Significantly enhancing fracture toughness of epoxy composite with promising γ-FeOOH@Fe2O3 hybrid nanoparticles by magnetic field assistance
Gun Chen ,Chunguo M,b,* ,Zeho Fu ,Jing Wng ,Peibng Di,b
a School of Material Science and Engineering,Guilin University of Electronic Technology,Guilin,541004,China
b Guangxi Key Laboratory of Information Materials,Guilin,541004,China
Keywords:A.polymer-matrix composites (PMCs)A.Carbon fibres A.Nanocomposites B.Fracture toughness
ABSTRACT The toughening of epoxy resin(EP)and the interlaminar toughening of carbon fiber reinforced composite(CF/EP)laminates have been widely concerned.In this work,the needle-like γ-FeOOH nanoparticles were prepared by liquid phase deposition-air oxidation method,and then were calcined under different conditions to obtain γ-FeOOH and γ-Fe2O3 hybrid nanoparticles (γ-FeOOH@Fe2O3).Effect of calcination condition of γ-FeOOH@-Fe2O3 and magnetic field assistance on fracture toughness (KIC) of EP was systematically investigated.Then the selected γ-FeOOH@Fe2O3 with the best toughening effect were used to improve the mode I interlaminar fracture toughness (GIC) of CF/EP laminate.The resulting γ-FeOOH@Fe2O3 have a length of around 1 μm,a diameter of around 100 nm and the Ms of 8.99–45.96 emu/g.After calcinated at 250 °C for 1 h,the γ-FeOOH@Fe2O3 containing 24 wt%FeOOH and 76 wt%Fe2O3 achieved the best toughening effect.Under a magnetic field of 0.09 T,the KIC of the γ-FeOOH@Fe2O3/EP composite(2.45 MPa m1/2)is 81.7%and 66.7%higher than that of neat epoxy and the composite without magnetic field induction,respectively.Furthermore,the GIC of the γ-FeOOH@Fe2O3/CF/EP composite (0.914 kJ/m2) is also significantly increased by 88.8% and 51.8% compared to that of CF/EP and the corresponding composite without magnetic field induction,respectively.
1.Introduction
As a kind of important thermosetting resin,epoxy resin(EP)has been widely used in many fields,and is the important matrix resin of carbon fiber (CF) reinforced composite materials due to its advantages of high rigidity,good adhesion,high thermal stability,good chemical resistance and low cost [1].However,due to the high crosslinking density,the fracture toughness of EP is usually poor,especially for low viscosity EP used in the resin transfer molding process [2].In CF reinforced EP composite undergoing high-speed impact or stress fatigue,cracks are easy to be induced between CF layers,resulting in the interlaminar failure of the composite,which is a widely concerned and urgent issue[3–6].
In the recent decades,toughening methods of EP have been the research hotspots,and extensive efforts have been made [1–3].In general,there are two main strategies for improving the fracture toughness of EP:one is to incorporate high toughness polymer into EP,such as elastomer [2] and particular engineering thermoplastic resins [7],and the other is to incorporate nanoparticles into EP,which can balance the contradiction between toughening and strengthening to achieve the purpose of toughening without declining strength and modulus.Inorganic nanoparticles like SiO2[8],TiO2[9],Fe2O3[10],Halloysite [11]and clay [12,13] are commonly used in the modification as well as carbon nanoparticles like carbon black (CB) [14],multi wall carbon nanotube (MWCNT) [15–18],carbon nanofiber (CNF) [19],graphene oxide(GO) [20,21],reduced graphene oxide (RGO) [20] and graphene nanoplate(GNP)[22].Table 1 shows comparison of fracture toughnessKICof representative EP composites with loading rigid nanoparticles in recent years.The involved different nanoparticles can significantly improve the fracture toughness of EP,and the type,content and surface modification of the nanoparticles are the important factors affecting the modification effect[2].
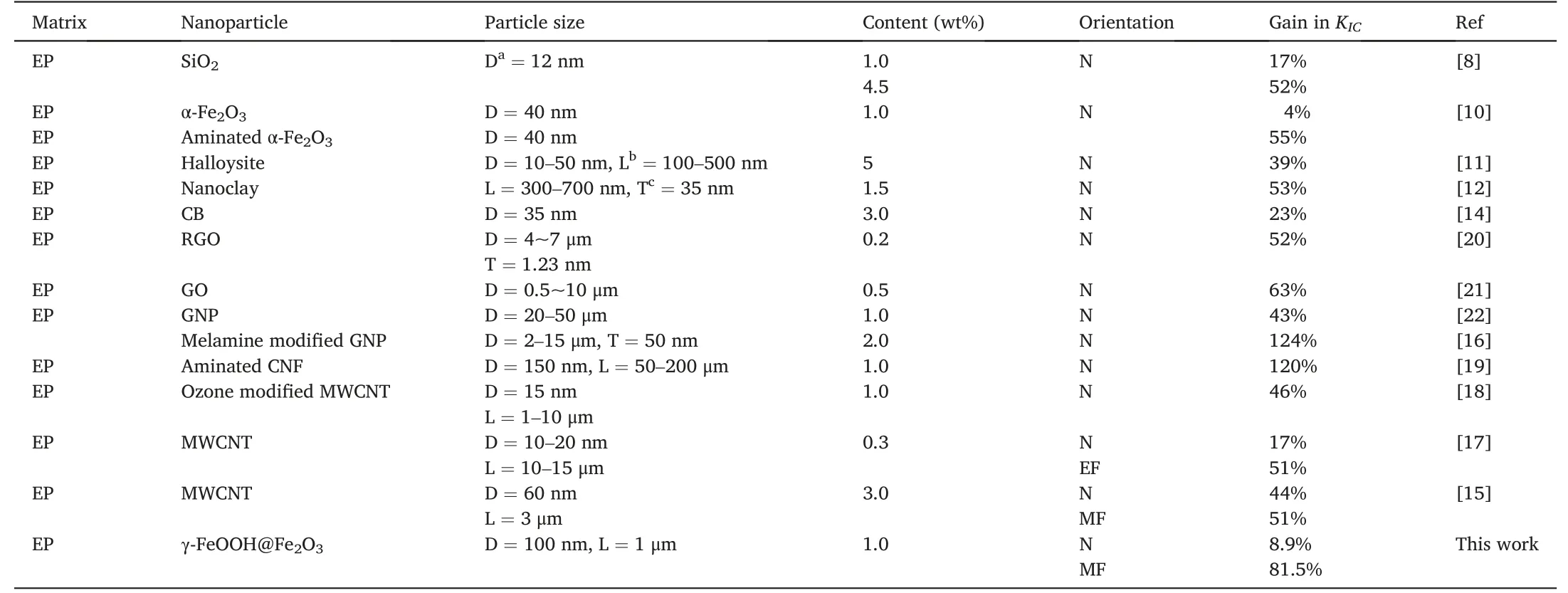
Table 1 Comparison of fracture toughness KIC of representative EP composites with loading rigid nanoparticles.
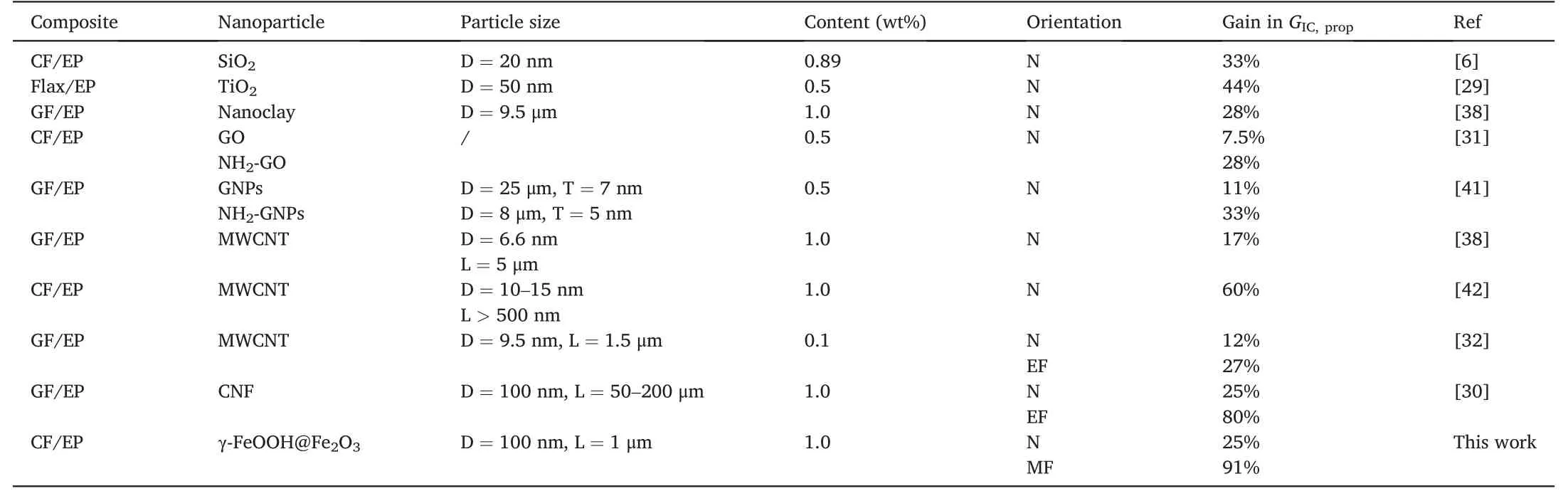
Table 2 Comparison of the mode I-interlaminar fracture toughness GIC,prop of representative fiber composites with loading rigid nanoparticles.
Compared with inorganic nanoparticles,carbon nanomaterials can achieve more remarkable toughening effect at a lower content[19–22].The fracture toughness of most composites is about 50%higher than that of pure epoxy resin.Generally,the toughening effect can be furtherachieved by appropriate surface modification such as the amino modification[10,19].Cha et al.[16]found that melamin functionalized GNP at 2 wt% content enhanced the fracture toughness of EP by 124%,which is the highest value reported in the current literature.Anwar et al.[19]showed that the fracture toughness of EP is increased by 120% with 1 wt% the amino CNF modified by silane coupling agent.In addition,under the induction of magnetic field(MF)[15],electric field(EF)[17]or stress field [23],the orientation of nanoparticles can further improve the toughening effect.Usually,magnetic nanoparticles modification is used to improve the MF responsiveness of carbon nanotubes or graphene,achieving more easy orientation under a low MF [23].In our previous study [15],it was found that the orientation of MWCNT containing catalytic Ni nanoparticles was perpendicular to the fracture surface under the induction of a low MF (0.4T),which improved theKICat 3 wt% MWCNT by 51% compared to pure EP.Khan et al.[17] reported that alignment of MWCNT under DC EF enhances theKICby 51% compared to pure EP,and more effective toughening than random orientation of MWCNT(17%).Moreover,the synergistic effect of the two nanoparticles has also been proved to be an effective way to significantly improve the fracture toughness of EP[24].
In the same way,different methods have been developed to improve the interlaminar failure of carbon fiber-reinforced composites,mainly including toughening matrix [4,25],Z-pin [26],stitching [26],3D weaving[27] and interleaf modification[28].As far as toughening matrix is concerned,it is still an important method to improve the interlaminar toughness (GIC) of fiber-reinforced composites.The above methods about toughening epoxy resin are also suitable for toughening the interlaminar toughness of fiber-reinforced composites.The toughening modification of rubber and thermoplastic engineering resin generally leads to a significant increase in viscosity of EP,which limits its application in the traditional manufacturing process of fiber composite.While the nanoparticle modification can achieve significant toughening effect at low loading with little negative effect on the manufacturing process.
Table 2 shows the comparison of the mode I interlaminar propagation fracture toughnessGIC,propof typical fiber composites enhanced by rigid nanoparticles.Overall,carbon nanoparticles have better improvement onGICthan inorganic nanoparticles.At the low loading of no more than 1 wt%,the improvement ofGICby the inorganic nanoparticles is generally no more than 44% [29],while the improvement ofGICby carbon nanoparticles is up to 80%[30].Both the surface modification[31]and the orientation of nanoparticles with the help of electric field[30,32]can also further improve the toughening effect.
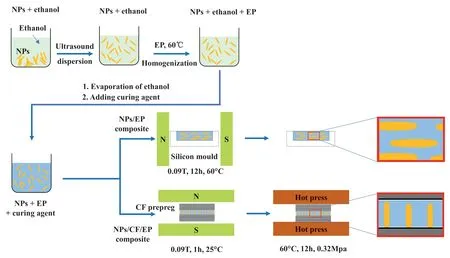
Fig.1.Schematic diagram of preparation of γ-FeOOH@Fe2O3 nanoparticles (NPs) modified EP composite and CF/EP composite laminate.
Fe2O3nanoparticles as a kind of common inorganic nanoparticles with many crystal forms such as α-Fe2O3,β-Fe2O3and γ-Fe2O3are widely used in catalysts,battery electrodes,gas sensors,magnetic materials and absorbing materials [33–35].In addition,Fe2O3nanoparticles are also promising modifiers to prepare high-performance coatings,reinforced plastics and multi-functional polymer composites[10,36].Sun et al.[10]synthesized a kind of cubic α-Fe2O3nanoparticles with a particle size of 40 nm,which increasesKICof EP by 55% at the content of 1 wt% after coupling agent modification.Balguri et al.[36]prepared a kind of flower like α-Fe2O3,which can increase the notched impact strength of the EP composite by 59% at the content of 0.1 wt%.These studies show that Fe2O3nanoparticles are potential for toughening epoxy resin.γ-Fe2O3nanoparticle are one kind of typical one-dimensional nanomaterial with needle-like structure,and are generally prepared by calcining its precursor γ-FeOOH in air [34].γ-Fe2O3nanoparticles have high saturation magnetization so that they are easy to be aligned in a low MF.However,it has rarely been reported that γ-Fe2O3or γ-FeOOH was used to enhance the fracture toughness of epoxy resin and the interlaminar toughness of reinforced fiber reinforced epoxy resin composite.
In our previous study[37],it was found that γ-FeOOH nanoparticles have relatively low saturation magnetization,and are difficult to be aligned under a weak MF (<0.1T).Although the saturation magnetization (Ms) of γ-Fe2O3obtained by calcination of γ-FeOOH at 350°C is significantly increased and their responsiveness to weak MF is enhanced,the calcination process causes serious agglomeration of the γ-Fe2O3nanoparticles.On the other hand,the hydroxyl content on the surface of the γ-Fe2O3is greatly decreased,resulting in poor dispersion in the epoxy resin and serious decline in the interface interaction with the epoxy resin.Accordingly,there is slightly improvement of 7% in theKICof γ-Fe2O3/EP composite compared to that of pure EP.Therefore,obtaining sufficiently highMs and good dispersion is the key to the good toughening effect of γ-Fe2O3nanoparticles.Surface modification of γ-Fe2O3is a commonly used strategy,such as coupling agent treatment,but this will inevitably lead to the complexity of the process and the increase in cost.
Herein,a simpler γ-Fe2O3modification strategy was proposed to achieve significant toughening of epoxy resin composites under a weak magnetic field assistance.Instead of surface modification of γ-Fe2O3,the γ-FeOOH@Fe2O3hybrid nanoparticles consisting γ-FeOOH and γ-Fe2O3components were prepared by optimizing the preparation process,retaining the needle-like shape.In the γ-FeOOH@Fe2O3,γ-Fe2O3component ensures that the nanoparticles have a good response to the weak MF,and γ-FeOOH component keeps a proper amount of surface hydroxyl on the nanoparticles to achieve its affinity to EP.Then the γ-FeOOH@Fe2O3hybrid nanoparticles were used to improve the fracture toughness of EP and the mode I interlaminar fracture toughness of CF/EP composite under MF induction.The relationship between the composition of the γ-FeOOH@Fe2O3hybrid nanoparticles and calcination conditions was studied systematically.The influence of the hybrid nanoparticles on the toughening of the composite was investigated to obtain the optimal preparation conditions for the hybrid nanoparticles were obtained,which is of significance for exploring new potential toughened nanomaterials.In addition,the corresponding toughening mechanism was clarified by SEM analysis.
2.Experimental
2.1.Materials
FeCl2·4H2O (ferrous chloride tetrahydrate),NaOH (sodium hydroxide) and ethanol were purchased from China Xilong Chemical Co.,Ltd.All the reagents were analytical grade and used as received without further purification.Epoxy resin(Bisphenol type,RIMR-135)and curing agent(Modified polyether amine,RIMH-134)were produced by Hexion Inc,and have relatively low viscosity at room temperature and widely used in vacuum assisted resin transfer molding process of fiber composite.Unidirectional carbon fiber fabric (T700-12K) was produced by Japan Toray Corporation.
2.2.Synthesis of needle-like nanoparticles
The needle-like γ-FeOOH@Fe2O3nanoparticles were prepared by the two-step method[34,35].The precursor γ-FeOOH was first prepared by chemical precipitation-air oxidation method,and then calcinated at different conditions of temperatures and time to obtain needle-like γ-FeOOH@Fe2O3hybrid nanoparticles with different components.
2.3.Preparation of composites
Fig.1 shows schematic diagram of preparation of γ-FeOOH@Fe2O3/EP composite and γ-FeOOH@Fe2O3/CF/EP composite laminate.The well dispersed EP suspension containing 5 wt% γ-FeOOH@Fe2O3nanoparticles was prepared by liquid transfer method [15],and was further mixed with EP in a certain proportion to obtain other diluents with different γ-FeOOH@Fe2O3contents.At the mass ratio of EP to curing agent 100:30,the corresponding diluent and curing agent were mixed evenly,followed by degassing for 15 min in vacuum oven.Then the mixture was poured into a silicon mold under the MF of 0.09 T and cured for 12 h at 60°C to obtain the γ-FeOOH@/EP composite with MF induction.As shown in Fig.1,the needle-like γ-FeOOH@Fe2O3nanoparticles were aligned in the direction of MF and kept in the alignment state after EP curing.
The γ-FeOOH@Fe2O3/CF/EP laminates were prepared by manual,and the typical process is as follows:firstly,the CF prepreg was prepared by manual brushing the mixture of EP and curing agent at room temperature,and consisted of the 6 layers of CF fabrics oriented in the same direction and had a fiber content of around 55 wt% by draining the excess mixture with vacuum assistance in a vacuum bag.Then the diluent with 1 wt%γ-FeOOH@Fe2O3content was coated onto one piece of the CF prepreg and covered with another one piece to obtain the composite prepregs with 12 layers of CF fabrics.A piece of PTFE film with thickness of 25 μm was inserted between the two pieces of the prepregs at the front to obtain a prefabricated crack.Then the composite prepregs was placed in a MF of 0.09T where the induced orientation of γ-FeOOH@Fe2O3in the diluent was carried out for 60 min.After that,the EP in the composite was partially cured and the orientation state of γ-FeOOH@Fe2O3was maintained,which is shown by the corresponding local enlargement in Fig.1.Finally,the composite was further cured for 12 h at 0.32 MPa pressure and 60°C to obtain 1 wt%γ-FeOOH@Fe2O3/CF/EP composite laminate with MF induction.
In addition,as control samples,pure EP,γ-FeOOH@Fe2O3/EP composite without MF induction,CF/EP and γ-FeOOH@Fe2O3/CF/EP composite laminates without MF induction were prepared at the same conditions.Measured according to ASTM D2734 method A,the fiber content and void content of the different composite laminates were around 73 wt% and 4%,respectively.Apparently,under the higher pressure in the molding stage,the resin was further drained and the fiber content was increased.
2.4.Characterization
The phase composition of γ-FeOOH@Fe2O3nanoparticles was characterized by X-ray diffraction (XRD,BRUKER,D8-2-Advance),using an X-ray diffractometer with a Cu Kα radiation (λ=1.5418 ?).The magnetization of the nanoparticles with respect to the applied field were carried out using a Vibrating Sample Magnetometer (VSM,Lakeshore,7400-S) with a maximum magnetic field of 20 kOe at room temperature.The thermal decomposition behavior of the nanoparticles sample(~5 mg)was studied using a thermogravimetry analysis(TGA,Netzsch,STA449 F3),which operated at argon atmosphere with the flow rate of 20 ml/min and scanning rate of 10°C.Field emission scanning electron microscope(FE-SEM,FEI,Quanta FEG 450)was employed to observe the surface morphology of nanoparticles with an operating voltage of 20.0 kV,and further to investigate the toughening mechanism of the nanoparticles by fracture surface morphological analysis of the composites with an operating voltage of 5.0 kV.Before examination,the nanoparticle and the fracture surface were coated with a thin evaporated gold layer to improve the electrical conductivity.
The mechanical testing was performed on a universal testing machine(Shimadzu,SPL-10 kN) at room temperature.The stress intensity factor(KIC) was used to evaluate the mode I fracture toughness of the nanoparticles modified EP composite according to ASTM D5045 in a threepoint end notch bending(3P-ENB) test mold.The single edge notched bending(SENB)specimen for 3P-ENB test had the geometry of 52.8 mm length (L) ×6 mm thickness (B) ×12 mm width (W).The tests were carried out at a displacement rate of 1 mm/min using a span of 48 mm.The specimens were pre-cracked using a razor blade before testing.At least six specimens from each set were tested.KICwas calculated using the formula(1) and(2)[14].
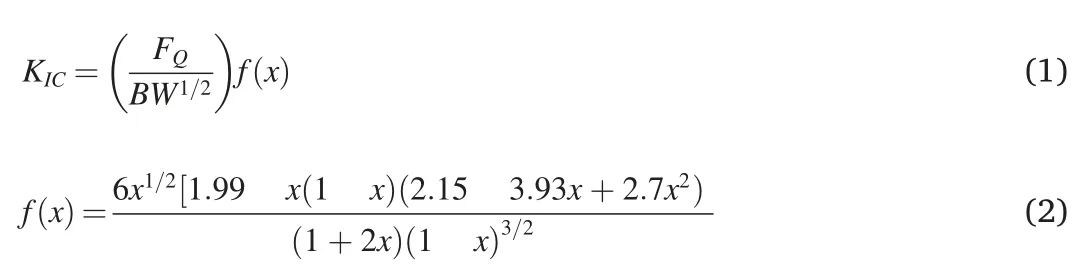
WhereFQis maximum load,BandWare the specimen thickness and width,respectively,andais the crack length andx=a/W(0.45<x<0.55).
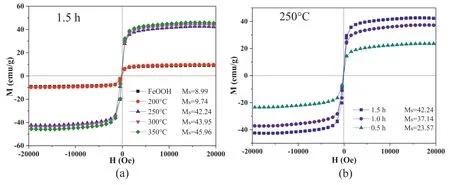
Fig.3.Hysteresis loops of γ-FeOOH and γ-FeOOH@Fe2O3 prepared at different calcination conditions:(a) at different temperature for 1.5 h and (b) at 250 °C for different time.
The interlaminar fracture toughness of the composite laminates was evaluated by the mode I strain energy release rate(GIC)according to the ASTM D5528 standard using the modified beam theory (MBT) method[38].In the static double cantilever beam(DCB)test,the specimens with geometry of 150 mm length×20 mm width×3 mm thickness and the initial crack lengths of 50 mm were loaded through two loading blocks at a displacement rate of 5 mm/min.At least three specimens from each set were tested.theGICvalue was calculated using the formula(3)[28,38].

WhereP,δ,b,aand Δ are the load,the deflection at the load point,the specimen width,the delamination length and the correction factor,respectively.
3.Result and discussion
3.1.Characterization of the nanoparticles
XRD patterns and hysteresis loops of the precursor γ-FeOOH and its derivatives calcinated at different conditions are shown in Fig.2 and Fig.3,respectively.After calcination at 350°C for 1.5 h,the diffraction peaks of γ-FeOOH disappeared completely and only the diffraction peaks of γ-Fe2O3were observed,which indicates that all γ-FeOOH produced to γ-Fe2O3due to dehydration of γ-FeOOH under this calcination condition.TheMs of γ-FeOOH and γ-Fe2O3was 8.99 emu/g and 45.96 emu/g,respectively,which suggests that γ-Fe2O3is obviously easier to be aligned by MF than γ-FeOOH.When γ-FeOOH was calcined at 200°C for 1.5 h,the diffraction peaks of γ-FeOOH did not change and no diffraction peaks of γ-Fe2O3were observed.In addition,theMs of the corresponding product was 9.74 emu/g and very close to that of γ-FeOOH.These results indicate that γ-FeOOH almost cannot produce Fe2O3under this calcination condition.
The diffraction peaks of γ-FeOOH almost disappeared when the calcination temperature was increased to 250°C and 300°C,and the diffraction peaks corresponding to (311) (400) (440) planes of γ-Fe2O3appeared.TheMs values of the corresponding products were close to that of the product γ-Fe2O3calcined at 350°C,showing 42.24 emu/g and 43.95 emu/g,respectively.However,compared to that of the calcined product at 350°C,the diffraction peaks were weaker and relatively broader,which implies that γ-FeOOH has also almost completely produced Fe2O3,but the crystallization was still not well developed.
After the calcination at 250°C for 0.5 h,the γ-FeOOH diffraction peaks were still obvious,and the three weak γ-Fe2O3diffraction peaks were observed,indicating that a part of γ-FeOOH was dehydrated to produce γ-Fe2O3.The resulting product exhibiting aMs of 23.57 emu/g was a γ-FeOOH@Fe2O3hybrid composed of the main component γ-FeOOH and the secondary component γ-Fe2O3.When the calcination time was extended to 1 h,only two weak γ-FeOOH diffraction peaks were retained,while the Fe2O3peaks were significantly enhanced.This indicates that Fe2O3became the main component in the γ-FeOOH@Fe2O3hybrid with aMs of 37.14 emu/g.When the calcination time was further extended to 1.5 h,the γ-FeOOH diffraction peaks disappeared,suggesting that almost all the γ-FeOOH produced γ-Fe2O3.
TheMs of the γ-FeOOH@Fe2O3hybrid is the sum of theMs of the component γ-Fe2O3and the component γ-FeOOH.Using theMs of pure γ-Fe2O3and pure γ-FeOOH,the weight percentage of the components in the γ-FeOOH@Fe2O3hybrid can be calculated.For example,At 250°C for 1 h,the γ-FeOOH@Fe2O3nanoparticles was composed of 24 wt% γ-FeOOH and 76 wt% γ-Fe2O3.The content of γ-FeOOH in γ-FeOOH@-Fe2O3was 61 wt% at 250°C for 0.5 h,10 wt% at 250°C for 1.5 h,5 wt% at 300°C for 1.5 h,and 0 wt% at 350°C for 1.5 h,respectively.Regarding the structure of hybrid nanoparticles,the core-shell structure is impossible for them because thermal decomposition and water loss of the γ-FeOOH nanoparticles cannot only occur on the surface of the nanoparticles.Therefore,the hybrid nanoparticles should be the mixture of γ-FeOOH and γ-Fe2O3.
Fig.4 shows the SEM images of γ-FeOOH,γ-FeOOH@Fe2O3and γ-Fe2O3.As seen in Fig.4,all the nanoparticles showed typical onedimensional needle-like structure with a diameter of around 100 nm and a length of around 1 μm.Due to the large amount of hydroxyl groups on the surface,the interaction between FeOOH nanoparticles was enhanced under the action of hydrogen bond,leading to the significant agglomeration of the nanoparticles.Compared with γ-FeOOH nanoparticles(Fig.4a),γ-FeOOH@Fe2O3nanoparticles(Fig.4b)had a clearer external profile,a rougher surface and a slight agglomeration,which may be related to the dehydration reaction in the calcination process.
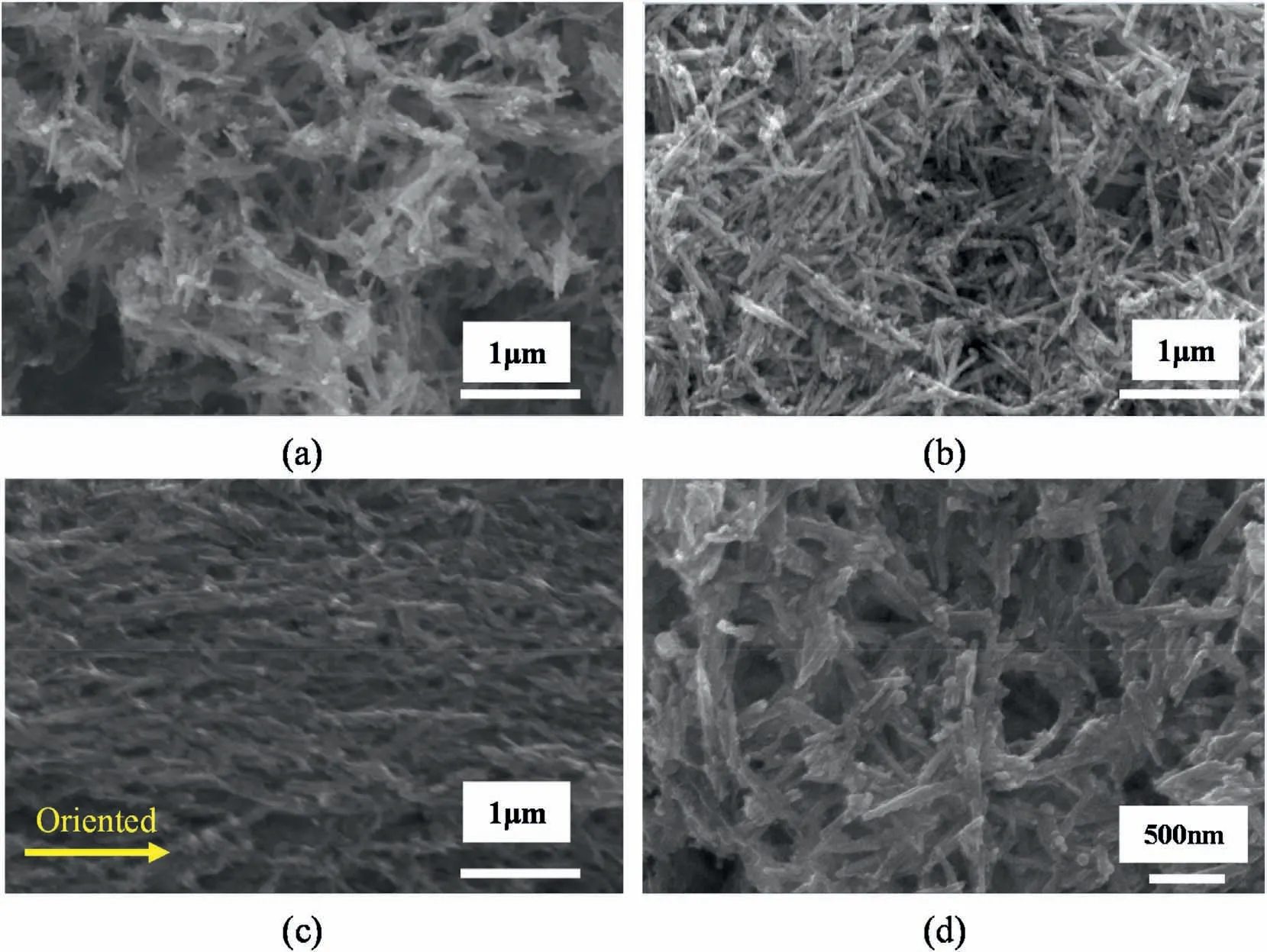
Fig.4.SEM images of (a) γ-FeOOH,(b) γ-FeOOH@Fe2O3 (250 °C,1 h),(c) γ-Fe2O3 (350 °C,1.5 h) and (d) γ-FeOOH@Fe2O3 (250 °C,1 h) under MF.
However,the agglomeration of γ-Fe2O3nanoparticles (Fig.4c) was more serious,which may be attributed to the partial recombination of γ-FeOOH nanoparticles under the higher calcination temperature.The serious agglomeration of γ-Fe2O3nanoparticles made them difficult to be dispersed well in EP,which is not conducive to the improvement of the properties of the composites.The dispersion of γ-FeOOH@Fe2O3nanoparticles in ethanol was dropped on the SEM sample platform and placed in the MF of 0.09 T to make the nanoparticles undergo the MF induction.As shown in Fig.4d,the γ-FeOOH@Fe2O3nanoparticles was oriented in the direction of the MF,which indicates that the nanoparticles can respond to the weak MF and provides feasibility for their alignment in the EP composite and the CF/EP composite.
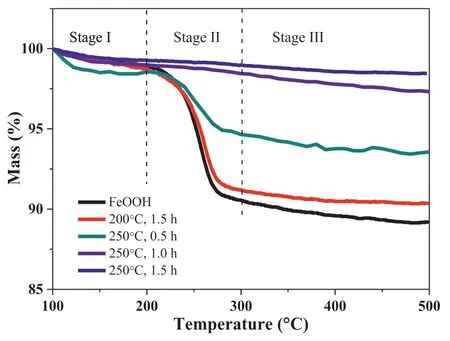
Fig.5.TGA curves of γ-FeOOH and γ-FeOOH@Fe2O3 prepared under different calcination conditions.
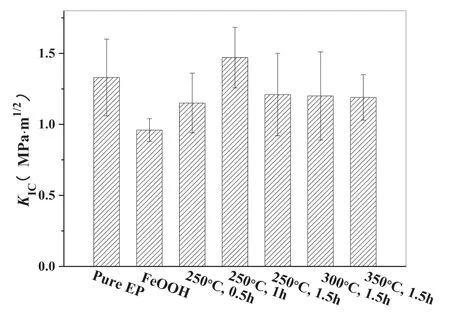
Fig.6.Comparison of KIC of pure EP and EP composites modified by γ-FeOOH@Fe2O3 prepared under different calcination conditions.
Fig.5 gives the TGA curves of γ-FeOOH and its derivatives under different calcination conditions.As shown in the figure,the weight loss of the samples mainly shows three stages.In the first stage (100–200°C),there was about 2% mass loss due to the weight loss of adsorbed water on the surface of samples.The second stage (200–300°C) corresponded to the formation of γ-Fe2O3from dehydration of γ-FeOOH with an initial decomposition temperature of 230°C,a maximum decomposition temperature of 260°C,and the termination temperature of 300°C.This is consistent with the data reported in the literature [34,35].The weight loss at this stage reflects the content of γ-FeOOH in the samples.The product calcined at 250°C for 1.5 h had almost no weight loss,indicating that γ-Fe2O3has been completely formed by γ-FeOOH.Other samples had a different degrees of weight loss,respectively.The third stage(300–500°C) corresponded to the high temperature removal process of the surface hydroxyl of Fe2O3.All the samples had a similar weight-loss behavior.
3.2.Fracture properties of the nanoparticles/EP composites
In order to obtain optimized calcination conditions and corresponding products,the comparison ofKICof pure epoxy and different nanoparticles modified EP composite with 1 wt% content is shown in Fig.6.Among these nanoparticles,only the γ-FeOOH@Fe2O3hybrid nanoparticles calcinated at 250°C for 1 h improved the fracture toughness of EP,while other nanoparticles decreased the fracture toughness of EP in different degrees.According to the above characterization of the nanoparticles,although the nanoparticles obtained under different calcination conditions had similar one-dimensional needle-like nanostructures,the surface properties and morphology caused by different compositions were quite different,which are the main factors affecting the fracture toughness of nanoparticles modified polymer composite[3].The amount of surface hydroxyl had an important influence on the surface property of the nanoparticles.Under the calcination conditions of 250°C,300°C and 350°C for 1.5 h,the corresponding product was γ-Fe2O3,on which the amount of surface hydroxyl group was greatly reduced as result of the dehydration of γ-FeOOH.
A small amount of hydroxyl groups may lead to poor affinity with EP,which is not conducive to the dispersion of nanoparticles and interface interaction between EP and nanoparticles.This reason,together with the serious agglomeration of nanoparticles themselves,resulted in the decrease of the fracture toughness of EP.In our previous work [37],it was found that Fe2O3nanoparticles have serious agglomeration in epoxy resin and form stress concentration point.On the contrary,γ-FeOOH and the hybrid nanoparticles containing more γ-FeOOH components at 250°C for 0.5 h have abundant hydroxyl groups on their surfaces,but the excessive hydroxyl groups enhanced the hydrophilicity of the nanoparticles.Therefore,excessive hydroxyl groups were not helpful for the dispersion of the particles and the interface adhesion between the particles and EP,which also leads to the decrease of the fracture toughness of EP,especially for γ-FeOOH.Obviously,the optimal calcination condition is at 250°C for 1 h and the resulting γ-FeOOH@Fe2O3nanoparticles had a moderate amount of surface hydroxyl,a slight self-agglomeration and relatively high saturation magnetization.Therefore,the nanoparticles calcinated at 250°C for 1 h were selected to further study the influence of MF induction on modification of EP and CF/EP composites.
Fig.7 shows theKICof the composite as a function of the content of nanoparticles,clearly indicating the significant influence of the nanoparticles and the MF induced orientation of the nanoparticles on theKIC.Without MF induction,theKICof the composite increased first and then decreased significantly with the increase of the nanoparticles content.The largest improvement ofKICwas observed at 0.5 wt%content.TheKICwas increased to 1.96 MPa m1/2,and was 45.2%higher than that of pure epoxy (1.35 MPa m1/2).At a higher content than 0.5 wt%,the improvement ofKICwas reduced.TheKICof the composite with 2 wt%content was even lower than that of the pure EP,which is usually attributed to the serious agglomeration of nanoparticles at high content[3].With the MF induction,theKICof the composites with all nanoparticle contents studied was higher than that of the composites without MF induction.TheKICof the composite with 1 wt% content was 2.45 MPa m1/2and achieved the largest improvement of 81.5%compared to that of pure EP,and also the remarkable improvement of 66.7% compared to that of the composite without MF induction.As shown in Table 1,the gain in theKICis a relatively high value and the toughening effect of the nanoparticle is at the same level as that of carbon nanomaterials.
With the further increase of the nanoparticle content,theKICdid not decrease significantly.It is noted that the magnetic induction did not obviously improve theKICof composites with low nanoparticle contents no more than 0.5 wt% compared to that of the composites without MF induction.For example,at 0.5 wt% content,theKICof the composite with MF induction was only 6.1% higher than that of the composite without MF induction.Additionally,the fracture toughness of the composite without MF induction reduced significantly when the nanoparticle content was more than 1 wt%.However,when the MF was applied,the fracture toughness was significantly increased by the nanoparticles even when the nanoparticle content reached 2 wt%.These indicate that the alignment and content of the nanoparticles has an important influence on the fracture mechanism of the composite.
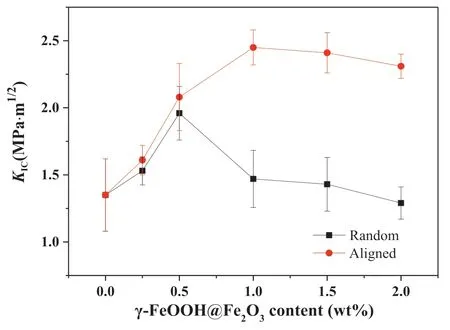
Fig.7.Effect of γ-FeOOH@Fe2O3 content on the KIC of composites.
Fig.8 gives the SEM images of pure EP and 1 wt% γ-FeOOH@Fe2O3/EP composites with and without MF induction,presenting the corresponding fracture surface morphology.Apparently,as seen in Fig.8a,the pure EP showed relatively smooth fracture surface with the sparse river line,which is a typical characteristic of brittle fracture despite its relatively highKICof 1.35 MPa m1/2.In the absence of MF induction(Fig.8b),the addition of the nanoparticles increased the surface roughness with more river lines,which is resulted from the crack deflection by the nanoparticles.Overall,the white nanoparticles were well dispersed in the matrix and no large aggregates were observed,which is helpfully contributing to the improvement of fracture toughness.As above analyzed,this is mainly due to the moderate surface properties of the hybrid nanoparticles.
Fig.8c further shows that,as indicated by the red dashed circles,the nanoparticles were distributed in the matrix in form of impregnated small aggregate containing several nanoparticles,suggesting that the matrix has a good affinity with the nanoparticles.Fig.8d gives more detailed information about the small aggregate.The nanoparticles were randomly oriented under no MF induction,and the toughening mechanisms such as pull-out and debonding of nanoparticles,and local plastic deformation of matrix around the nanoparticles were identified.These are also the main mechanisms in CNT modified composite[39].
As shown in Fig.8 (e and f),compared with the composites without MF induction,the surface roughness of the composites with MF induction was further improved,and more torturous fracture paths appeared as well,suggesting the further improvement of fracture toughness.As pointed out by the arrows and dashed ellipses,it can be noted that the distribution of the nanoparticles in the matrix had changed greatly to be in form of chain clusters as the main structure with a little amount of the small aggregates,which is undoubtedly the important reason for the significant difference in toughening effect of the composites with and without MF induction.The chain cluster structure has also been found in the previous studies on electric field induced carbon nanotubes [17]modified EP composites,but the particularity of the chain cluster in the toughening behavior has not been concerned.
Here,the formation of the chain cluster can be attributed to the highMs of the hybrid nanoparticles resulting in the strong magnetic force on the nanoparticles.Under MF induction,the randomly oriented needlelike nanoparticles with good dispersion in the matrix were not only rotated and aligned along the direction of MF,but also attracted each other and gathered together,thus forming the chain cluster.When theMs of the nanoparticles is relatively low,or the nanoparticles content is low,which is equivalent to a large distance between the nanoparticles,the interaction force between the nanoparticles is not enough to drive them together,resulting in the failure to form the chain cluster.This should be the reason why the improvement ofKICis not significant for the composites with the low content less than 0.5 wt%.
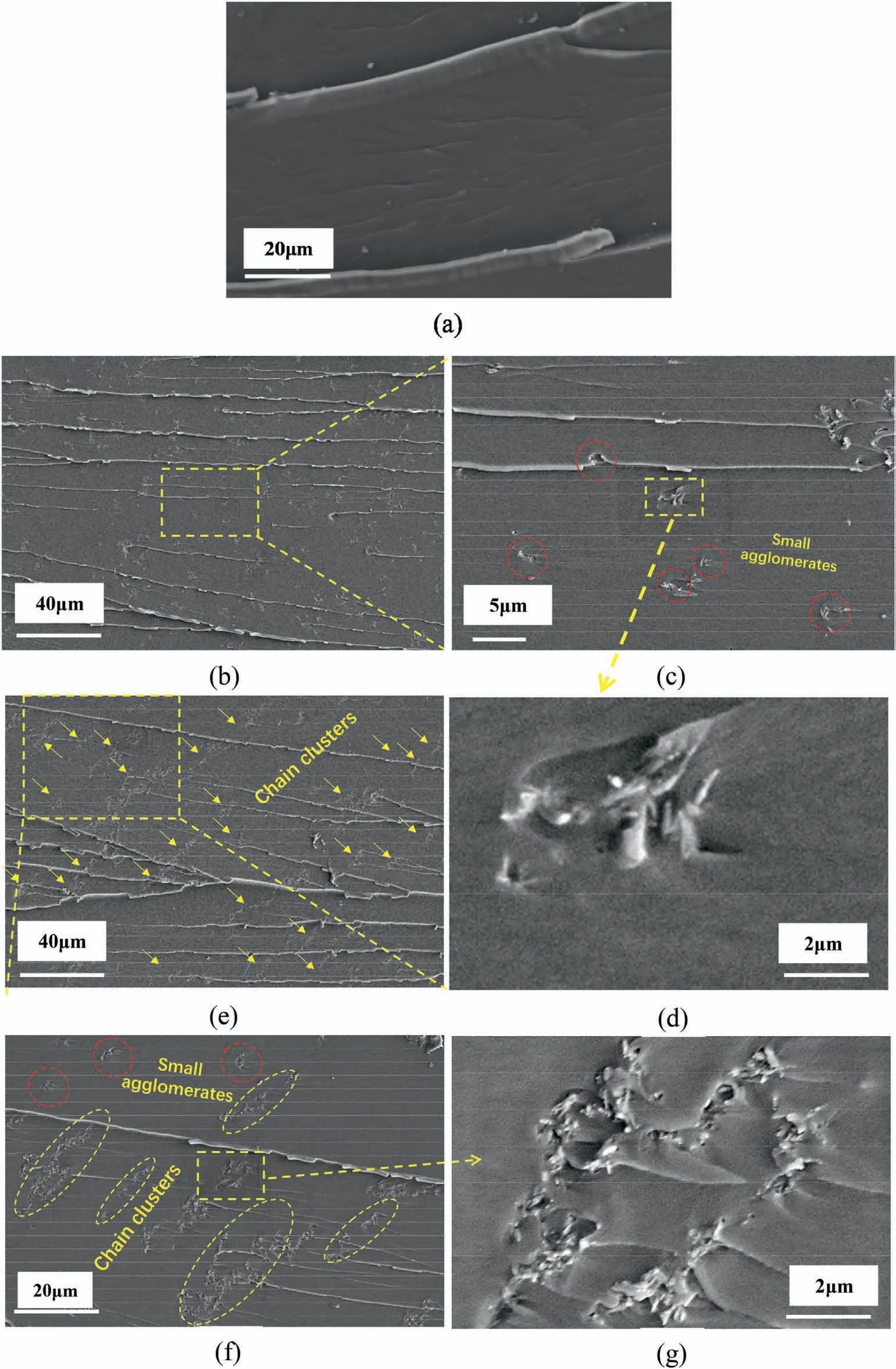
Fig.8.SEM images of fracture surface of different samples:(a)pure epoxy resin,(b,c,d)1 wt% γ-FeOOH@Fe2O3/EP,and(e,f,g)1 wt%γ-FeOOH@Fe2O3/EP under without MF induction.
The nanoparticle content should be an important factor influencing the formation of the chain cluster.Moreover,our previous study [15]reported that the MWCNTs wrapping nickel particles catalyst with a lowMs of 8.9 emu/g have been partially aligned under MF of 0.4T in form of small aggregates rather than chain cluster.In addition,the viscosity of resin,magnetic strength,and shape,size and surface modification of nanoparticles are also important factors for the formation of the chain cluster and alignment of the nanoparticles[17].At the same time,based on the preparation process of the composite in this work,controlling the size of the chain clusters can be easily achieved by changing the intensity of the applied magnetic field.
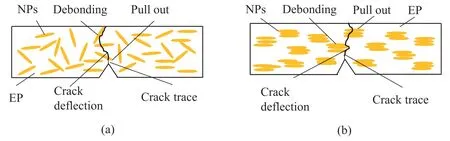
Fig.9.Schematic diagram of toughening mechanism of γ-FeOOH@Fe2O3 nanoparticles(NPs):(a)randomly oriented and(b)aligned perpendicular to the direction of crack growth.
Fig.8g shows more detailed information about the chain cluster and its toughening behavior.The size of the chain clusters was 20–40 μm in length and 0.5–2 μm in diameter.The chain clusters significantly induced the crack deflection with many tail-like cracks behind them,which indicates that the chain cluster more effectively prevented the propagation of the crack than the small aggregate.On the contrary,it is difficult for the small aggregates to induce the plastic deformation and crack deflection of the matrix because of their relatively weak resistance to the propagation of crack.Inset in Fig.8e shows that most of the nanoparticles in the cluster pointed out of fracture surface,which indicates the alignment of the nanoparticles induced by MF.The alignment of the nanoparticles was perpendicular to the direction of crack growth,which helps to bridge the propagation of crack[39].Moreover,the many holes left by nanoparticles pulled out and the more obvious plastic deformation of the matrix around the nanoparticles were observed.Therefore,the nanoparticles aligned perpendicular to the direction of crack growth can improve the fracture toughness of composite more efficiently than the random oriented nanoparticles.
Based on the clear toughening mechanism,the difference in the nanoparticle content corresponding to the decrease in fracture toughness with and without MF is mainly attributed to the difference in toughening ability between the chain cluster aggregates and the common aggregates.As shown in Fig.8(b and e),at 1 wt% content,the nanoparticles in the composite with and without MF induction were all agglomerated.However,with MF induction,the nanoparticles were mainly the chain cluster-like agglomerates,and their toughening effect is significantly better than the common small agglomerates formed without MF induction.In fact,the toughening advantage of the chain clusters overcame the negative effect of the common agglomerates,so it can still have a significant toughening effect at more than 1 wt% content.However,at higher nanoparticle content,the toughening advantage of chain clusters cannot balance the negative effect of the common agglomerates,and the fracture toughness also reduced.
Fig.9 shows the schematic mechanism of the nanoparticles toughening the EP composite.The toughening mechanisms of the composite without MF induction include pull-out,debonding and bridging of the nanoparticles,accompanied by slight crack deflection and matrix plastic deformation.The chain cluster like a bundle are formed and aligned perpendicular to the direction of the crack growth under MF induction,so pull-out and bridging of nanoparticles become the dominant toughening mechanisms,which leads to more crack deflection and more matrix plastic deformation.Therefore,the fracture toughness of the composite with MF induction can be improved more significantly compared to that of the composite without MF induction.
3.3.Mode I interlaminar fracture toughness of the composite
The mode I delamination resistance of composite laminates was investigated in order to evaluate the influence of γ-FeOOH@Fe2O3and MF induction on improvement of the interlaminar fracture toughness.Fig.10a shows the load versus displacement curves during mode I crack propagation for the representative specimens of the EP/CF and 1 wt%γ-FeOOH@Fe2O3/CF/EP composite with and without MF induction.With the increase of the displacement,the load linearly increased to the maximum value and then decreased in a jag-shaped curve,which conforms to the common interlaminar failure characteristics of carbon fiber reinforced composite[40].The maximum load and the load at the stage of jag-shaped curve reflect the interlaminar resistance of crack initiation and the interlaminar resistance of crack propagation,respectively.Compared with CF/EP,γ-FeOOH@Fe2O3/CF/EP without MF induction was slightly improved in the interlaminar resistance of crack initiation and crack propagation.But γ-FeOOH@Fe2O3/CF/EP with MF induction was significantly improved,which suggests that the nanoparticles induced by MF significantly improved interlaminar fracture toughness of the composite.
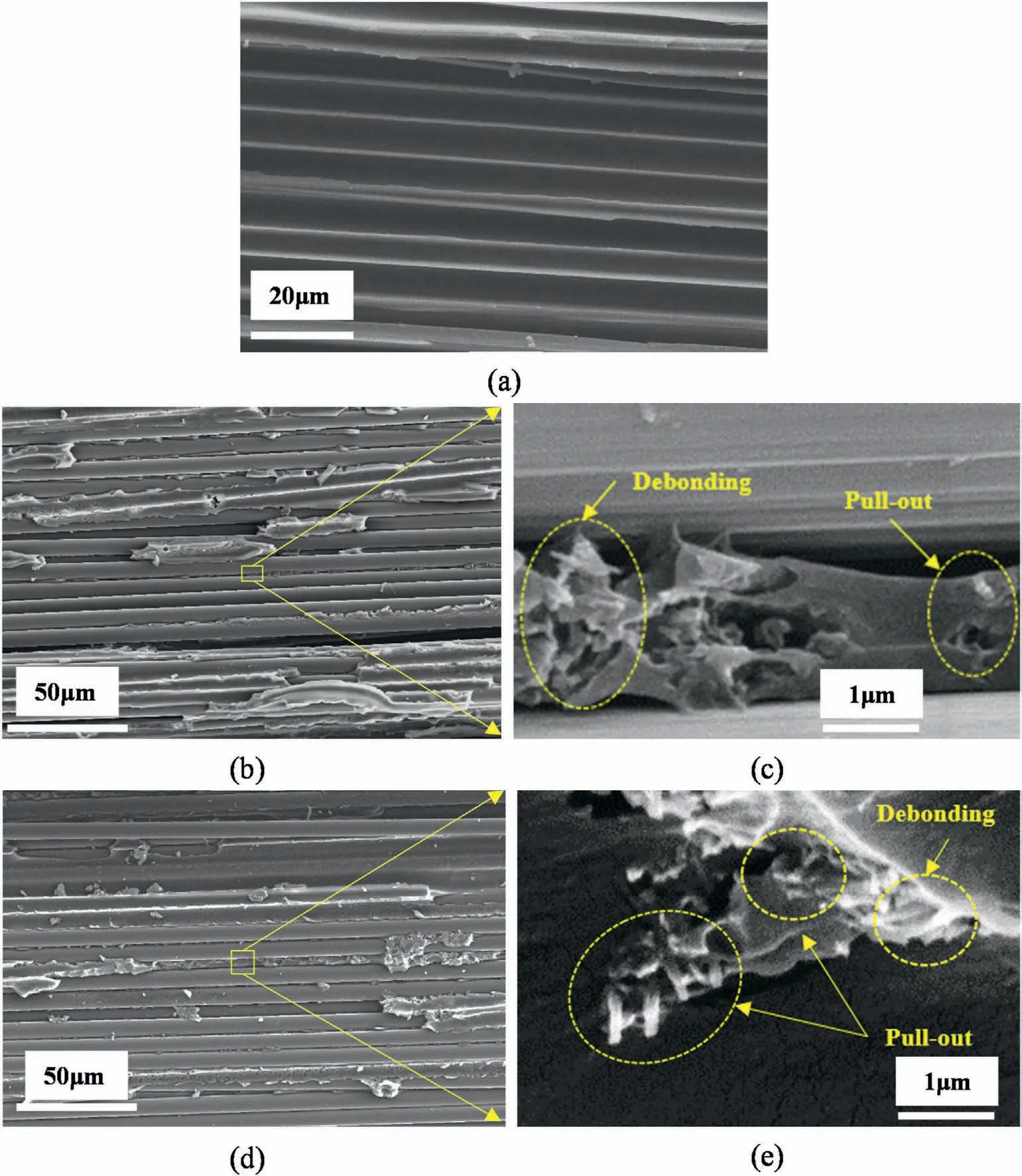
Fig.11.SEM images of fracture surface of different composite laminates:(a)CF/EP,(b,c)1 wt% γ-FeOOH@Fe2O3/CF/EP and(d,e)1 wt% γ-FeOOH@Fe2O3/CF/EP with MF induction.
Fig.10b illustrates theGICas function of crack increment for the corresponding representative laminate specimens.TheGICof all the three composite laminates first increased and then tend to be stable,which may be attributed to the CF fiber bridging.At the initiation stage of crack,as shown by dashed circles,the first data of each curve indicates the interlaminar initial fracture toughness(GIC,init).TheGIC,initvalues were determined as 0.371,0.405 and 0.804 kJ/m2for CF/EP,γ-FeOOH@-Fe2O3/CF/EP without and with MF induction,respectively.Namely,the composite laminates with the nanoparticles before and after MF induction were improved inGIC,initby 9.1% and 117% compared to the control composite.
At the stable propagation stage of crack,as shown by the horizontal dashed lines,the interlaminar propagation fracture toughnessGIC,propcorresponding to the average ofGICvalue of the plateau part of the curves(crack length 70 mm–100 mm)was found to be 0.602 and 0.914 kJ/m2for γ-FeOOH@Fe2O3/CF/EP without and with MF induction,respectively,which corresponds to the improvement of 24.4% and 88.8%compared to the that of the control composite(0.484 kJ/m2).Obviously,the influence of the nanoparticle and MF induction on the interlaminar fracture toughness of the CF/EP composite is consistent with that of the above the nanoparticle toughened EP composite.It is that at 1 wt%content,the nanoparticles induced by MF more significantly improved the interlaminar fracture toughness of CF/EP than those without MF induction.Additionally,as shown in Table 2,the gain value of 91% by the magnetic induced nanoparticles is the highest in those by other common rigid nanoparticles,which indicates that the toughening effect of the nanoparticles is superior,and at least at the same level as carbon nanomaterials.
In fiber reinforced composite with nanoparticles modified matrix by blending method,the contribution of the nanoparticles to interlaminar fracture toughness is generally attributed to the toughening of the matrix[5].To further identify the toughening mechanism in the composites,the fracture surface morphology of the mode I specimens was analyzed by SEM observation,and the corresponding SEM images were shown in Fig.11.As seen in Fig.11a,the CF/EP exhibited smooth and featureless failure characteristics,and the debonding of carbon fibers from the matrix and regular fracture surface of the matrix were observed.This indicates the low interfacial interaction between carbon fibers and the matrix,as well as the brittle characteristics of the matrix.In contrast,the composites with the nanoparticles,whether or not induced by MF,as shown in Fig.11(b and d),exhibits significantly different failure surfaces and irregular fracture surface of the matrix.This indicates that cracks are subject to more resistance when passing through the matrix in the delamination of the composite laminate,apparently as result of the toughening of the matrix by the nanoparticles.
The toughening mechanism of the matrix by the nanoparticles can be clearly seen in the local magnification Fig.11(c and d) of the matrix fracture surface between fibers.As indicated by arrows and dashed circles,in the composite without MF induction,the toughening of the matrix was achieved by the mechanism of debonding,pull-out of the nanoparticles and local plastic deformation of the matrix.On the other hand,in the composite with MF induction,most of the nanoparticles were aligned perpendicular to the direction of crack propagation.In addition to the mechanisms of nanoparticle debonding and matrix local plastic deformation,the nanoparticles toughen the matrix by pull-out and bridging as dominant mechanisms,which are consistent with the toughening mechanism of the nanoparticles/EP composite above.However,the chain clusters of the nanoparticles were absent in the composite with MF induction.The possible reason for this is that the movement of the nanoparticles are limited in the thin EP resin layer between carbon fibers.
4.Conclusions
In this work,the needle-like γ-FeOOH nanoparticles were prepared by chemical precipitation-air oxidation method,and the needle-like γ-FeOOH@Fe2O3hybrid nanoparticles composed of γ-FeOOH and γ-Fe2O3was obtained by optimizing the calcination process.Under the mild calcination condition of 250°C for 1 h,γ-FeOOH@Fe2O3hybrid nanoparticles with γ-Fe2O3as the main component have a high saturation magnetization of 37.14 emu/g and an aspect ratio of about 10,which ensure their responsiveness to a weak MF of 0.09T and make them be aligned along the direction of MF.Meanwhile,the γ-FeOOH component provides the particle with good dispersion in the matrix and good affinity for EP.In addition,the γ-FeOOH@Fe2O3nanoparticles were in a relatively loose state with a slight agglomeration,which is conducive to their dispersion in EP.
The γ-FeOOH@Fe2O3hybrid nanoparticles were successfully used for the first time to significantly improve the fracture toughnessKICof epoxy resins and the mode I interlaminar fracture toughnessGICof CF/EP laminates under MF induction.At the calcination condition of 250°C for 1 h,the γ-FeOOH@Fe2O3nanoparticles composed of 24 wt%FeOOH and 76 wt%Fe2O3has the best toughening effect.TheKICof the nanoparticles modified EP is 81.5%higher than that of pure EP,reaching 2.47 MPa m1/2,and 66.7% higher than that of the corresponding composite without MF induction.TheGICof CF/EP with modification of MF induced nanoparticles was also increased to 0.914 kJ/m2,88.8% and 51.8%higher compared that of CF/EP and γ-FeOOH@Fe2O3/CF/EP without MF induction,respectively.Compared to common rigid nanoparticles,the hybrid nanoparticles have remarkable toughening effect at the same level as carbon nanomaterials.Additionally,the hybrid nanoparticles also have simple preparation process and low cost,indicating potential application in polymer toughening modification.
According to SEM analysis,the main toughening mechanisms of the nanoparticles mainly included pull-out,debonding and bridging,crack deflection and local plastic deformation of the matrix,which are similar to those of common one-dimensional nanoparticles or two-dimensional nanoparticles such as CNT and graphene.Under the induction of MF,the pull-out and debonding of particles became the dominant mechanism and made a major contribution to the remarkable toughening of composites.In the nanoparticle toughened epoxy composite it is also noted that the toughening mechanism of nanoparticle chain clusters has been found.So the further study on the factors affecting the chain clusters and related toughening mechanism are worthy of attention in the future.The proper surface modification may further improve the dispersion of the nanoparticles and enhance the interface interaction between nanoparticles and matrix,which may obtain more significant toughening effect.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to the National Natural Science Foundation of China (51763006),the Foundation of Guangxi Key Laboratory of Structure Activity Relationship for Electronic Information Materials(201018-K) and the Natural Science Foundation of Guangxi Province(2019GXNSFGA245005)for financial support for this work.
- Namo Materials Science的其它文章
- Preface of “Trends in Nanomaterials and Nanocomposites:Fundamentals,Modelling and Applications”
--Festschrift in honor of Prof Yiu-Wing Mai's 75th birthday - A comparative study of 85 hyperelastic constitutive models for both unfilled rubber and highly filled rubber nanocomposite material
- On mechanical properties of nanocomposite hydrogels:Searching for superior properties
- Ultra-transparent nanostructured coatings via flow-induced one-step coassembly
- VN nanoparticle-assembled hollow microspheres/N-doped carbon nanofibers:An anode material for superior potassium storage
- Molecular dynamics study on mechanical behaviors of Ti/Ni nanolaminate with a pre-existing void

