Iron supplementation for non-anaemic pregnant women and the incidence of hypertensive disorders in pregnancy: A systematic review and meta-analysis
Farida Fitriana, Phoebe Pallotti
1Midwifery Study Program, Faculty of Medicine, Universitas Airlangga, Indonesia
2Division of Midwifery, School of Health Sciences, University of Nottingham, United Kingdom
ABSTRACT
KEYWORDS: Iron supplementation; Non-anaemic pregnancy;Healthy pregnancy; Hypertensive disorders in pregnancy;Gestational hypertension; Preeclampsia; Systematic review
1. Introduction
Iron is a vital trace element, making up 60% of erythrocyte’s haemoglobin (Hb)[1]. The daily intake of iron should be increased to at least 27 mg, compared to 1 to 8 mg for adults in general[2]to support the development of placenta, fetus, and to prepare the mother for birth[1,3]. If this minimum requirement is not met,the body will use its own iron stores, causing iron deficiency anaemia[4,5] which is the major contributor to maternal and neonatal morbidity and mortality[1,6,7].
The World Health Organization (WHO)’s definition of anaemia in pregnancy is a Hb level lower than 11 g/dL in the first and third trimester, and lower than 10.5 g/dL in the second trimester[8,9]. The WHO recommends daily oral supplementation with 30 mg to 60 mg of elemental iron and 0.4 mg of folic acid[10]. However, if the sideeffects make daily supplementation unappealing and the prevalence of anaemia in pregnancy is less than 20% of the total pregnant women, intermittent supplementation with 120 mg of elemental iron and 2.8 mg of folic acid once weekly is recommended[10].
Hypertensive disorders in pregnancy (HDP) is a group of diseases which include gestational hypertension, preeclampsia and eclampsia, and chronic hypertension[11,12]. The adverse effects caused by HDP are also major causes of maternal and neonatal morbidity and mortality[13]. Unfortunately, the etiology of HDP is still poorly understood[14,15], but poor placentation during the early stage of pregnancy and endothelial dysfunction are considered to be the leading causes of hypertension in pregnancy[14,16].
Despite its beneficial effect on preventing and treating irondeficiency anaemia, iron supplementation may be unnecessary for women who are not anaemic due to its potential adverse effect, particularly in developing HDP[15]. Some previous quasiexperimental studies have found that a high Hb, which means≥13.2 g/dL during the first[17,18], second[19] and third trimester[20],correlated with the incidence of HDP at the end of pregnancy. Using slightly different cut-off points, other quasi-experimental studies found that Hb of >13 g/dL in the first trimester of pregnancy may increase the risk of HDP at the end of pregnancy[14,21].
Two meta-analyses of observational studies have found that pregnant women with a high serum iron level have an increased risk of HDP[22,23]. The free radicals due to iron overload can react with fatty acids to form lipid peroxides[24], leading up to placental cell injury and increased oxidative stress[25]. Clearly, in preeclampsia,an increased level of oxidative stress due to excess iron plays a key role in developing endothelial cell dysfunction[16]. Furthermore, iron supplementation may increase the risk of HDP because it potentially deteriorates of hypoxia-inducible transcription factor which is essential for placental development[26]. Hence, iron prophylaxis given during pregnancy might be seen not only as an effort to prevent“TLTL (too little too late)” causing iron deficiency anaemia, but also“TMTS (too much too soon)” leading to unnecessary medicalisation and potentially causing harm for normal pregnancy[27].
Some existing evidence has looked at the effect of daily oral iron supplementation on HDP outcomes by including women in general,not specific for only pregnant women who are not diagnosed as anaemic, and has found that iron supplementation may have little or no effect on the preeclampsia outcome[28,29]. A recommendation states that intermittent oral iron supplementation during pregnancy may be a feasible alternative for pregnant women who are not anaemic; however, the recommendation was generated without having HDP as part of the outcomes analysis[30]. While some studies have concluded that iron supplementation for non-anaemic pregnant women may increase the incidence of HDP[31,32], a contradictory result has been found by another study[33].
The absence of knowledge and consensus about the benefits and harm of routine iron prophylaxis for the non-anaemic may create uncertainty in giving care to women and potentially lead to suboptimal care[27]. Therefore, the research question of this study is: What is the association between iron supplementation for nonanaemic pregnant women and the incidence of HDP?
2. Materials and methods
This study used a systematic review and meta-analysis guided by the Preferred Reporting Items for Systematic Reviews and Metaanalyses (PRISMA) 2020 statement[34]. The treatment comparison pair in this meta-analysis was an experimental treatment (ironcontaining versus non-iron supplementation or high dose versus low dose iron supplementation). This review analysed the incidence of HDP among non-anaemic pregnancy according to the guidelines used in the included studies and pregnant women having high iron status (Hb ≥13.2 g/dL). Ethical approval was unnecessary for this review.
2.1. Search strategy
The search was conducted from February 2019 to August 2019 in the eight electronic databases as follows: MEDLINE(OVID), Cumulative Index to Nursing and Allied Health Literature (CINAHL), PubMed, Cochrane Library, Scopus, Web of Science; and in two clinical trial registration databases: the WHO's International Clinical Trial Registry Platform (ICTRP) and ClinicalTrials.gov. Besides, a ‘snowball sampling’ method through a manual search of reference lists of the potential resources and the included studies was used to find other relevant literatures.
Three categories of terms with its synonyms or related words were used to search the literatures: 1) Iron, Ferrum, Ferrous, Fe,Ferri*, Sidero*; 2) Supplement*, Tablet*; 3) Hypertensive Disorders in Pregnancy, Pregnancy-induced hypertension, Gestational hypertension, Pregnancy hypertension, Mother hypertension,Maternal hypertension, Pre-eclampsia, Preeclampsia, Eclampsia,Maternal blood pressure, and Mother blood pressure. Literature search process was started by searching each term in the same category. The results of each term in the same category were combined using “OR”.After the process was conducted for all categories, the final results from each category were combined using “AND”.
2.2. Defining eligibility criteria
Randomized control trials (RCTs) were used for this review because it provides the best understanding of the treatment’s effect[35]. Besides RCT, other inclusion criteria for this study were:1) research published in English; 2) full-text available; 3) studies published up to August 2019; 4) pregnant women without anaemia as the eligibility criteria for study participant and having HDP at the end of pregnancy as the outcome. The exclusion criteria of this study were: 1) non-research studies; 2) having complications in pregnancy,including chronic hypertension; 3) having the same dose of iron supplementation between treatment and control arm (in the context of multi-drug trials).
2.3. Quality assessment and data extraction
We assessed the quality of the included papers by assessing the risk of bias using an instrument developed by Cochrane (http://handbook-5-1.cochrane.org/ in Chapter 8). The results of risk of bias assessment were presented in the risk of bias graph and risk of bias summary, which were developed using Review Manager software(RevMan 5.3 version) following Cochrane’s review[28-30]. It consists of seven questions regarding random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting,and other biases.
For each RCT study included in this meta-analysis, we extracted the following information: The first author, year, outcome, trial design,number of centres, cut-off Hb level used, total initial and final participants, treatment and control arm, co-treatment, start and end of treatment, ethical approval, participant consent, and assessment of compliance.
2.4. Statistical analysis
Individual study’s odds ratio (OR) and 95% confidence interval (CI)were calculated by using the statistical software ‘Epi Info’[36]. The pooled data were subsequently analysed using Comprehensive Meta Analysis (CMA) version 3.3.070 software by Biostat Inc, USA[37].In a meta-analysis, there are always varieties across studies, causing heterogeneity (I2)[38]. If I2values were >50% indicating that the studies were heterogeneous, the random-effects model was used for meta-analysis, whereas if I2values were 50% or less showing that the studies were homogenous, then the fixed-effects model was used. The percentage of heterogeneity in this review was obtained automatically from CMA software. Then, the result of the analysis was presented as forest plot. Each forest plot displayed OR, 95%CI, and weight from each article. The summaries of OR and 95% CI from the pooled analysis carried out according to the model (fixed/random effects model) were also displayed to describe the overall treatment effect.
3. Results
3.1. Selection of studies
We identified 1 066 articles from the initial search from eight electronic databases and seven potential sources (Figure 1). Of these articles, 1 051 articles were excluded from three screenings,remaining 15 articles. After the first three screenings, 11 of 15 articles were excluded due to observational design, and four RCTs remained. After conducting a manual search of potential articles from the 4 RCTs, no additional articles were found. Finally, four RCT trials were included in this review[24,31-33].
3.2. Characteristics of the included studies
The characteristics of the included literature are summarized in Table 1. Three trials found gestational hypertension as the pregnancy outcome[31-33], and two trials found preeclampsia as the pregnancy outcome[24,32]. Total participants in the analysis were 13 425 women(13 365 women for gestational hypertension and 842 women for preeclampsia, with 782 women having both gestational hypertension and preeclampsia outcome in the study by Ouladsahebmadarek et al[32] (Table 1). The lowest number of participants was 60 women by Bhatla et al[24] and the highest number of participants was 11 856 women by Chen et al[33].
3.3. Quality appraisal of the included studies
The risks of bias assessment of each included study are provided in Table 2. The risks of bias assessment results are presented in the risk of bias graph (Figure 2A) and the risk of bias summary (Figure 2B).
3.4. Data analysis results
Four RCT trials were included in this review[24,31-33], which analysed the association between iron supplementation in nonanaemic pregnancy and the incidence of HDP. Trials by Ziaei et al[31]and Chen et al[33] investigated gestational hypertension outcomes,whereas the study by Bhatla et al[24] evaluated the incidence of preeclampsia, and the research by Ouladsahebmadarek et al[32]examined for both gestational hypertension and preeclampsia outcomes. The pooled analysis of HDP outcomes from all included studies can be seen in Figure 3. The study by Chen et al[33] and Bhatla et al[24] respectively contributed the highest (91%) and lowest(0.75%) statistical weight to the overall analysis. The pooled effect size or OR found that the iron supplementation for non-anaemic pregnancy might not affect the incidence of HDP (OR 0.93, 95% CI 0.81-1.07; P=0.30). There was a moderate heterogeneity between the studies (I2=53.893%), so the pooled analysis was a random-effects model, meaning that the effect estimate of iron supplementation for non-anaemic pregnant women between the studies was randomly distributed to the incidence of HDP, and the differences of the studies’ findings were possibly due to both by chance and real distribution.
Three trials analysed the association between iron supplementation for non-anaemic pregnant women and the incidence of gestational hypertension (Figure 4)[31-33]. The study by Chen et al[33] contributed the highest statistical weight (48.47%), whereas the study by Ziaei et al[31] contributed the lowest statistical weight (17.87%) to the overall analysis. The pooled effect size or OR showed that the iron supplementation for non-anaemic pregnant women might not affect the incidence of gestational hypertension (OR 1.37, 95% CI 0.69-2.73; P=0.36). In addition, there was substantial heterogeneity between the studies (I2=71.923%), so the pooled analysis was a random-effects model.
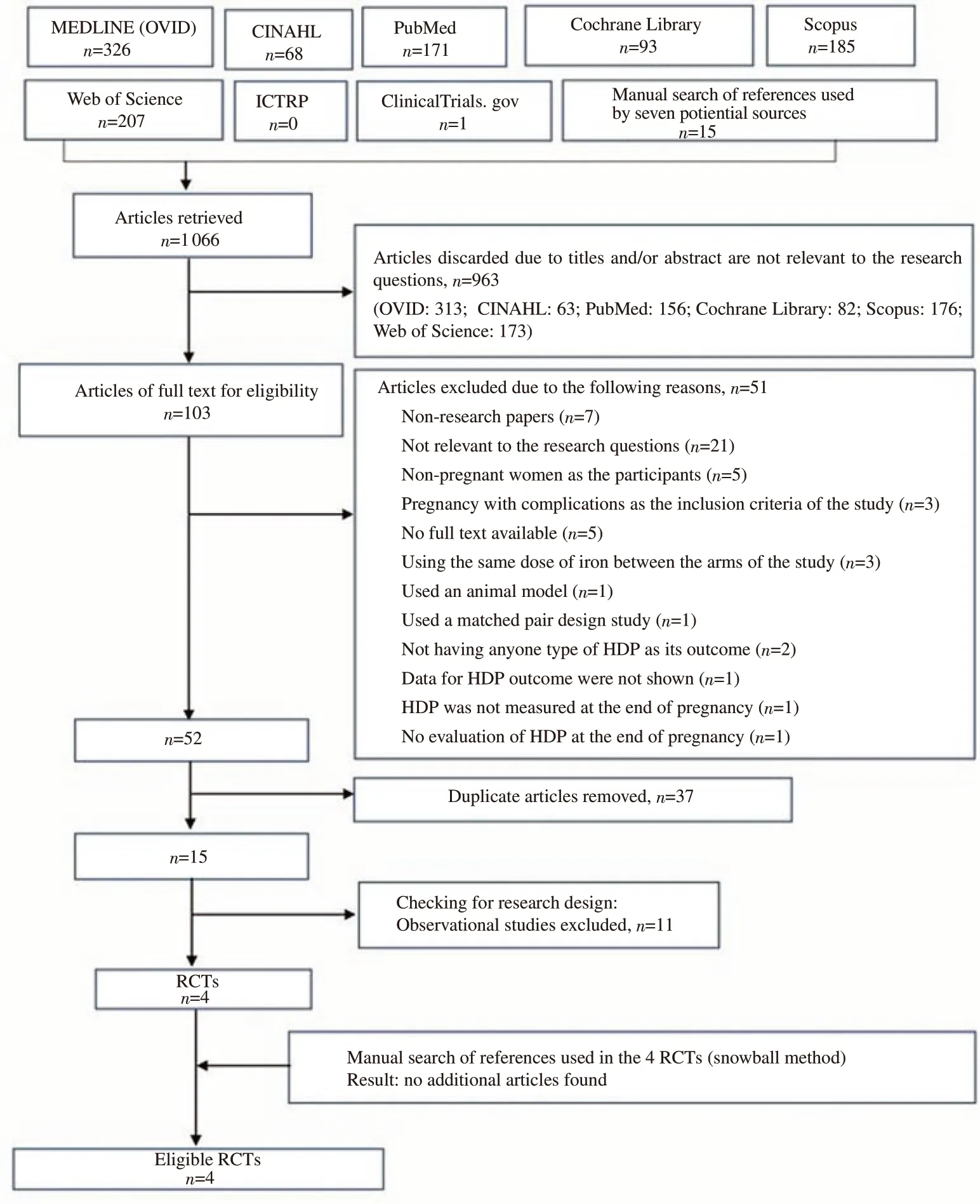
Figure 1. Flow chart of the selection of studies. HDP: hypertensive disorders in pregnancy; RCTs: randomized control trials.
Two trials analysed the association between iron supplementation for non-anaemic pregnant women and the incidence of preeclampsia[24,32] (Figure 5). The study by Ouladsahebmadarek et al[32] contributed a higher statistical weight (79.70%) than the study by Bhatla et al[24] (20.30%) to the overall analysis. The pooled OR showed that the iron supplementation might not affect the incidence of preeclampsia (OR 1.45, 95% CI 0.71-2.97; P=0.31). There was no heterogeneity between the studies (I2=0.000%), so the pooled analysis was a fixed-effects model, meaning that the studies had a common true effect size on the incidence of preeclampsia, and differences among study results were entirely due to play of chance.
There were two trials that looked at the association between iron supplementation and gestational hypertension by including pregnant women with Hb ≥13.2 g/dL before 20 weeks of gestation[31,33] (Figure 6). The study by Chen et al[33] contributed a higher statistical weight (63.86%) than the study by Ziaei et al[31](36.14%) to the overall analysis. The pooled OR showed that the iron supplementation might not affect the incidence of gestational hypertension (OR 1.50, 95% CI 0.47-4.75; P=0.49) with substantial heterogeneity (I2= 69.486%) and random-effects model.
3.5. Publication bias
We could not assess the publication bias using funnel plots in this study because there should be a minimum of five studies to assess it[39]. Therefore, the publication bias for HDP and preeclampsia outcomes could not be assessed in this review due to the lack of studies.
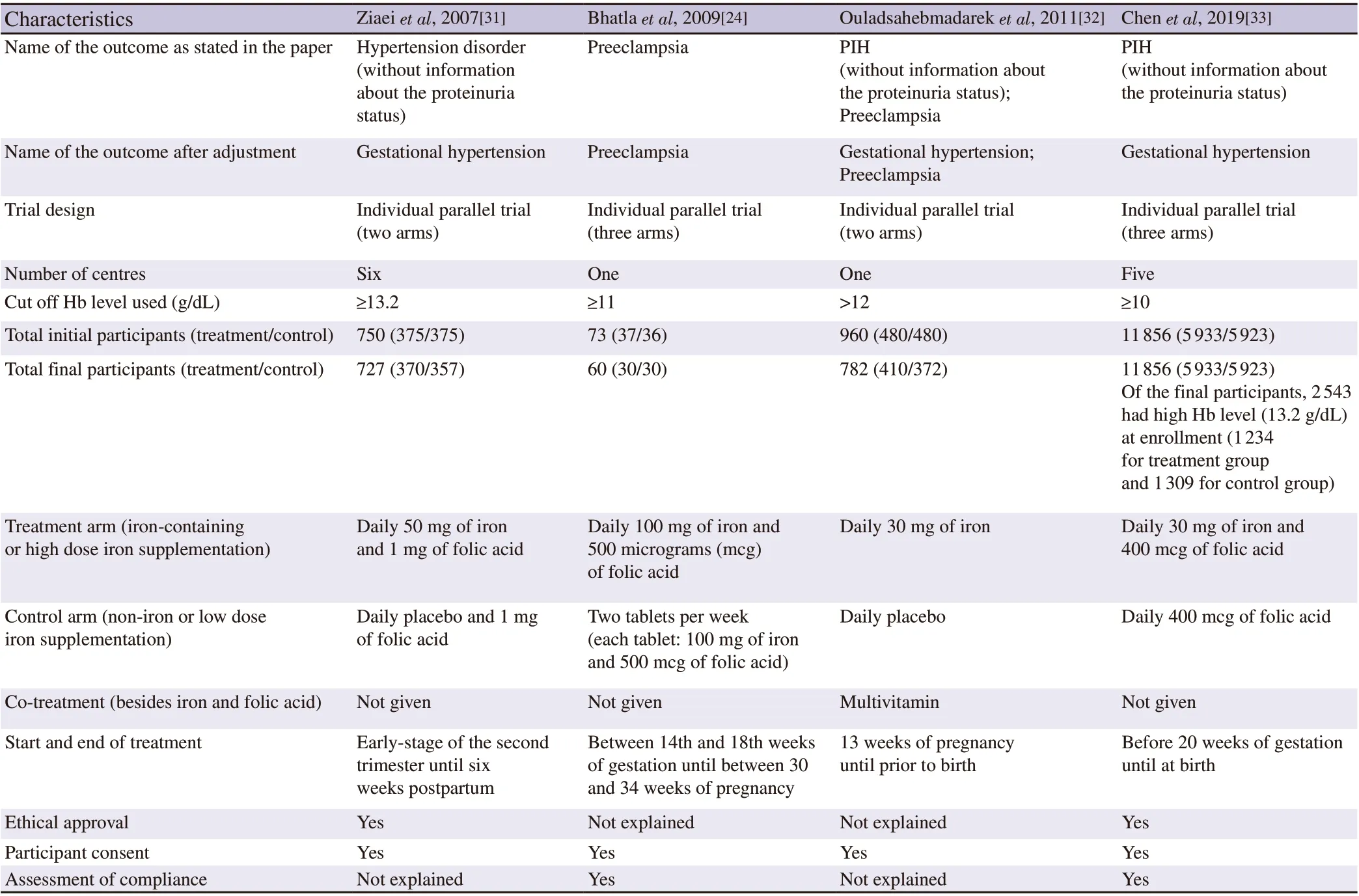
Table 1. Main characteristics of the included studies.
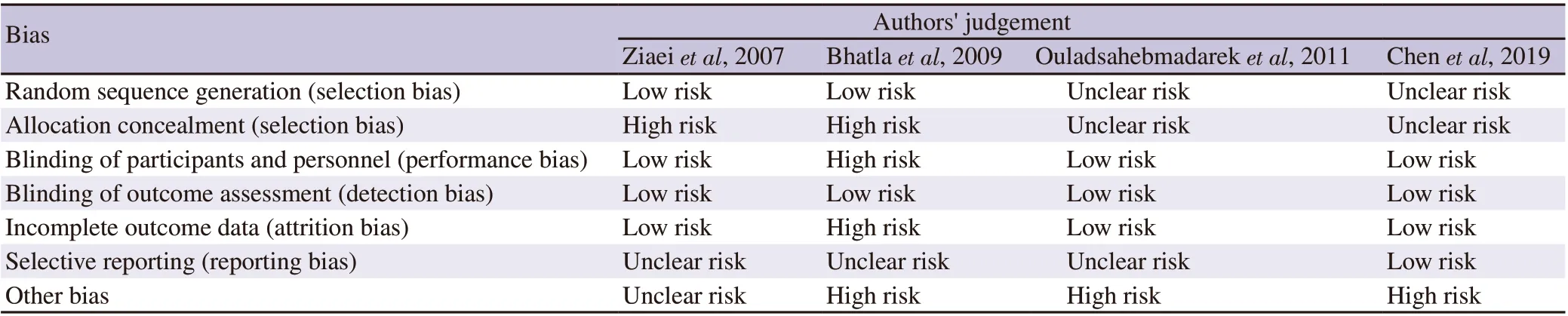
Table 2. Risk of bias assessment.
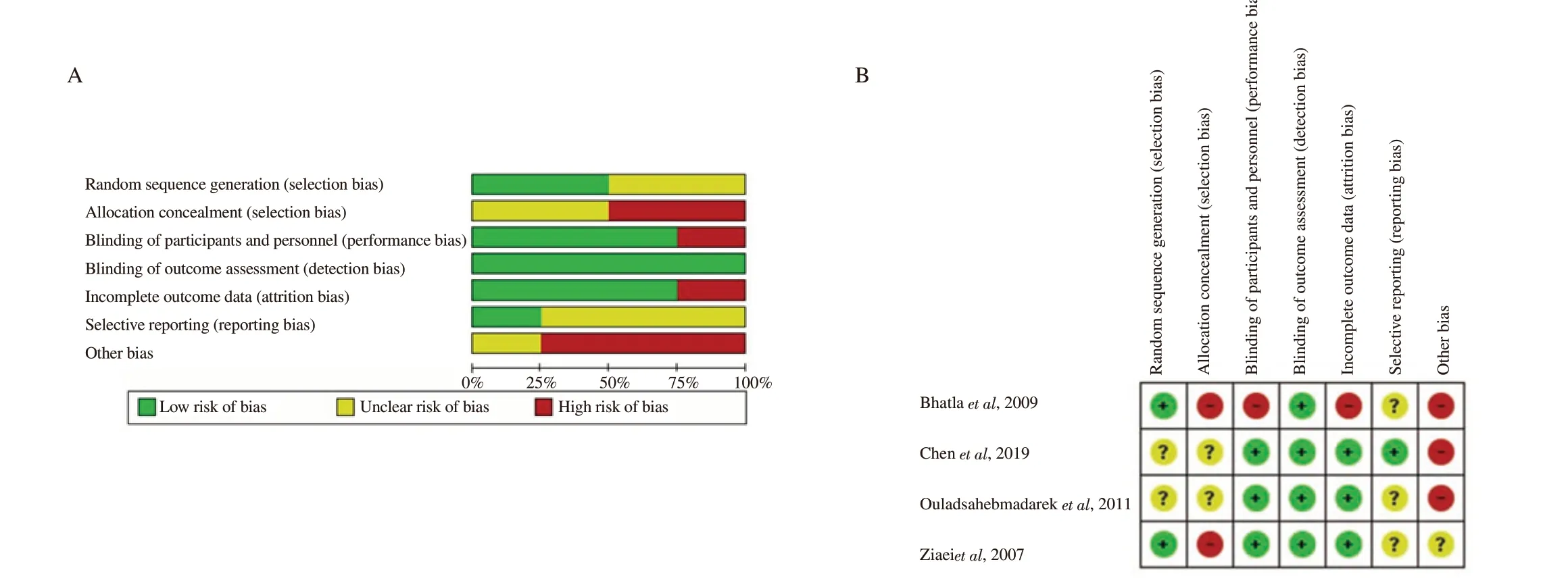
Figure 2. Risk of bias graph (A) and summary of risk of bias item for each included study (B).
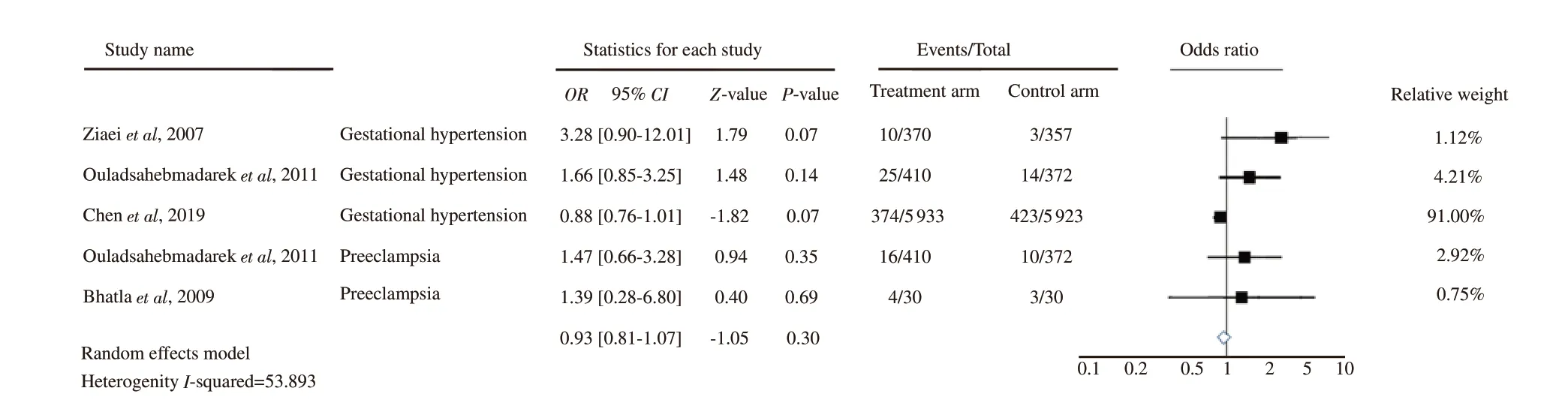
Figure 3. Forest plot analysis for hypertensive disorders in pregnancy outcomes.
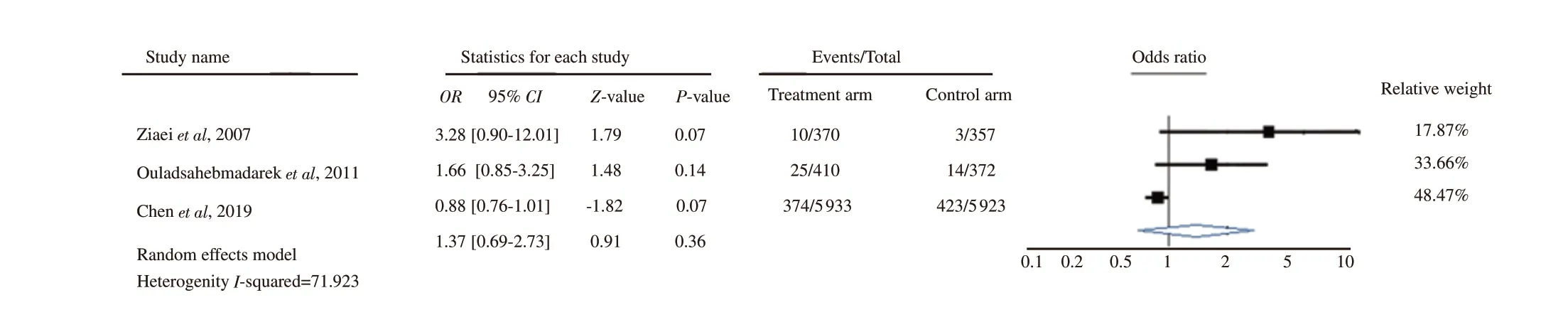
Figure 4. Forest plot analysis for gestational hypertension outcomes.

Figure 5. Forest plot analysis for preeclampsia outcomes.

Figure 6. Forest plot analysis for studies that included participants with Hb ≥13.2 g/dL and the incidence of gestational hypertension.
4. Discussion
This review uses the HDP term instead of pregnancy-induced hypertension (PIH) to follow international standards[11,12]. Ziaei et al[31] used the term ‘hypertension disorder’ without information about the proteinuria status, and therefore, it was categorized as gestational hypertension[11]. A similar case also happened in the study by Ouladsahebmadarek et al[32] and Chen et al[33] for their‘PIH’ outcomes. Due to the absence of definition of preeclampsia in the study by Bhatla et al[24] and Ouladsahebmadarek et al[32], the preeclampsia terms in these studies were assumed to be the same as the international guidelines, i.e., hypertension after 20 weeks of pregnancy with substantial proteinuria (>0.3 g in a-24-hour urine sample)[11,12].
4.1. Iron supplementation, initial Hb level, and potential HDP
Iron supplementation may be beneficial for pregnant women with low initial iron status and may be harmful to those with high initial iron status[19]. The trial by Ziaei et al[31] used Hb level 13.2 g/dL as the cut-off, and a study by Chen et al[33] had participants with initial Hb level ≥13.2 g/dL. The total participants from both studies were 24% (3 270 women) of the total participants in this review(13 425 women). The pooled analysis of the two studies found that there might be no association between mothers’ initially high Hb and the incidence of gestational hypertension at the end of pregnancy (OR 1.50, 95% CI 0.47-4.75; P=0.49; I2: 69.486%). The low precision of such a pooled analysis may be caused by the small number of trials and participants[40]. The substantial heterogeneity may be due to the various iron doses (daily 50 mg versus daily 30 mg of elemental iron) and the duration of supplementation (±22 weeks versus ±34 weeks[38]. Hence, more studies are needed to reach a definite conclusion. In addition, this pooled result could not adequately support or against the quasi-experimental studies, suggesting that a high Hb level in the first trimester is correlated with the development of HDP[14,17,18].
It is widely considered that HDP is caused by poor placentation[41].A high level of Hb possibly increases the blood viscosity, leading to impaired placental circulation[21], placental cells injury, and oxidative stress[42], which eventually induce HDP[21]. Therefore,iron supplementation for mothers with high initial Hb levels requires reconsideration[14,17,18]. Despite weak pooled estimates of experimental studies in this review, the result still benefits the understanding of iron supplementation impacts on women with initial high Hb and is complementary to the previous quasiexperimental research. Furthermore, these results suggest that screening Hb level and iron status in the first antenatal care visit is essential to recognise any potential HDP, and the decision to provide iron supplementation should be based on the screening’s result.Further study is needed to analyse the effect of iron supplementation on women with initial high Hb.
4.2. Iron supplementation, high Hb level during pregnancy,and potential HDP
Similar to early pregnancy, the threshold (≥13 g/dL) is also considered as a high Hb level in the second and third trimester of pregnancy which potentially impacts on adverse pregnancy outcome[30]. Trials by Ziaei et al[31], Bhatla et al[24] and Ouladsahebmadarek et al[32] provided the Hb level data at the start and at the end of the trial. The studies individually show no significant difference between Hb levels before and after iron supplementation among participants in the treatment arms. The highest Hb after treatment observed by Ziaei at al[31] was (13.75±1.05) mg/dL, with a baseline Hb was (13.98±0.56) mg/dL. The finding indicates that Hb level might not entirely reflect the maternal iron status. The estimation of iron status is best when multiple indicators are used[43,44]. Some resources suggest that serum ferritin may be a more accurate indicator of iron status in pregnancy[45-48]. In this review, only Ouladsahebmadarek et al[32] examined the serum ferritin: (41.05±2.16) μg/dL in the treatment arm as the baseline.By using the threshold of ≥20 μg/L for normal iron status[49],Ouladsahebmadarek et al’s study had more confidence in including non-iron deficiency anaemia participants. At the end of pregnancy,the serum ferritin was (26.91±2.11) μg/dL in the treatment arm.These results indicate that the serum ferritin decreased significantly,whereas the Hb level remains stable during pregnancy in the daily iron-supplemented arm.
The relation between iron supplementation, high Hb level during pregnancy, and potential HDP can be described in two approaches.First, iron supplementation may lead to excess iron because maternal hepcidin is suppressed during pregnancy[50,51]. The excess of iron can elevate the oxidative stress level[25] and lipid peroxides[24],leading up to endothelial cell dysfunction[16], which is known as a major presumption of the aetiology of HDP[41]. Second, a high Hb concentration may lead to HDP due to haemodilution disturbance[17,21]. However, the possibility of iron supplementation leading to a high Hb level is still unclear. Whereas Yip[45] has stated that iron supplementation may not lead to a higher Hb level than optimal concentration, the study by Pe?a-Rosas et al[29] has found that women receiving iron supplementation may have an increased risk of Hb level >13 g/dL during pregnancy. Therefore, further studies are needed.
4.3. Correlation between administering iron supplementation for non-anaemic pregnant women and the incidence of HDP
Korkmaz et al[52] found that iron supplementation for non-anaemic pregnant women during the first trimester significantly elevated the level of oxidative stress at 14th weeks of gestation and increased the negative pregnancy outcome. This approach may explain why a cohort study by Jirakittidul et al[15] found that the effect of iron supplementation (60 mg of iron/day) given before 16 weeks of pregnancy was significantly associated with the development of preeclampsia among women with initial Hb level of(12.58±0.79) g/dL.
A trial by Viteri et al[53] suggested that a weekly iron supplementation scheme (120 mg iron and 0.4 mg folic acid)from 20 until 28 weeks of pregnancy might prevent anaemia and decrease lipid peroxidation and oxidative stress. This approach could be an alternative HDP prevention for non-anaemic pregnant women, but further research should be directed to understand the impact of this scheme if used until the end of pregnancy.Similarly, a trial by Casanueva et al[54] found that a weekly intake (2 tablets of 60 mg elemental iron) for nonanaemic pregnant women (Hb >11.5 g/dL) at 20 weeks of gestation potentially give better outcomes, particularly decreasing the risk for haemoconcentration at 28 weeks of pregnancy onwards. This effort could prevent severe anaemia as well as oxidative stress damage[53].The recommendations proposed in these two studies are in line with a Cochrane systematic review by Pe?a-Rosas et al[30] which has found that intermittent oral iron supplementation (weekly 120 mg elemental iron starting at the second trimester of pregnancy) did not increase haemoglobin level higher than 13 g/dL in the second and third trimester. These analyses suggest that iron supplementation in pregnancy may give more benefit if it is administered basis on the needs as indicated in previous research[55-57]. Given that intermittent oral iron supplementation started in the second trimester might not increase maternal high Hb, lipid peroxidation, and oxidative stress,this could be an alternative for preventing HDP among non-anaemic pregnant women. Further experimental studies, however, remain needed to understand more thoroughly the effect of intermittent iron supplementation for non-anaemic pregnant women.
There are some strengths and limitations of this review. To the best of the authors' knowledge, there is no previous meta-analysis on the association between iron supplementation and the incidence of HDP using experimental studies focused on non-anaemic pregnant women only. Although its weak pooled estimates, this research still benefits from the evidence of the U-shaped curve for HDP outcome from the effect of excess iron's side by including experimental studies. This review only included studies published in English, which may be any other potential studies presented in different languages.
In conclusion, iron supplementation may have no association with HDP, gestational hypertension, and preeclampsia. In general, the evidence of the effect of iron supplementation among non-anaemic pregnant women to the incidence of HDP is still inconclusive due to lack of studies, and further research are needed.
Conflict of interest statement
The authors declare that there is no conflict of interest.
FundingThis study was funded by the Indonesia Endowment Fund for Education (LPDP) with Reference number S-422/LPDP.3/2018.
Authors’contributors
Farida Fitriana and Phoebe Pallotti prepared, conceived, and designed the research. Farida Fitriana performed the statistical analyses. All authors contributed to the study.
 Asian Pacific Journal of Reproduction2022年4期
Asian Pacific Journal of Reproduction2022年4期
- Asian Pacific Journal of Reproduction的其它文章
- Reproductive health and rights in the COVID-19 era: Why and how are rights and choices still the answer?
- Assisted reproduction in the COVID-19 era: Dilemmas and conundrums
- Testicular vascularization at two locations in relation to hormonal levels, and pixel echotexture in bulls at different ages
- Season modulates endocrinological profiles and sex behavioural characteristics in indigenous male goats under tropical humid island ecosystem
- Impact of geographical and seasonal temperature on sperm parameters in Indian men who were partners in subfertile couples - A retrospective analysis
- Conventional treatment options and herbal remedies for male infertility: An overview
