Increasing angiotensin-converting enzyme (ACE) 2/ACE axes ratio alleviates early pulmonary vascular remodeling in a porcine model of acute pulmonary embolism with cardiac arrest
Hong-li Xiao, Lian-xing Zhao, Jun Yang, Nan Tong, Le An, Guo-xing Wang, Miao-rong Xie, Chun-sheng Li
1 Department of Emergency Medicine, Beijing Friendship Hospital, Capital Medical University, Beijing 100050, China
2 Department of Emergency Internal Medicine, the Affi liated Hospital of Qingdao University, Qingdao 266000, China
3 Department of Emergency Medicine, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100050, China
KEYWORDS: Acute pulmonary embolism; Cardiac arrest; Early pulmonary vascular remodeling;Angiotensin-converting enzyme
INTRODUCTION
Acute pulmonary embolism (APE) is a syndrome that causes pulmonary circulation disturbance due to endogenous or exogenous embolus blocking the main stem or branch of pulmonary artery.It is the third most common cardiovascular disease with an annual incidence rate from 39 to 115 per 100,000 population.The mortality rate of cardiac arrest (CA) caused by APE is as high as 65%–95%.The main rescue measures for CA patients with suspected APE are cardiopulmonary resuscitation (CPR)and intravenous thrombolysis.The main cause of death is pulmonary arterial hypertension (PAH) following APE,which leads to acute right heart failure.The pathological and physiological characteristics of PAH are abnormal pulmonary vascular contraction and vascular remodeling in chronic pulmonary thromboembolism.Early inhibition of pulmonary vascular remodeling may reduce acute persistent PAH and hold up the formation of chronic PAH following APE. The pulmonary artery remodeling is initiated by pulmonary vascular endothelial cell dysfunction.The pathological manifestations of this dysfunction include endothelial cell proliferation and concurrent neoangiogenesis,leading to the formation of glomeruloid structures known as plexiform lesions.However, in massive APE, whether early pulmonary vascular remodeling has initiated and what are the pathological manifestations on pulmonary vascular endothelial cell remain to be established.
Endothelial dysfunction is caused by an imbalance in endothelial cell production of vasoconstrictors and vasodilators.In the renin angiotensin system (RAS),angiotensin-converting enzyme (ACE)-angiotensin(Ang) II-Ang II type 1 receptor (AT1) axis comprises vasoconstrictors that promote cell growth and vascular remodeling in PAH.The recently discovered ACE2-Ang (1-7)-Mas receptor axis of the RAS contributes to vasodilation and inhibits apoptosis, angiogenesis, and proliferation.Imbalance of the ACE2 and ACE axes is an important factor in endothelial dysfunction and vascular pathology.Whether the ACE and ACE2 axes participate in endothelial dysfunction during the early pulmonary arterial remodeling caused by massive APE with CA is not clear.
The ACE inhibitor captopril is a vasodilator that prevents the conversion of Ang I to Ang II. The result from our previous study confirmed that the pulmonary vascular resistance was reduced and the ACE2/ACE axes ratio was increased by captopril after ROSC in APE.To test the hypothesis that increasing ACE2/ACE axes ratio alleviates the early pulmonary arterial remodeling in APE,we examined the endothelial pathological changes and expression of ACE2/ACE axes in pulmonary artery with or without captopril in a porcine massive APE with CA model.
METHODS
Ethical approval
The experimental procedures were permitted by the Capital Medical University Institutional Animal Care Committee (permit number: 2010-D-013). All animals were housed individually in cages (80 cm×80 cm×90 cm) and fed with standard pig chow and free access to water. We kept room temperature at 26 ℃ and body temperature at 37 ℃using an infrared lamp.
Animal preparation
All procedures were performed on Landrace pigs, because of their similarity to humans in terms of the pathological and physiological characteristics of cardiovascular and endocrine diseases. Twenty-nine pigs from Beijing Experimental Animal Center (license number:SCXK 11-00-002) were used (both sexes, age 3 months,weight 28±2 kg). The midazolam (0.2 mg/kg) was used for intramuscular sedation, and then 3% pentobarbital (8 mg/[kg·h]) was intravenous continuously after propofol (1.0 mg/kg) injection through ear vein for general anesthesia.All pigs received mechanical ventilation by a ventilator(Evita 4, Draeger Medical, Germany) which delivered a tidal volume of 8 mL/kg at a respiratory rate of 12 to 20 breaths/min after endotracheal intubation. The end-tidal COwas maintained at 30–40 mmHg (1 mmHg=0.133 kPa)and oxygen saturation (SpO) 95%–100%. Central venous pressure was monitored through a triple-lumen central venous catheter inserting into the left femoral vein. Aortic pressure was observed through an arterial catheter inserting into the left femoral artery. Mean pulmonary artery pressure was measured by a Swan-Ganz catheter (7-Fr, Edwards Life Sciences, USA) inserting into the pulmonary artery through the right external jugular vein. All hemodynamic parameters were recorded under an M8001A monitor (Philips Medizin Systeme Boeblingen GmbH, Germany). The central venous pressure was maintained at 5–12 mmHg by infusion of normal saline (8 mL/[kg·h]) through the right femoral vein. A large-bore catheter (1.0-cm internal diameter) was manipulated into the left external jugular vein under the guidance of computed tomography (CT) so that its tip was f inally positioned very close to the orif ice of the pulmonary artery trunk. The M8001A monitor (Philips Medizin Systeme Boeblingen GmbH, Germany) was also used to visualize the electrocardiogram. All wounds were partially sutured. Catheter tips were left outside the body after the operation.
Experimental protocol
After penetration of the femoral vein, 100 mL of blood was obtained and left at room temperature for 2–3 h for selfcoagulation. About 10–15 mL blood clots were chopped into small clots (1.5 cm×1.0 cm×1.0 cm) which were then suspended in normal saline in a large catheter-tip syringe.The suspension was then injected into the left external jugular vein over the next 2 min, until mean arterial pressure(MAP) dropped below 30 mmHg.Animals were injected with 15,000 U/kg urokinase (Nanjing Nanda Pharmaceutical Co., Ltd., China) through a Swan-Ganz catheter that had been inserted into the pulmonary artery and received CPR(as dictated by the 2010 American Heart Association guidelines). If systolic blood pressure remained >50 mmHg for more than 10 consecutive minutes, we considered the animals to obtain the ROSC.Pigs were considered dead if spontaneous circulation was not restored within 30 min.CT-guided pulmonary angiography was used to diagnose pulmonary embolism.
Twenty-nine animals were randomly divided into the following four groups. In the control group (=5), the 10 mL of potassium chloride (15%) was injected intravenously into pigs after a bolus of propofol (3.0 mg/kg, 6 h after experimental preparation). In the APE-CA group (=5), pigs were injected quickly with thrombus until MAP dropped below 30 mmHg.Nineteen animals received thrombus injections until MAP dropped below 30 mmHg and then were treated with urokinase and CPR. Ten successfully resuscitated animals were randomly divided into two groups.In the ROSC-captopril group (=5), animals received captopril (Sigma-Aldrich, C8856-1G) with 22.22 mg/kg intravenously at 30 min after ROSC.Two pigs died at 6 h after ROSC. In the ROSC-saline group (=5), the animals were injected with the same volume of saline (SA) at 30 min after ROSC. One pig died at 4 h after ROSC, and another died 6 h after ROSC. At 6 h after ROSC, animals were euthanized with potassium chloride (15%, 10 mL) intravenously following injection of propofol with 3.0 mg/kg.
Histological evaluation
Pulmonary artery samples obtained at 6 h after ROSC were fixed with 10% buffered formalin, embedded in paraffin, sectioned with 5-μm thickness and processed for routine hematoxylin and eosin staining.
Western blotting anal ysis
Pulmonary artery tissue was homogenized in radioimmunoprecipitation assay (RIPA) lysis buff er plus protease inhibitors (Roche, Switzerland) and phosphatase inhibitors(Roche, Switzerland). About 20 mg of the tissue was cut into small pieces and homogenized in RIPA lysis buff er (200 μL).Tissue lysate was separated by centrifugation with 13,000×for 20 min at 4 °C, and swiftly harvested and kept in reserve at ?80 °C. The total protein concentration was measured with the bicinchoninic acid (BCA) assay (Pierce, USA).Homogenates were heated in the sample buff er at 95 °C for 5 min. The total protein (40 μg) was then run on the 10%and 12% sodium dodecyl sulfate (SDS)-polyacrylamide gels and wet-transferred to a polyvinylidene fluoride membrane(0.45 μm, Millipore, USA). Blots were blocked using 5%nonfat dry milk in tris-buff ered saline with Tween-20 (TBST)buff er (1 mol/L Tris-HCl [pH 7.5], 100 mmol/L NaCl, 20%Tween 20) for 2 h and incubated overnight at 4 °C with 1:1000 B-cell lymphoma-2 (Bcl-2), Bcl-2-associated X(Bax), angiopoietin-2, Tie 2, or 1:5000 cleaved caspase-3(Cell Signaling Technology, Inc., USA), or 1:10000 angiopoietin-1 antibody (Santa Cruz Biotechnology, Inc.,USA), vascular endothelial growth factor (VEGF), β-actin antibody (Abcam, UK). Following incubation, the blots were washed in TBST, and then incubated for 40 min at room temperature with secondary anti-rabbit or anti-mouse IgG-horseradish peroxidase-linked antibody (1:10000,Jackson ImmunoResearch Laboratories, Inc., USA).Bands were detected using enhanced chemiluminescence(Millipore, USA). The optical density for quantif ication was obtained with Gel Image system ver.4.0 (Tanon Science &Technology Co., Ltd., China).
lmmunohistochemical staining
The 3-μm-thickness pulmonary artery tissue embedded in paraffin was deparaffinized and serially dehydrated in ethanol. After blocking with 5% bovine serum albumin for 4 h, sections were then incubated overnight at 4 ℃ with anti-Bax (anti-mouse, 1: 250, Santa Cruz Biotechnology, Inc., USA), anti-angiopoietin-2(anti-rabbit, 1:200, Abcam, UK), and anti-VEGF (antirabbit,1:200, Abcam, UK). The sections were washed with phosphate-buff ered saline (PBS) (5 min, 3 times) and incubated with biotinylated secondary antibodies (1:200)for 2 h at room temperature, followed by incubation with avidin-biotin-peroxidase solution. The primary antibody with normal rabbit serum served as negative controls.Peroxidase conjugate localization was determined by diaminobenzidine tetrahydrochloride (DAB, Sigma, USA)as the chromogen and counterstained with hematoxylin.The positive areas showed the color of brown-yellow.Sections were viewed using an IX80 microscopy(Olympus, Japan) and analyzed using Image-Pro Insight 6.0 software (Media Cybernetics, USA).
Ultrastructure evaluation
Ultrastructure of pulmonary artery endothelial cells was observed by electron microscopy. Samples were obtained within 1–2 min of sacrifice. Pulmonary artery tissues were sliced (approximately 1-mm thickness) on ice, and then f ixed for 30 min at 4 ℃ with glutaraldehyde(2.5%). Samples were postfixed for 1 h with 1% (v/v)osmic acid, then dehydrated and embedded using Epon812 at 40 °C for 4 h, 50 °C for 2 h, or 90 °C for 12 h. Sections(0.5–1.0 μm thickness) were cut and stained using toluidine blue (0.5%), and then observed using a light microscope(Nikon Corporation, Japan). Ultra-thin (approximately 60 nm) sections were double-stained using uranyl acetate(2.0%) for 20 min and lead citrate (2.0%) for 15 min, and then examined by blinded observers under a HITACHI HT7700 transmission electron microscope (Hitachi Scientif ic Instruments, USA).
Statistical analysis
The results were expressed as mean±standard deviation(SD). The difference between groups was calculated by Student’s-test or analysis of variance (ANOVA). Pearson correlation was used to analyze correlations between the ACE2/ACE axes ratio and angiopoietin, ACE2/ACE axes ratio and VEGF, ACE2/ACE axes ratio and Bcl-2/Bax,ACE2/ACE axes ratio and cleaved caspase-3.<0.05 was considered statistically significant. All the analyses were conducted using the SPSS 25.0 software (SPSS Inc, USA).
RESULTS
Proliferative and apoptotic changes of endothelial cells in the APE-CA model
As shown in Figure 1A, the pulmonary arteries were almost completely occluded by accumulated endothelial cells in the APE-CA and ROSC-saline groups, while no angiostenosis due to endothelial cell proliferation was displayed in the control group. Fewer clogged arteries were discovered in the ROSC-captopril group than in the ROSCsaline group. The arteriolar lesions were characterized by plexiform lesion and concentric lesion composed of endothelial cells in “onion-skin” layers in APE (Figure 1A).Electron microscopy was used to observe the endothelial cells apoptosis and migration into vascular lumen in the APE-CA group, unlike the normal endothelial cell structure in the control group (Figure 1B).
Pulmonary arterial expression of ACE2/ACE axes ratio after APE-CA
Compared with the control group, at the time of CA caused by APE, expression levels of ACE (=0.001) and AT1 receptor (<0.001) in the pulmonary artery increased,while the expression of ACE2 and Mas receptor declined dramatically (=0.019, 0.029, respectively; Figure 2A). The expression of ACE (=0.004) and ACE2 (=0.001) declined significantly in the ROSC-saline group, compared to the APE-CA group (Figure 2A). To conf irm the opposing eff ects of the ACE2-Ang (1-7)-Mas receptor axis and the ACEAng II-AT1 receptor axis in massive APE, the correlation between the two axes was analyzed in the control group,APE-CA group, and ROSC-saline group. The results showed positive correlations between ACE2 and Mas receptor(=0.748,=0.005), and between ACE and AT1 receptor(=0.716,=0.009). Negative correlations were observed between Mas and AT1 receptors (= –0.665,=0.018).Immunohistochemical staining showed that ACE2 and Mas receptor were expressed in vascular endothelial cells and abnormally proliferative endothelial cells, which formed plexiform lesions (Figure 2B).
Pulmonary artery expression of angiopoietin-2/angiopoietin-1, VEGF, and Bax in APE-CA
The angiopoietin-2/angiopoietin-1 ratio increased 2.80 fold in the APE-CA group compared with that in the control group (<0.05; supplementary Figure 1A). There was no significant difference in p-Tie 2 expression between groups.The expression of VEGF and Bax was signif icantly higher in the APE-CA and ROSC-saline groups than in the control group(all<0.01; supplementary Figures 1 A and B). Compared with the control group, cleaved caspase-3 levels were higher in the ROSC-saline groups (<0.001; supplementary Figure 1B). Correlation analysis showed that levels of angiopoietin-1 and angiopoietin-2 presented opposing trends (= –0.747,=0.008), while angiopoietin-2 and VEGF expression were coincident (=0.927,<0.001). Immunohistochemical staining showed that Bax was expressed in pulmonary artery endothelial cells (supplementary Figure 2A); angiopoietin-2(supplementary Figure 2B) and VEGF (supplementary Figure 2C) were expressed in the abnormally proliferative endothelial cells of plexiform lesions during APE.
Eff ect of increasing ACE2/ACE axes ratio on postresuscitation angiopoietin-2/angiopoietin-1 and VEGF expression in APE-CA
In contrast to the APE-CA group, the ROSCsaline group showed a slight decrease in angiopoietin-2/angiopoietin-1 ratio (>0.05) and a substantial decrease after captopril treatment (<0.05; supplementary Figure 1A).Similarly, captopril inhibited VEGF activation compared with saline after ROSC (<0.05; supplementary Figure 1A). Correlations between ACE2/ACE axes ratio and angiopoietins/VEGF were analyzed. There were positive correlations between ACE and angiopoietin-2 (=0.645,=0.032), and between ACE and VEGF (=0.612,=0.045). There were negative correlations between ACE and angiopoietin-1 (= –0.665,=0.026), ACE2/ACE axes ratio and angiopoietin-2 (= –0.833,=0.001), and between ACE2/ACE axes ratio and VEGF (= –0.893,<0.001).
Eff ect of increasing ACE2/ACE axes ratio on postresuscitation apoptosis of endothelial cells in APE
In the APE-CA group, there were signif icant correlations between Bcl-2/Bax and ACE2/ACE axes ratio (=0.981,<0.001), and between cleaved caspase-3 and ACE2/ACE axes ratio (= –0.602,=0.038). Captopril increased the Bcl-2/Bax ratio (=0.006) and decreased cleaved caspase-3 levels (=0.004; supplementary Figure 1B).
DISCUSSION
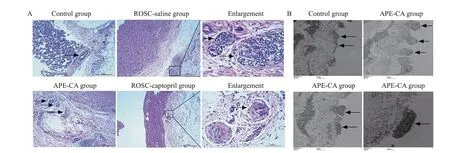
Figure 1. Mor phological and ultrastructure evaluation of pulmonary artery in APE. A: morphological evaluation of pulmonary artery in APE.Pulmonary arteries were occluded in the APE-CA and ROSC-saline groups (black arrow, ×100); fewer pulmonary arteries were occluded in the ROSC-captopril group (×100); plexiform lesions in the ROSC-saline group (enlargement, black arrows, ×400) and concentric lesions in ROSCcaptopril group (enlargement, black arrows, ×400) were found. B: ultrastructure of endothelial cells in APE. Normal endothelial cell structure in the control group (black arrows, ×7,000), endothelial cell migration into vascular lumen in the APE-CA group (black arrows, ×10,000),and endothelial cell apoptosis in the APE-CA group (black arrows, ×10,000 and ×30,000). APE: acute pulmonary embolism; ROSC: return of spontaneous circulation; CA: cardiac arrest.

Figure 2. P rotein expression of RAS in the pulmonary artery and eff ects of captopril on APE. A: protein expression and quantitative analysis of RAS in the pulmonary artery and eff ects of captopril on APE, as revealed by Western blotting analysis; B: immunohistochemistry analysis of protein expression of ACE2 in the pulmonary artery in the control, APE-CA (black arrow pointing to expression of ACE2 in vascular endothelial cells), ROSC-saline (black arrow showing expression of ACE2 in vascular endothelial cells), and ROSC-captopril groups (red arrow showing expression of ACE2 in proliferative endotheliocytes); protein expression of Mas receptor in the pulmonary artery in the control, APE-CA (red arrow showing expression of Mas in proliferative endotheliocytes), ROSC-saline, and ROSC-captopril groups (black arrow showing expression of Mas receptor in vascular endothelial cells; red arrow showing expression of Mas receptor in proliferative endotheliocytes). Compared with the control group, *P<0.05, **P<0.01, ***P<0.001; compared with the APE-CA group, ##P<0.01, ###P<0.001; compared with the ROSC-saline group,^P<0.05. Scale bar in B: 100 μm. RAS: renin angiotensin system; APE: acute pulmonary embolism; ACE2: angiotensin-converting enzyme 2; CA:cardiac arrest; ROSC: return of spontaneous circulation.
In this study, we described endothelial proliferation and apoptosis during early pulmonary arterial remodeling and imbalance of the ACE2 and ACE axes and increases in the angiopoietin-2/angiopoietin-1 ratio, VEGF, and pro-apoptotic factors. Increasing ACE2/ACE axes ratio with captopril could inhibit the activation of angiopoietin-2/angiopoietin-1,VEGF, and pro-apoptotic factors after ROSC. This finding implicates a critical role for increasing ACE2/ACE axes ratio in alleviating endothelial proliferation and apoptosis during the early pulmonary arterial remodeling of acute PAH due to massive APE.
The endothelial dysfunction triggers vascular remodeling,which leads to the formation of PAH.The pathological characteristics of pulmonary endothelial dysfunction in PAH include disordered proliferation, abnormal intimal thickening,and the formation of fibrotic, plexiform, concentric, and dilation/angiomatoid lesions. Plexiform lesions are formed by irregular endothelial cell masses and sparse myofibroblasts.Concentric lesions are composed of endothelial cells and myofibroblasts in “onion-skin” layers.These findings are in partial accordance with our morphological analysis of the pulmonary artery in the APE-CA model which showed that endothelial cells proliferated randomly, forming plexiform and concentric lesions. In addition, electron microscopy revealed endothelial cell apoptosis. These results illustrate that endothelial cell apoptosis and proliferation contribute to early pulmonary arterial remodeling in APE.
Besides pathological abnormalities, we found increased angiopoietin-2/angiopoietin-1 ratio and VEGF expression,which represent endothelial proliferation. Angiopoietin-1 is a Tie-2 agonist that protects against endothelial cell apoptosis to maintain homeostasis of the vasculature in PAH.Angiopoietin-2 is an antagonist to Tie 2, blocking receptor activation by angiopoietin-1. Angiopoietin-2 participates in the formation of pathological neovascularization and vascular leakage together with VEGF by stimulating disordered endothelial cell proliferation,and is up-regulated in plexiform lesions in idiopathic PAH patients.VEGF is a specific mitogen of endothelial cells that contributes to the formation of plexiform lesions or endothelial cell clusters in PAH.These results are in accordance with our present f indings that angiopoietin-2 and VEGF were both expressed in plexiform lesions and VEGF was also expressed in concentric lesions by immunohistochemical analysis. Moreover, the protein expression of angiopoietin-2/angiopoietin-1 and VEGF increased in APE-CA by Western blotting analysis.These findings indicated that the activation of VEGF and increased angiopoietin-2/angiopoietin-1 ratio were associated with pulmonary arterial remodeling, which presented as plexiform and concentric lesions formed by disordered endothelial cell proliferation.
The classic ACE-Ang II-AT1 receptor axis of RAS is known to cause endothelial dysfunction.ACE2-Ang (1-7)-Mas receptor axis opposed the detrimental effects of ACE-Ang II-AT1 receptor axis.In a model of severe PAH induced by monocrotaline, neointimal occlusive lesions and impaired endothelium-dependent relaxation in pulmonary arteries were alleviated by ACE2.However,it remains unclear the roles of pulmonary artery ACE2 and ACE axes in APE. In the present study, Western blotting analysis revealed increased ACE levels and reduced ACE2 levels in the pulmonary artery after CA due to APE.Immunohistochemistry revealed expression of ACE2 and Mas receptors in plexiform lesions after CA due to APE.The results indicated that ACE2/ACE axes imbalance participated in the pulmonary artery remodeling in APE.
The ACE inhibitor captopril infusion was associated with signif icantly lower mean right ventricular pressure and pulmonary vascular resistance, and higher serum Ang (1-7) levels, ACE2/ACE ratio, and Ang (1-7)/Ang II ratio after ROSC.This study is an extension of previous research and we found that increasing ACE2/ACE axes ratio by captopril could alleviate pulmonary artery remodeling after ROSC caused by APE. The ACE axis has been shown to contribute to the activation of angiopoietin, VEGF, and proapoptotic factors. Otani et alshowed that Ang II induced the expression of angiopoietin-2, but not angiopoietin-1 or VEGF, in cultured bovine retinal endothelial cells. Madsen et alfound that AT1 receptor antagonism inhibited the transcription of VEGF, angiopoietin-1, angiopoietin-2,and Tie 2 in cortex and medulla in postnatal kidney injury and diabetic nephropathy.In our investigation of the pulmonary artery remodeling in APE-CA, captopril alleviated histological endothelial cell proliferation and apoptosis, and reduced the angiopoietin-2/angiopoietin-1 ratio and VEGF levels as well as pro-apoptotic factor cleaved caspase-3 expression. The present f indings might suggest a novel protective effect of increasing ACE2/ACE axes ratio on the inhibition of early pulmonary artery remodeling in APE. Our study had a certain limitation. The sample size is not enough to observe mortality.
CONCLUSIONS
The present findings show that the early pulmonary artery remodeling including endothelial cell proliferation and apoptosis occurs in massive APE pigs with CA.Increasing ACE2/ACE axes ratio reduces endothelial cell proliferation by inhibiting VEGF and angiopoietin-2/angiopoietin-1 ratio after ROSC. In addition, increasing ACE2/ACE axes ratio restrains endothelial cell apoptosis by reversing the imbalance in Bcl-2/Bax levels and decreasing cleaved caspase-3 expression.
This work was supported by grants from the National Natural Science Foundation of China (81773931 and 81374004);the Beijing Municipal Administration of Hospitals’ Youth Program(QML20170105); the Natural Science Foundation of Beijing Municipality (7173253); and the Beijing Municipal Administration of Hospitals Clinical Medicine Development of Special Funding Support “Yangfan” Project (ZYLX201802).
The experimental procedures were permitted by the Capital Medical University Institutional Animal Care Committee (permit number: 2010-D-013).
No conf licts of interest declared.
HLX: literature search, study design, animal care, data collection, data analysis, data interpretation, writing,and critical revision; LXZ: animal care, pathology methods, data collection, data analysis, and data interpretation; JY, NT, LA,and GXW: animal care, data collection, data analysis, and data interpretation; MRX and CSL: literature search, study design, data analysis, data interpretation, writing, and critical revision.
All the supplementary files in this paper are available at http://wjem.com.cn.
 World Journal of Emergency Medicine2022年3期
World Journal of Emergency Medicine2022年3期
- World Journal of Emergency Medicine的其它文章
- Mortality-related electrocardiogram indices in methanol toxicity
- The combination of creatine kinase-myocardial band isoenzyme and point-of-care cardiac troponin/contemporary cardiac troponin for the early diagnosis of acute myocardial infarction
- Shrinking lung syndrome in autoimmune inflammatory diseases: A case series and review of literature
- Traumatic tension pneumocephalus: A case report
- Blunt myocardial injury and gastrointestinal hemorrhage following Heimlich maneuver: A case report and literature review
- Successful treatment of a patient with diff use alveolar hemorrhage and anti-neutrophil cytoplasmic antibody-associated vasculitis
