination children than in the pre-vaccination children.Conclusions Rates of rotavirus-attributed acute gastroenteritis hospitalizations declined from the pre- to the post-vaccination period. Higher rates of dehydration were found in the post-vaccination children. Ongoing surveillance is warranted to better understand the implications of the vaccine.
Keywords Acute gastroenteritis · Booster dose · Hospitalization · Rotavirus · Vaccination ? Hussein Zaitoon
Introduction
Rotavirus is the most common cause of acute gastroenteritis in infants aged 6 months to 2 years [ 1]. The vast majority of children with rotavirus experience typical symptoms of acute gastroenteritis including non-bloody diarrhea, vomiting, and fever, but some develop more severe illness resulting in dehydration, seizures, and even death [ 2]. Diagnosis of rotavirus is based primarily on detecting rotavirus antigen in the feces of symptomatic patients (typically non-bloody diarrhea, vomiting, and fever). Tests used for diagnosis include enzyme-linked immunosorbent assay and latex agglutination testing which are the most common, and polymerase chain reaction which is the most sensitive [ 3, 4]. Dehydration is an important clinical sign of moderate-to-severe rotavirus infection and was traditionally a major cause of mortality from rotavirus-attributed gastroenteritis. The development and implementation of oral rehydration therapy has led to a significant decrease in mortality, with deaths falling from 4.6 million globally in 1982 [ 5] to 2.5 million in 2003 [ 6].
Multiple studies worldwide have shown declines in hospitalization rates following introduction of rotavirus vaccination into national immunization programs [ 7– 10]. Currently, four World Health Organization (WHO)-approved rotavirus vaccines are globally available: RotaTeq?(Merck & Co., Inc.), Rotarix?(GlaxoSmithKline Biologicals), ROTAVAC?(Bharat Biotech), and ROTASIIL (Serum Institute of India) [ 11].RotaTeq?was introduced into the Israeli national vaccination program from December 2010 [ 12].RotaTeq?is a live, oral human-bovine reassortant pentavalent rotavirus given as a three-dose series at 2, 4, and 6 months, and is administered at government well-child clinics (Tipat Chalav). Today, both RotaTeq?and Rotarix?are licensed for use in Israel.
While studies show a clear decline in rotavirus-attributed acute gastroenteritis following introduction of the vaccine, little is known about the vaccine’s protective efficacy over time, particularly among patients with varying background characteristics. A better understanding of how demographic, clinical, and laboratory characteristics may be predictive of disease severity is important in that it may guide clinical decision-making for patients presenting to the emergency department with rotavirus-attributed gastroenteritis.
The purpose of this study is thus twofold. First, we examine the impact of vaccination on rates of rotavirus-attributed acute gastroenteritis emergency room visits and illness severity at Bnai Zion Medical Center in the years following the introduction of routine rotavirus vaccinations compared to the pre-vaccination period. Second, we assess the protective impact of rotavirus vaccination among patients with varying risk factors and backgrounds (e.g., age, breast-feeding, and daycare attendance).
Methods
The current study is a descriptive, observational, and retrospective study. The study was conducted in the pediatric ward at Bnai Zion Medical Center, and included children who presented to the emergency room or were hospitalized with acute gastroenteritis and a stool positive antigen test for rotavirus between the years 2007 and 2019. As the RotaTeq?vaccine was added to the national vaccination program on December 1, 2010, the years 2010–2011 were excluded from the analysis. Demographic, clinical, and laboratory data, including stool antigen results, were collected and entered into an electronic database. Demographic and clinical data included age, sex, breast-feeding status, siblings at home, daycare attendance, duration of hospitalization, and need for intravenous fluid infusion during hospitalization. Laboratory tests included blood gas analysis, as well as creatinine and urea levels at admission and discharge.
Degree of dehydration was determined using the WHO scale of dehydration [ 13] on the basis of blood pressure, heart rate, skin turgor, skin perfusion, fontanelles, mucous membranes, tears, respiration, and urine output. Following the WHO, patients were divided into three degrees of dehydration: mild, moderate, and severe. However, as there were only six cases of severe dehydration, the moderate and severe dehydration groups were combined for the statistical analysis and compared to the mild dehydration group.
Parental reporting was used to ascertain the vaccination status of the recruited children. A study conducted in 2019 in Israel investigated the reliability of parental reports of rotavirus vaccination compared to the national immunization registry. The sensitively of parent reported was found to be high (up to 97%), with specificity of 75% [ 14]. Those findings validate the use of parental reporting in the current study. The study was approved by the institutional review board (approval number: 020-37BNZ). Informed consent was not needed.
Statistical analysis
Poisson regression was performed to evaluate the rate of yearly positive rotavirus cases. Comparisons of the two groups (pre- and post-vaccination) were performed via Chi-square test or Fisher’s exact test where appropriate for the categorical data and by the Wilcoxon two-sample test for the continuous variables. Odds ratios (ORs) and their 95% confidence intervals (CIs) were computed for select outcomes. Multivariate logistic regression was performed to predict dehydration level. Significance was considered to be P < 0.05. Statistical analysis was performed using SPSS software version 24 (SPSS, Chicago, IL).
Results
During the pre-vaccination period (2007–2009), 114 infants and young children (median age: 14 months, range: 1–72 months; 59 male, 55 female) were hospitalized for rotavirus-attributed acute gastroenteritis. The post-vaccination group (2012–2019) included 168 infants and young children (median age: 17 months, range: 0–84 months; 90 male, 78 female). There were a total of 23,296 emergency room visits in the pre-vaccination period (median 7705 a year) and 50,464 emergency room visits in the post-vaccination period (median 6248 a year). Across the entire study period, the rate of positive rotavirus tests was 7.34 per 1000 emergency room visits (95% CI 4.22–10.46). This represents a rate of 11.71 per 1000 (95% CI 7.21–14.67) during the pre-vaccination period (2007–2009), and 4.18 (95% CI 2.79–5.59) in the post-vaccination years (2012–2019)–a statistically significant decline ( χ 2 = 155.34, P < 0.001). Note that the pre-vaccine positivity rate was significantly lower in 2009 than in the previous 2 years (Fig.1).
Nearly two-thirds of the participants had siblings (190; 63.8%) and 53.8% (193) attended daycare. Nearly onethird of those aged 0–11 months were breast-feeding (45; 33.0%). There were 6 cases of severe dehydration and 51 cases of moderate dehydration. Table 1 presents patient characteristics at the time of hospitalization by time period. Although there was no difference in median age pre- and post-vaccination, there was a difference in age group distribution with a significantly greater proportion of patients 2 years or older in the post-vaccination group (35.1%) compared to the pre-vaccination group (21.1%; P < 0.01), and a significantly lower percentage of patients aged 1–2 years in the post- vs. pre-vaccination group (29.8% vs. 44.7%; P < 0.01).
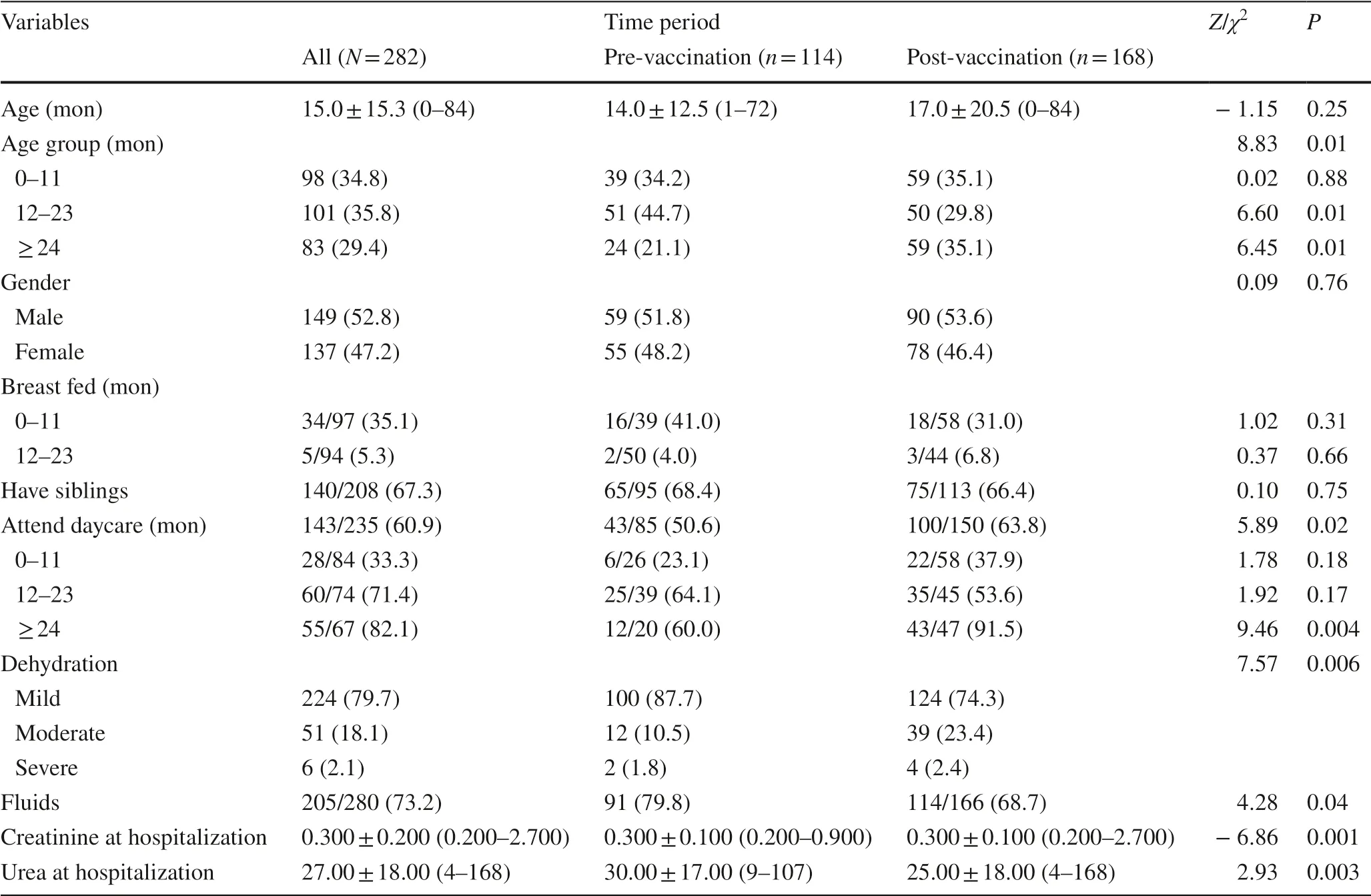
Table 1 Participant characteristics at hospitalization
There was no statistically significant distribution of gender, rate of breast-feeding, or siblings. A statistically significant higher percentage of post-vaccination patients attended daycare/nursery school (63.8% vs. 50.6%, P < 0.02). This difference in daycare/nursery school attendance was only evident in the patients 2 years or older (pre: 60.0% vs. post: 91.5%, χ 2 = 9.46, P < 0.002). There was a statistically significant distribution of severity in dehydration between the two time periods with a greater percentage of moderate or severe cases post-vaccination ( χ 2 = 7.57, P < 0.01). This was evident in patients 2 years or older (Fig.2; χ 2 = 4.07, P < 0.04). Creatinine and urea levels were significantly lower in postvaccination patients. There was a significantly lower use of fluids in the post-vaccination group.
Within the post-vaccination group, most of the patients had been fully or partially vaccinated against rotavirus. Of the 162 patients with known vaccination status, 70.4% (114/162) were fully vaccinated, 12.3% (20/162) were partially vaccinated, and 17.3% (28/162) were not vaccinated at all. There was no statistically significant difference in vaccination status between children aged 1–2 years of age and those 2 years and older ( χ 2 = 2.36, P > 0.31). In children over 1 year of age (103/162), 87.4% were fully vaccinated, 1.0% were partially vaccinated, and only 11.7% were not vaccinated. In infants younger than 1 year of age (59/162), 40.7% were fully vaccinated, 32.2% were partially vaccinated, and 27.1% were not vaccinated at all (most of the latter were infants younger than 2 months old).
Patient characteristics by dehydration status are presented in Table 2. There was a statistically significant difference in gender ( P < 0.02) with male patients 1.9 times more likely to suffer from moderate-to-severe dehydration than female patients (OR = 1.93, 95% CI 1.10–3.39). The odds of moderate-to-severe dehydration in the post-vaccination period were 2.4 times greater than in the pre-vaccination period (OR = 2.40, 95% CI 1.16–4.17). There was no significant difference between the different age groups in dehydration severity ( P > 0.10) or breast-feeding ( P > 0.46). There was a borderline significant difference in daycare attendance ( P > 0.08) with daycare attendance in the youngest group a significant risk factor for moderate-to-severe dehydration (OR = 2.71, 95% CI 0.97–7.53, P < 0.05).
Multivariate logistic regression of patient characteristics revealed that gender ( χ 2 = 5.11, P < 0.02) and time period ( χ 2 = 7.20, P < 0.007) both predicted moderate or severe dehydration. Male patients were more than twice as likely as females to suffer from moderate or severe dehydration (OR = 2.04, 95% CI 1.10–3.38). Patients in the post-vaccination period were nearly 2.5 times more likely to suffer from moderate or severe dehydration than in the pre-vaccination (OR = 2.48, 95% CI 1.28–4.82). Patients with moderate or severe dehydration had significantly lower pH and significantly higher creatinine and urea at admission as compared to patients with mild dehydration. After adjusting for age and gender, pH ( P < 0.007) and urea ( P < 0.001) were significant predictors of moderate or severe dehydration, while creatinine level was not ( P > 0.46).
Table 3 presents the outcomes by dehydration status. Nearly, all the patients with moderate or severe dehydration required fluids (96.5%), while only two-thirds (67.6%) of those with mild dehydration did so. Patients who suffered from moderate or severe dehydration had significantly higher creatinine at release, and also showed a greater change in creatinine, compared to patients with mild dehydration. While there was no difference in urea at release, patients who suffered from moderate or severe dehydration had a significantly greater change in urea levels compared to patients with mild dehydration. There was no difference in length of stay between the two groups with both having a median 2-day stay.
Patients’ characteristics by dehydration level and age group are presented in Table 4. Among patients under a year old, a significantly greater percentage of those with moderate/severe dehydration required fluids ( χ 2 = 12.71, P < 0.001) and they had a significantly longer length of stay ( Z = 2.01, P < 0.04). At hospitalization, pH levels were lower ( Z = 4.19, P < 0.001) and creatinine and urea levels higher in patients with moderate/severe dehydration as compared to their mild dehydration counterparts ( Z = 3.69, Z = 4.43, P < 0.001). In addition, creatine level was higher at release ( Z = 2.69, P < 0.01), and there was a significantly greater change in creatinine levels among patients with moderate/severe dehydration compared to their mild dehydration counterparts ( Z = 2.28, P < 0.02). The change in urea levels was also significantly different ( Z = 3.00, P < 0.003). There were no statistically significant differences among patients aged 1–2 years. In patients aged 2–7 years, there was a statistically significant difference in severe dehydration between the two periods with children aged 2–7 in the post-vaccination period having a significantly higher rates of severe dehydration compared to their pre-vaccination counterparts. At hospitalization, pH levels were lower ( Z = 3.18, P < 0.001) and creatinine levels higher in patients with moderate/severe dehydration compared to their mild dehydration counterparts ( Z = 1.96, P < 0.05).
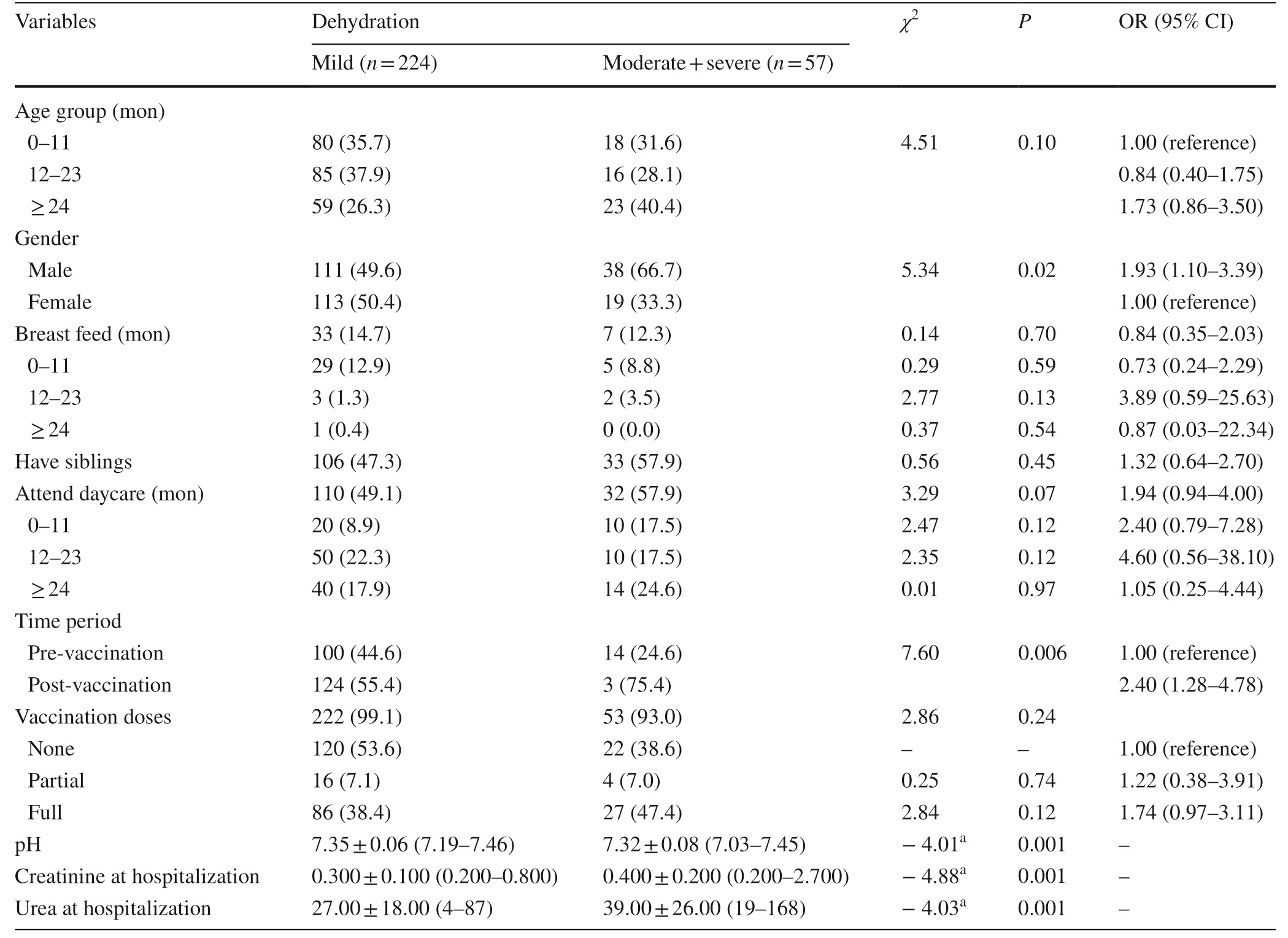
Table 2 Patient characteristics and lab data at hospitalization by dehydration status
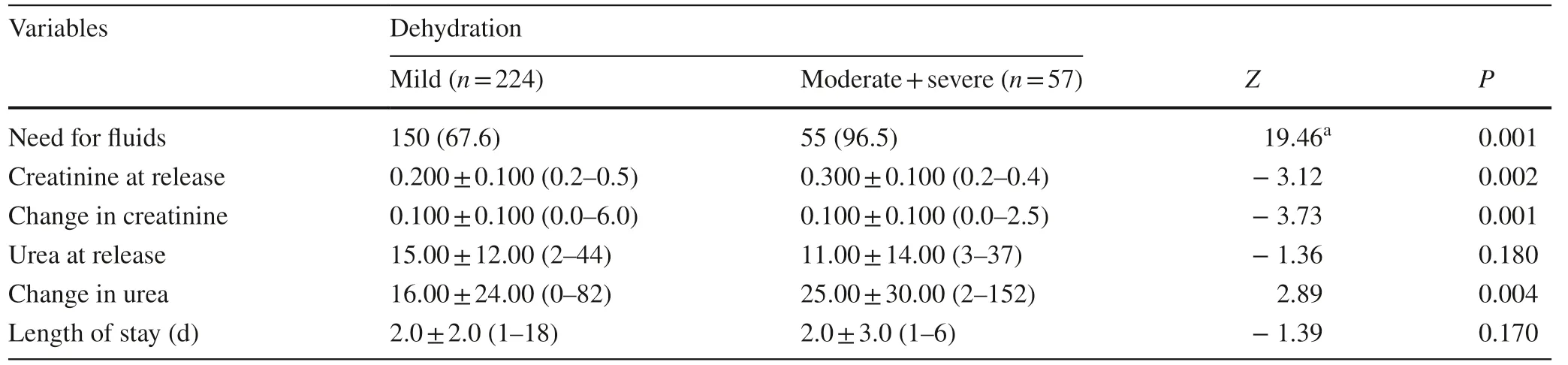
Table 3 Patient outcomes by dehydration status
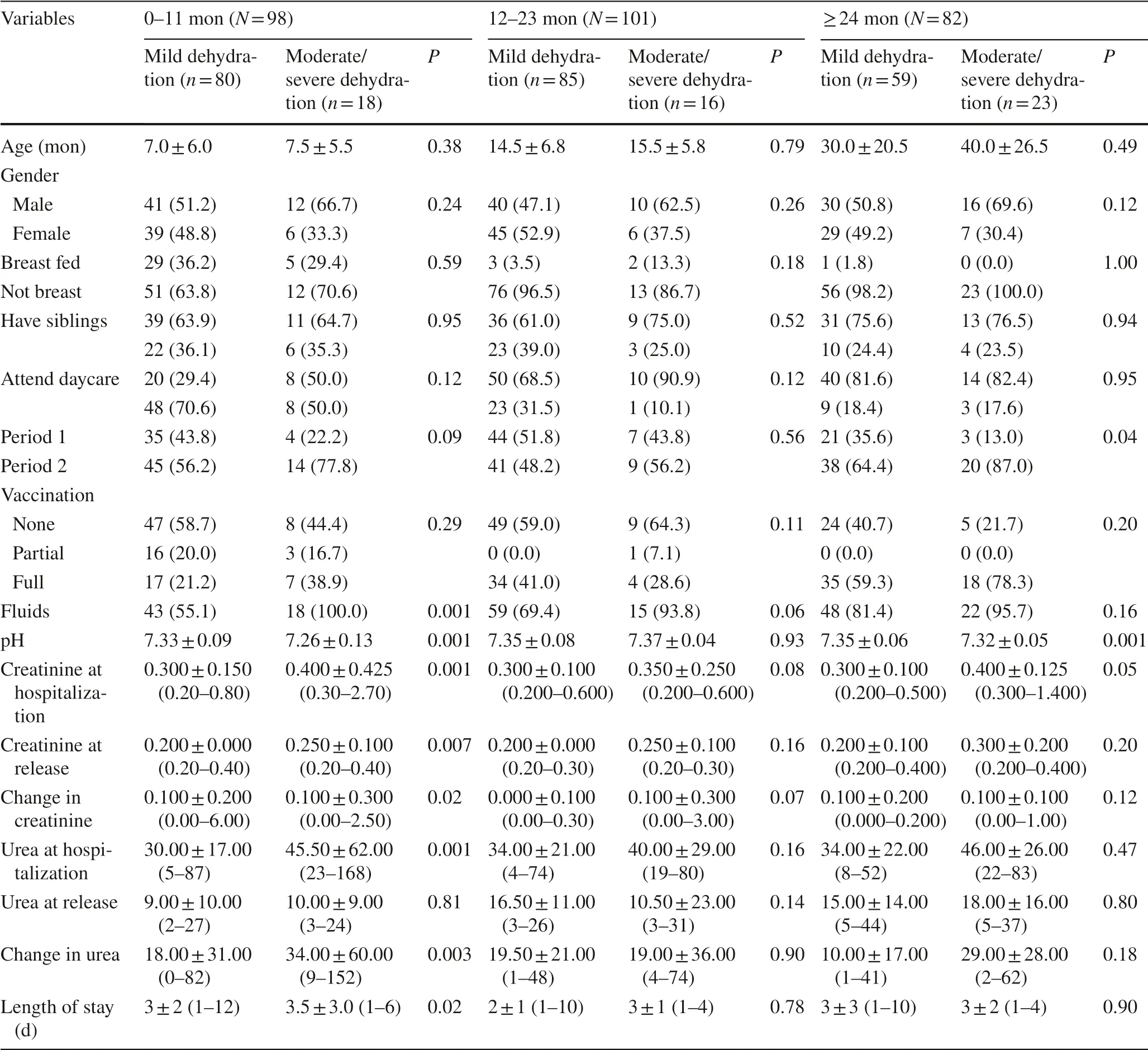
Table 4 Dehydration by age group
Table 5 presents the percentage of patients with moderate or severe dehydration in the post-vaccination era. Of the 161 patients in the post-vaccine period with known vaccination status, 8 (28.6%) patients who suffered from moderate/severe dehydration were not vaccinated, 4 (20.0%) were partially vaccinated, and 27 (23.9%) were fully vaccinated. Over all ages, there was no statistically significant difference between vaccine status and dehydration. A statically significant difference ( P < 0.04) was found in patients 12–23 months old, but post-hoc multiple comparison testing did not show any pairwise differences.
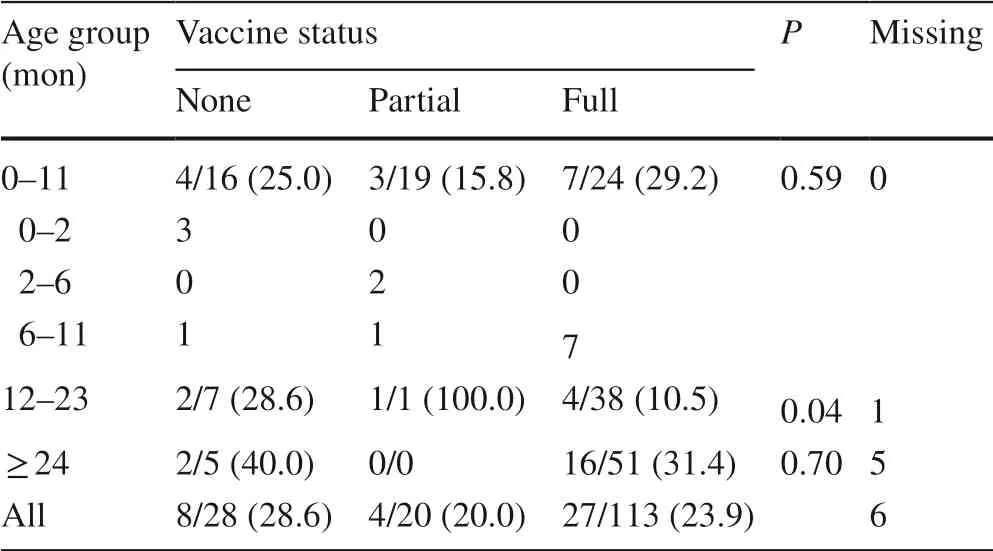
Table 5 Percentage of patients with moderate/severe dehydration in post-vaccination period
Discussion
Globally, the introduction of rotavirus vaccination has resulted in decreasing rates of acute rotavirus gastroenteritis [ 15– 20], albeit varying degrees [ 21]. Our study shows that this decreasing trend in morbidity also applies to emergency room visits (18.8%) and hospitalizations (31.98%). In Ireland, the use of Rotarix led to an 85.5% decrease in hospitalizations due to acute gastroenteritis in children under one of age, and an 86.5% decrease in children younger than 2 years old [ 22]. In addition, that study showed a decline in hospitalizations among children aged 2–5 who had not received the vaccine, a finding that suggests the existence of herd immunity. Those results are similar to the results of the current study showing a decrease in hospitalizations, although we note an age difference in hospitalized patient. Most hospitalized patients in Ireland study were children too young to have been vaccinated, while in our study, hospitalization was mainly children between 1 and 2 years of age who had been vaccinated. In New Zealand, the same trend of a decline in hospitalizations in the post-vaccination era was observed. The use of the RotaTeq?vaccine contributed to significant reductions in rotavirus gastroenteritis, at a rate of up to 92.6% in the vaccinated group [ 18].
We found that the chance of moderate or severe dehydration in the post-vaccination era was 2.4 times greater than in the period before. Moreover, children attending day care centers were more likely to be infected with rotavirus in the post-vaccination era, and those children were more likely to present with moderate or severe dehydration. Burke and colleagues [ 23] described the rotavirus outbreak in California in 2017. They found that children aged 2–3 years were particularly likely to suffer from rotavirus at rates of 43% and 37%, respectively. Those findings point to the waning effect of the vaccine and probably the need for another booster dose specifically for daycare children.
Concerns regarding the waning immunity of rotavirus vaccines were raised in several observations and clinical trials [ 24– 31]. Efficacy of the vaccine declined beyond the second year of life and this decline was more apparent in low- and middle-income countries, though it was also observed in high-income countries. Studies from low- and middleincome countries support the hypothesis that administering a booster beyond the first year of life will extend protection, and reduce morbidity and mortality rates [ 32– 34]. This picture should become clearer when results are reported from an ongoing study examining the efficacy of a third scheduled dose of Rotarix in Australian Indigenous infants, currently in phase IV of the clinical trial [ 35]
The Ruska Vesikari Clinical Severity Scoring System allows clinician to assess dehydration in children with diarrhea without the use of blood tests. However, several studies report improved accuracy in assessing the degree of dehydration by measuring serum urea and creatinine, particularly in young patients [ 36, 37]. Briefly, a rise in levels of urea and creatinine in severe hypovolemia reflects the decreasing rate of glomerular filtration. High urea levels are attributed to the increase in sodium and water reabsorption and urea recycling. However, only very high values of creatinine and urea can efficiently predict severe dehydration [ 36, 37]. In other study, blood gas was found to be better correlated with severity of dehydration [ 38]. In our study, we showed that low levels of pH and high levels of urea were more associated with moderate or severe disease than creatinine levels.
The current study has several limitations. First, it is a retrospective study conducted in a single secondary hospital in the north of Israel. Second, this study does not include children who suffered from mild-to-moderate disease and were managed in community clinics. Therefore, we have limited data regarding children who did not develop severe illness and who might have benefited from vaccination. Also, in our center, rotavirus testing is not a routine for all children with gastroenteritis, but is included in the workup only for children with suspected rotavirus infection. Thus, it is possible that some children with rotavirus seen in our center were not detected. Another limitation is the periods studied. We compare the immediate pre-vaccination period (2007–2009) and somewhat longer post-vaccination period (2012–2019). However, we do not have information regarding rotavirus hospitalizations in earlier years, before 2007, a period that could have included higher rates of hospitalizations due to rotavirus. In addition, some patients received the vaccine shortly before hospitalization, and this could have been the cause of their mild symptoms. Unfortunately, we did not have access to genotypic data which would have allowed us to distinguish between vaccine strains and circulating strains of disease. No moderate or severe disease was attributed to vaccine side effects.
In conclusion, we found a decline in the rate of rotavirus-attributed acute gastroenteritis hospitalizations in 2012–2019 following introduction of rotavirus vaccination, compared with the pre-vaccination period 2007–2009. In addition, the introduction of the rotavirus vaccine raised the average age of children diagnosed with rotavirus gastroenteritis. When comparing the degree of dehydration between the two groups, the proportion of cases with moderate-tosevere dehydration was higher in the post-vaccination children than in the pre-vaccination children. Ongoing surveillance is warranted to better understand the implications of rotavirus vaccination in the post-vaccination era and the need for a booster dose in vulnerable children. Author contributions All authors contributed to the study conception and design. ZH contributed to writing of the draft of the manuscript, material preparation, data collection, and analysis. HS and BE contributed to material preparation, data collection, and analysis. All authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding None.
Data availability The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical approval This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Institutional Review Board of Bnai Zion Medical Center (approval number: 020-37BNZ).
Conflict of interest No financial or non-financial benefits have been received or will be received from any party directly or indirectly to the subject of this article. The authors have no conflict of interest to declare.
 World Journal of Pediatrics2022年6期
World Journal of Pediatrics2022年6期
- World Journal of Pediatrics的其它文章
- Instructions for Authors
- Applications of mPCR testing reduced initial antibiotic use and duration of mechanical ventilation in virus-infected children with severe community-acquired pneumonia admitted to the PICU
- Serum homocysteine, lipid profile and BMI as atherosclerotic risk factors in children with numerical chromosomal aberrations
- Neonates are more vulnerable to symptomatic SARS-CoV-2 infection than children: a matched cohort study in Brazil
- Trichotillomania occurs during the COVID-19 pandemic in an adolescent
- Short-term effects of air pollutants on hospital admissions for acute bronchitis in children: a multi-city time-series study in Southwest China
