Clinical lesson learned from genetic analysis in patients prior to surgical repair of hypospadias
Nurin A.Listysri ,Gorjn Roevsk ,Ktie L.Ayers ,c,Tiong Yng Tn ,Andrew H.Sinclir ,c,Sultn M.H.Frdz ,e,*
a Division of Human Genetics,Center for Biomedical Research,Faculty of Medicine Diponegoro University,Semarang,Indonesia
b Murdoch Children’s Research Institute,Melbourne,Australia
c Department of Paediatrics,University of Melbourne,Melbourne,Australia
d Victorian Clinical Genetics Services,Murdoch Children’s Research Institute,Melbourne,Australia
e Diponegoro National Hospital,Semarang,Indonesia
In Indonesia,undervirilisation in 46,XY males is the most common form of difference of sex development(DSD).This can include hypospadias(misplacement of the urethra),micropenis,bifid scrotum,and undescended testis[1].Undervirilisation or 46,XY DSD can be associated with a number of congenital syndromes,including Smith-Lemli-Opitz Syndrome(OMIM 602858),caused by an inborn error of cholesterol synthesis,and characterised by growth delay,intellectual disability,microcephaly,distinctive facial features,cleft palate,limb anomalies,and hypospadias[2]or Opitz syndrome(also known as Opitz G/BBB syndrome).Opitz syndrome can be caused by variants in the X-linked midline 1(MID1)gene(Type I)or in an autosomal dominant manner by monoallelic variants in sperm antigen with calponin homology and coiled-coil domains 1-like(SPECC1L)on chromosome 22q11.2(Type II)[3].Opitz syndrome is characterised by hypospadias,hypertelorism,cleft lip/palate,and heart defects[4].The prevalence of X-linked Opitz syndrome is estimated to be from 1 in 50 000 to 1 in 100 000 males[5].Recognition of a syndrome informs appropriate clinical management and patient care.Therefore,although these syndromes are rare,hypospadias may be diagnosed before the emergence of other comorbidities meaning that it is crucial for clinicians to perform a thorough clinical evaluation with syndromic causes in mind.
We report two patients that were referred to our clinic with severe hypospadias.Thorough clinical and genetic investigation led to the confirmation of Opitz syndrome in both individuals.The study was approved by Health Research Ethics Committee,Faculty of Medicine Diponegoro University,Semarang,Indonesia(No.24/EC/FK-RSDK/I/2017).Written informed consents were obtained from all participants.Two unrelated male infants from two nonconsanguineous families were admitted to Diponegoro National Hospital with severe hypospadias.A detailed review of medical and family history was obtained,and a comprehensive clinical evaluation was carried out.
Cytogenetic analysis on G-banded metaphases from peripheral blood lymphocytes was carried out according to standard procedures.Genomic DNA was isolated from the peripheral blood lymphocytes using salting out technique.All nine exons and intron-exon boundaries of MID1 gene were amplified by polymerase chain reaction using primers pairs as reported by Hu et al.[6]except for exon 4 which was amplified using reverse primer (5′-GTATCACAGGTCTTCACAGGAGAC-3′).Polymerase chain reaction amplification was performed using high-fidelity Phusion polymerase(NEB,Ipswich,MD,USA).
The results were aligned with reference sequences GenBank NM_000381.3 and NP_000372.1.Variants were curated based on the American College of Medical Genetics(ACMG)guidelines[7],with classifications into one of five categories:Pathogenic,likely pathogenic,variant of uncertain significance,likely benign,or benign.Variants were evaluated for pathogenicity using Mutation Taster(http://www.mutationtaster.org),SIFT(http://sift.jcvi.org/www/SIFT_enst_submit.html),and PolyPhen-2(http://genetics.bwh.harvard.edu/pph2/).
An 8-month-old boy(individual 1)presented with micropenis,perineal hypospadias,left cryptorchidism,and 5-mL right palpable testis.He was the first-born child to nonconsanguineous parents with no relevant family history(Fig.1A—1D).He was born with left-sided cleft lip and had it repaired when he was 3 months old.Additional features include hypertelorism,broad nasal bridge,and anteverted nostril(Fig.1B).Developmental delay was noted;he required support to sit and was unable to keep his head level with his body whilst sitting,which may be a symptom of a stridor of presumed laryngomalacia.His mother was healthy with normal intelligence,but she had hypertelorism(Fig.1C).His serum follicle-stimulating hormone(FSH),luteinizing hormone(LH),and testosterone were normal for his age(FSH:1.34 IU/L[normal range 0.16—3.50 IU/L];LH:0.35 IU/L[normal range 0.70—1.20 IU/L];testosterone:<2.5 nmol/L[normal range<2.5 nmol/L]).
A normal 46,XY karyotype was confirmed.Sequencing revealed a novel single base pair duplication of a thymine at position 904 of the MID1 gene cDNA c.904dupT.The change is predicted to lead to a frameshift at cysteine 302 of the protein to a leucine premature stop codon 6 amino acids later in the coiled-coil protein domain p.Cys302Leufs*6(Fig.1D).This variant was absent in dbSNP,the 1000G,ESP,ClinVar,ExAC,and gnomAD databases.The mother is a heterozygous carrier,suggesting X-linked inheritance.Curation using ACMG guidelines placed this variant as likely pathogenic.

Figure 1 The characteristics of the X-linked Opitz syndrome patients in this study.(A)The pedigrees of Family 1;(B and C)Hypertelorism showing in the frontal view of the proband and mother in Family 1;(D)Chromatograms of MID1 gene sequence and the affected index of the proband and mother in Family 1.Family 1 showed c.904dupT leading to p.Cys302Leufs*6 with mother is a heterozygous carrier.(E)The pedigrees of Family 2;(F and G)Hypertelorism showing in the frontal view of the proband and mother in Family 2;(H)Chromatograms of MID1 gene sequence and the affected index of the proband and mother in Family 2.Family 2 showed c.1322C>G leading to p.Pro441Arg with mother is a heterozygous carrier.Probands are hemizygous for the variant alleles.Arrow,the pathogenic variant position.
A 2-year-old boy(individual 2)presented at our university hospital with severe penoscrotal hypospadias and palpable scrotal testes confirmed by ultrasound.Parents were non-consanguineous but had a history of miscarriages(Fig.1E).He had hypertelorism,prominent forehead,flat occiput,broad nasal bridge,and scars from previous surgery for a right-sided cleft lip(Fig.1F),and had surgically repaired anal atresia.FSH and LH were 0.17 IU/L(normal range 0.70—1.20 IU/L)and 2.05 IU/L(normal range 0.16—3.50 IU/L),respectively,while his testosterone at 8 months of age was normal(<2.5 nmol/L,normal range<2.5 nmol/L).He had a 46,XY karyotype.Opitz syndrome was suspected.Furthermore,his mother had hypertelorism without any other dysmorphic findings(Fig.1G).Sequence analysis of MID1 revealed a novel missense variant,c.1322C>G in exon 8(Fig.1H).The p.Pro441Arg change falls within the fibronectin type III domain of the MID1 protein,a region of missense constraint.Sanger sequencing confirmed that his mother was a heterozygous carrier suggesting an X-linked inheritance pattern.This variant was absent from dbSNP,the 1000G,ESP,ClinVar,ExAC,and gnomAD databases.Curation placed this variant as of uncertain significance(unambiguously classifiable with predominantly pathogenic evidence)—something that could be resolved with further segregation in family members.
Opitz syndrome was diagnosed in two patients initially referred to our clinic for undervirilisation or hypospadias.Our patients were examined initially by a paediatric urologist;however,recognition of a possible syndromic association led to referral to our multidisciplinary gender team.Identification of dysmorphic features included hypertelorism and cleft lip in our patients leading to targeted sequencing of MID1 gene.A diagnosis of syndromic conditions allows more comprehensive medical intervention and clinical care.Thus,with DSDs often identified in neonates sometimes before the emergence of additional comorbidities,it is important to carry out a thorough clinical evaluation for additional dysmorphic features that may suggest a wider syndrome.
The identification of a pathogenic MID1 variant need prompt consideration of genetic testing in other family members including for X-linked inheritance from mothers.Here,both mothers had only hypertelorism in accordance with previously published data suggesting this is the most common feature in female carriers[8].Other clinical features in carriers have been reported such as short uvula or lack of an incisor to more severe presentation such as cleft lip and palate[9].For the children and families reported here,the impact of the diagnosis led to changes in the management plan which had to not only consider the surgical schedule but also optimise growth and cognitive ability as well as precise genetic counselling.
In a review by Maia et al.[8]describing 91 individuals with X-linked Opitz syndrome,the major clinical characteristics were hypertelorism(98.9%),hypospadias(91.9%),laryngotracheo-esophageal defects(86.4%),clefts of the lip and/or palate(68.9%),and intellectual disability(56.9%).Minor features include structural anal deformities(44.4%),brain(42.9%),and heart defects(38.9%).To the best of our knowledge,91 different variants of MID1 have been reported so far[8].Our study has identified two new variants.The clinical features observed in our patients were generally concordant with those previously published with pathogenic variants affecting the same exon(Table 1)[4,5,8].Patient 2 had anal atresia,a feature of OS,but not previously observed in patients with a variant in exon 8 of MID1.
Our findings emphasized the significance of obtaining a molecular diagnosis,which was informed by thorough phenotyping of both the proband and their parents.This is especially true in developing countries where molecularservices for genetic diseases are still limited,and syndromic cases may first present to specialist teams concerned with a specific organ system(in this case the DSD with paediatric urologist).
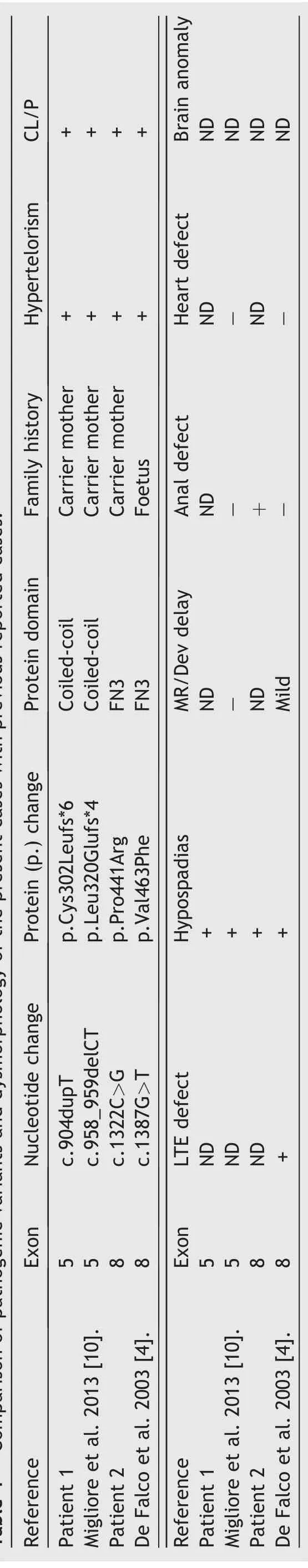
Table 1 Comparison of pathogenic variants and dysmorphology of the present cases with previous reported cases.
In conclusion,we identified two familial cases of Xlinked Opitz syndrome that were caused by novel hemizygous variants in the MID1 gene,and only after patients presented to our clinic with hypospadias and additional dysmorphologies were noted.It highlighted the importance of carrying out a thorough clinical analysis and demonstrated how a molecular diagnosis gives a foundation for genetic counselling for the families.
Author contributions
Study concept and design:Sultana M.H.Faradz,Andrew H.Sinclair.
Data acquisition:Nurin A.Listyasari,Tiong Yang Tan.
Data analysis:Nurin A.Listyasari,Gorjana Robevska.
Drafting of manuscript:Nurin A.Listyasari,Gorjana Robevska,Tiong Yang Tan.
Critical revision of the manuscript:Katie L.Ayers,Sultana M.H.Faradz,Andrew H.Sinclair.
Conflicts of interest
The authors declare no conflict of interest.
Acknowledgements
We would like to thank the patients and their families for their participations in this study.We also thank the members of gender team.Kariadi Hospital/Faculty of Medicine Diponegoro University who helped us in patient assessment.This study was funded by the Diponegoro University WCRU grant(No.118-03/UN7.6.1/PP/2021).
 Asian Journal of Urology2022年2期
Asian Journal of Urology2022年2期
- Asian Journal of Urology的其它文章
- Manuscript Guide for Authors
- Encrusted cystitis and ascites due to urethral calculus
- Clinical features and management of ureteric stump syndrome:Singlecentre experience and contemporary literature review
- Impact of coronavirus disease 2019 on semen parameters
- Factors influencing the degree of participation in surgical decision-making among Chinese patients with prostate cancer:A qualitative research
- Impact of delay from transperineal biopsy to radical prostatectomy upon objective measures of cancer control
