Effects of Iranian Polyherbal Syrup (Zufa syrup)on oxygen saturation and clinical symptoms in suspected patients with COVID-19:a triple-blinded,randomized,placebo-controlled trial
Razieh Borujerdi,Seyed Hasan Adeli,Abolfazl Mohammadbeigi,Fatemeh Aliasl,Akram Asghari,Ahmad Hormati,Hosein Moradi Dehnavi,Farhad Hoseini,Majid Asghari,*
1 Department of Persian Medicine,School of Persian Medicine,Qom University of Medical Sciences,Qom,Iran
2 Department of Internal Medicine,School of Medicine,Spiritual Health Research Center,Qom University of Medical Sciences,Qom,Iran,
3 Department of Epidemiology and Biostatistics,School of Health,Qom University of Medical Sciences,Qom,Iran
4 Gastroenterology and Hepatology Disease Research Center,Qom University of Medical Science,Qom,Iran
Abstract Coronavirus disease 2019 (COVID-19) pandemic has caused an urgent need for investigating potential treatments.Traditional medicine off ers many potential remedies that have been historically used and have the advantage of bypassing the cultural obstacles in the practice of medicine.We aimed to investigate the effi cacy of Zufa syrup in the treatment of suspected patients with mild to moderate symptoms of COVID-19.This triple-blind randomized controlled trial recruited patients with evidence of COVID-19 on chest computed tomography without an indication of hospital admission from March 2020 until April 2020.Participants were assessed by a physician and completed a pre-specif ied form to assess the duration and severity of symptoms.Patients were randomized to receive Zufa syrup (a combination of herbal medicines:Nepetabracteata,Ziziphus jujube, Glycyrrhizaglabra,Ficuscarica,Cordia myxa,Papaver somniferum,Fennel,Adiantumcapillusveneris,Viola,Viper’s-buglosses,Lavender,Iris,and sugar) or identical-looking placebo syrup at a dose of 7.5 mL (one tablespoon) every 4 hours for 10 days.After applying the eligibility criteria,116 patients (49.1% male) were randomized to trial arms with a mean age of 44.3.During the follow-up,Cough,dyspnea,headache,myalgia,anorexia,anxiety,and insomnia improved gradually in both groups,and showed no diff erence between Zufa syrup and placebo.Oxygen saturation and pulse rate had stable trends throughout the follow-up and were similar between study arms.No patient required hospital admission or supplemental oxygen therapy during the study period.To conclude,in patients with mild to moderate symptoms of COVID-19,Zufa syrup did not show any diff erence in symptomatology over a 10 days’ period when compared with placebo.Due to potential eff ects of medicinal plants in the treatment of respiratory infections,further studies are warranted to clarify their role in COVID-19.The study was approved by the Ethics Committee of the Qom University of Medical Science (Ethics committee reference number IR.MUQ.REC.1398.165) on March 10,2020 and was registered in Iranian Clinical Trial Center (approval ID:IRCT20200404046934N1) on April 13,2020.
Key words: corona;COVID-19;herbal drug;Iran;Nepetabracteata;pandemic;respiratory disease;traditional medicine
INTRODUCTION
Coronavirus disease 2019 (COVID-19) emerged in late 2019,1and has since become a global threat and provoked a pandemic.2As of July 16,2020,more than 13 million individuals have contracted the virus globally and over 574,000 people have died from COVID-19.2,3Although,vaccination have already started and is on its way to cover major population in some of the western countries,but it is still awaiting sensitivity analyses to evaluate the eff ectiveness of the vaccine after diff erent intervals following vaccination.3Besides lethal forms of COVID-19,many patients show mild to moderate symptoms and do not require hospitalization.4Such patients may require appropriate treatments to alleviate symptoms and stop the progression of disease.
Traditional and alternative medicine approaches have been employed in the care of COVID-19 patients during this pandemic,especially in China.5The traditional Persian medicine is a widely practiced alternative medicine intertwined with the culture and history of Iran,which has the advantage of bypassing the cultural obstacles in the practice of medicine.6Several herbs are used in the practice of traditional Persian medicine for alleviation of symptoms associated with common viral respiratory infections.Nepetaspecies have been found to be eff ective in chronic productive cough and in patients with chronic bronchitis.7,8In another study,Viola syrup reduced coughing in children who suff ered from asthma.9A mixture of several traditional herbs was also proven eff ective to ameliorate the symptoms of common cold in asthmatic children.10Moreover,studies have observed evidence of antiviral eff ects in a handful of these traditionally used herbal remedies,which might prove helpful with future investigations.11,12
The Zufa syrup is an herbal mixture of traditional Persian medicine remedies used for the treatment of respiratory illnesses which contains elements from several plants.As the search for eff ective treatments for COVID-19 continues,we decided to conduct a randomized clinical trial to investigate the eff ectiveness of Zufa syrup in patients with mild to moderate manifestations of COVID-19.
SUBJECTS AND METHODS
This randomized,placebo-controlled,triple-blind clinical trial was conducted in compliance with the principles of theDeclaration of Helsinkiand was approved by the Ethics Committee of the Qom University of Medical Science (Ethics committee reference number IR.MUQ.REC.1398.165) on March 10,2020 and was registered in Iranian Clinical Trial Center (approval ID:IRCT20200404046934N1) on April 13,2020.All participants were required to provide informed written consent.
Study design and participants
Patients were recruited from Shahid Beheshti Hospital of respiratory disease with mild to moderate symptoms suggestive of COVID-19 without an indication for hospital admission from March 2020 until April 2020.The def inition of mild to moderate symptoms was based on physician examination and oxygen saturation,respiratory rate and self-declaration of patients based on a checklist (Additional f ile 1).All patients who were visited by a physician in one of the assigned hospital outpatient clinics or offi ces were initially screened for eligibility and a chest computed tomography (CT) was requested.Inclusion in the study required the following criteria:20-70 years of age,presence of COVID-19 lung involvement on chest CT,and indication of outpatient pharmacotherapy for COVID-19 with moderate symptoms.Key exclusion criteria included a history of heart,lung,kidney,or liver disease,diabetes mellitus,systolic blood pressure higher than 160 mmHg,fever of 39°C or higher,pregnancy,and breastfeeding.
Following the conf irmation of eligibility and review of the CT scan,the demographic and baseline clinical data were recorded.A table of relevant symptoms was provided to patients as they were educated about each symptom and instructed to f ill the table for each day during follow-up.The patients rated each symptom as mild,moderate,or severe.Following enrollment,patients were randomized 1:1 to receive either the Zufa syrup or placebo syrup.The randomization code was generated by computer in permuted blocks of 4.The patients,the recruiting physicians who provided the syrups,the researchers who conducted the follow-ups and those who analyzed the data were blind to the treatment arms.Each syrup bottle had a unique code on its label and the recruiting physicians were instructed to provide bottles in the chronological order of the label code.The recruiting physicians were not aware of the randomization sequence or the block size.After the completion of data collection,a member of the staff ,who was not at any stage involved in the process of patient enrollment and follow-up,used the label codes to assign patients to treatment arms named A and B.Un-blinding was done after the conduction of statistical analysis.
Study medication
Zufa syrup (IRC:2129211562044973) was manufactured by Booalidaroo Pharmaceutical Company (Qom,Iran) and the placebo was prepared at the same appearance too.Zufa syrup is a poly herbal medicine that is a combination ofNepetabracteata,Ziziphus jujube,Glycyrrhizaglabra,Ficuscarica,Cordia myxa,Papaver somniferum,Fennel,Adiantumcapillusveneris,Viola,Viper’s-buglosses,Lavender,Iris,and sugar.Patients were instructed to take 7.5 mL of their syrup every 4 hours for 10 days.The remaining treatments except for Zufa syrup were the same between two groups and the patients were on the recommended treatments based Ministry of Health of Iran protocols.Routine treatment is generally considered according to protocol of COVID-19.13,14
Follow-up and endpoints
The time from the onset of COVID-19 symptoms to the presentation to investigating physicians was determined at f irst visit.Every other day,patients were followed through phone calls which inquired about treatment adherence and changes in symptomatology.Each symptom was assessed by the patient assigning a number describing its severity (1 = mild,2 = moderate,3 = severe).Oxygen saturation was recorded with a pulse oximetry (ChoiceMMed,Beijing,China) through home-visits on alternate days.The indications of hospital admission were oxygen saturation < 93%,respiratory rate > 30 breaths/min and acute respiratory disease.
Some symptoms include of fever,shaking,cough,dyspnea,headache,myalgia,fatigue,weakness,anorexia,and insomnia were registered.We have not any side eff ect in patients,one of them has headache and been excluded.
Statistical analysis
Statistical analysis conducted using SPSS version 20 (IBM,Armonk,NY,USA) and sample size calculation estimated by MedCalc Statistical Software version 15.8 (MedCalc Software bvba,Ostend,Belgium).Categorical variables are shown as number (percentage) and compared by Chi-square test.Continuous variables are shown as mean ± standard deviation (SD) and compared by independent samplest-test.For comparison of the follow-up variables,the symptoms were assessed by severity scores of 1 to 3.In each group,repeated measurements analysis of variance was performed according to measurements on each day of the follow-up.Tukey’spost hoctest was used for further pairwise comparison.The Zufa syrup and placebo arms were compared by independent samplest-tests.
Assuming a treatment eff ect size with a diff erence of at least 10% in symptoms between the two arms,with a power of 80% and a type 1 error of 5%,the appropriate sample size was calculated to be 65 participants in each group.
Six of participants were excluded,three of whom were excluded because of interference with other herbal medicine,and two of whom were admitted in hospital later for headache.
RESULTS
Trial population
Figure 1 shows the CONsolidated Standards Of Reporting Trials (CONSORT) trial f lowchart of participants through each stage of a randomized trial.From March 2020 until April 2020,were screened among whom 116 patients (49.1% male)with evidence of COVID-19 on chest CT were randomized.
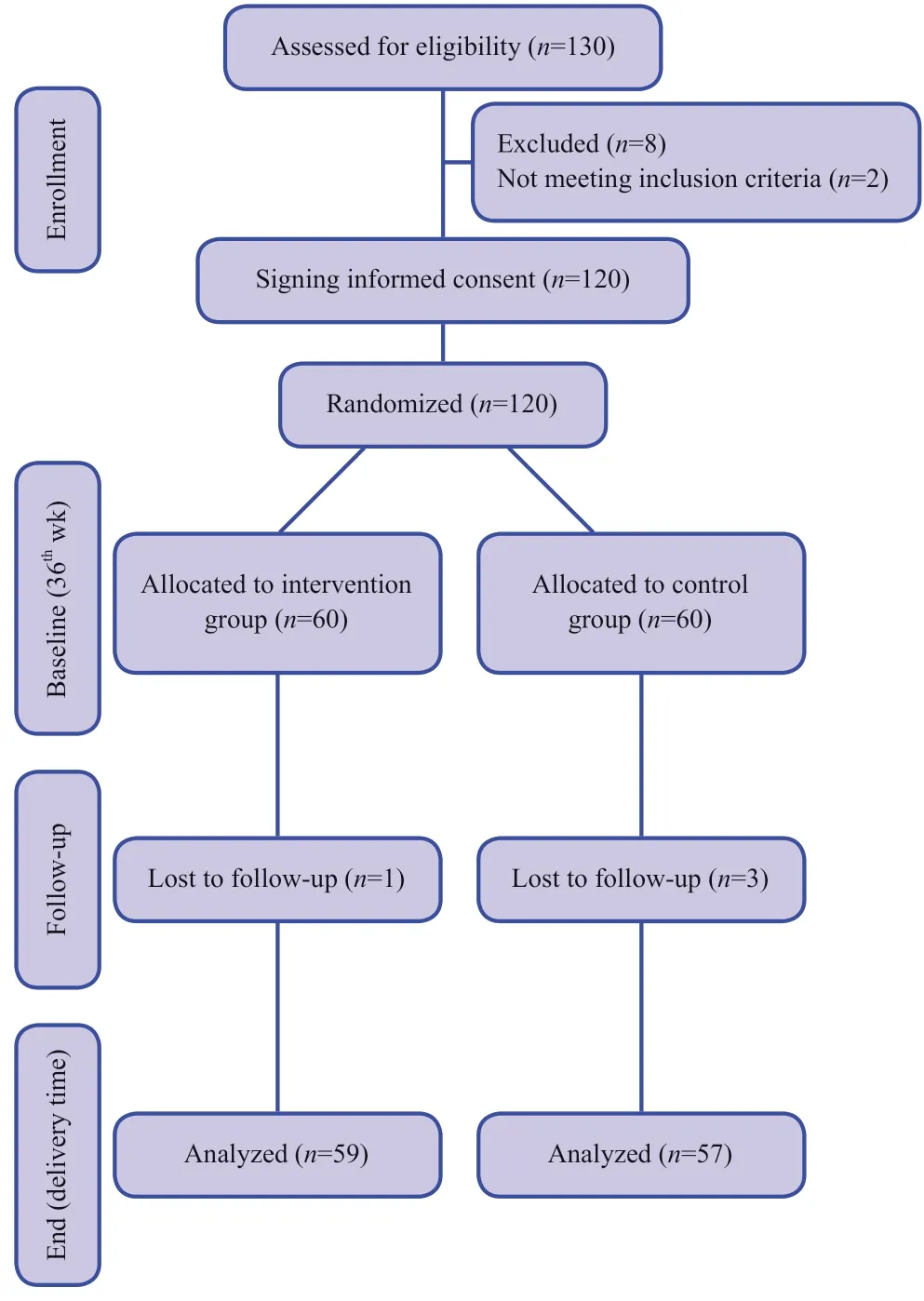
Figure 1:CONsolidated Standards Of Reporting Trials (CONSORT)diagram showing the flow of participants through each stage of a randomized trial.
The age of the population was 44.3 ± 11.5 years.Table 1 summarizes the baseline characteristics of study participants.At the f irst visit,the time from symptom onset until presentation was documented for each symptom and is presented in Table 2.The mean duration of symptoms was the longest for dry cough (5.4 days),followed by dyspnea (4.3 days),and was the least for productive cough (2.2 days).All patients were assessed through the 10 days’ follow-up period and data gathering was completed without loss to follow-up.No major adverse events were reported by study participants.

Table 1:Baseline characteristics of COVID-19 participants
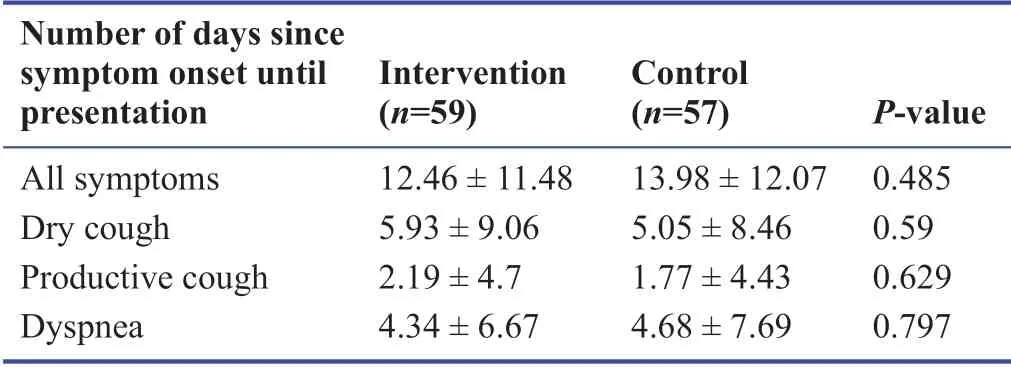
Table 2:Time from COVID-19 symptoms onset to presentation
COVID-19 symptomatology
Among the study population during the follow-up,none were admitted to the hospital or require oxygen therapy.Most patients improved during the 10 days’ treatment period.The symptomatology follow-ups assigned scored according to the severity of each symptom,and the data are demonstrated in Tables 3-9.The cough,dyspnea,headache,myalgia,anorexia,anxiety,and insomnia were improved signif icantly over the 10 days’ period in both the intervention and placebo group compared with those before treatment (P< 0.001).There was no signif icant diff erence in the severity of symptoms at each time point between those who the patients the Zufa syrup and placebo (Tables 3-9),with the exception of dyspnea on the 10thday of treatment which was reported to be less severe by the placebo group patients (P= 0.007).
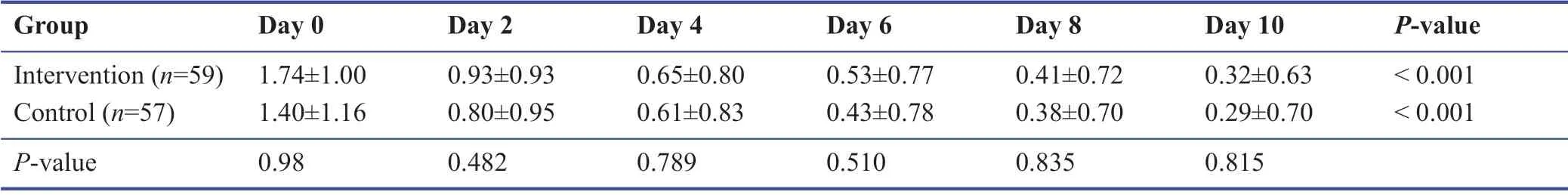
Table 3:Cough during the study period
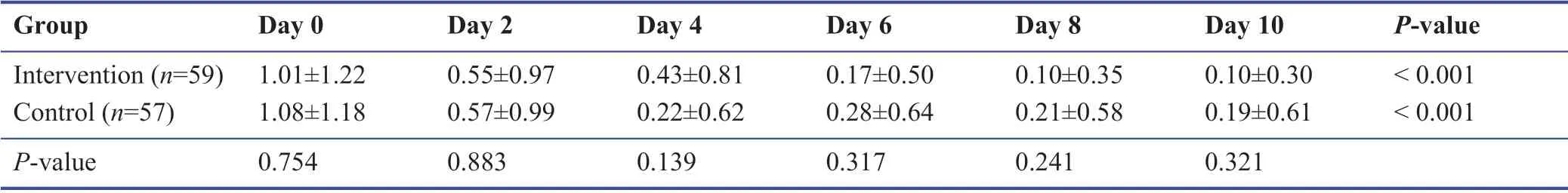
Table 4:Headache during the study period
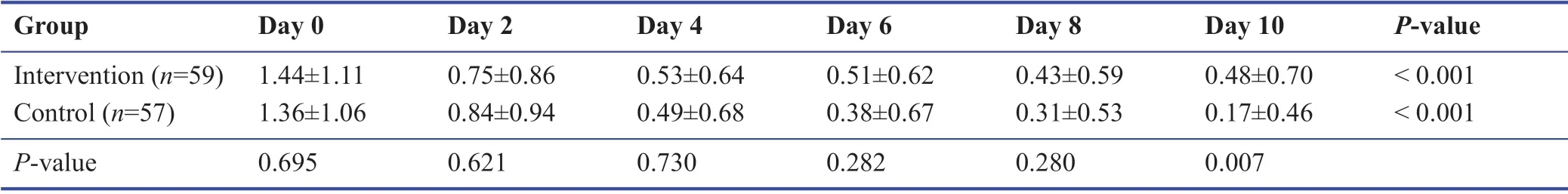
Table 5:Dyspnea during the study period
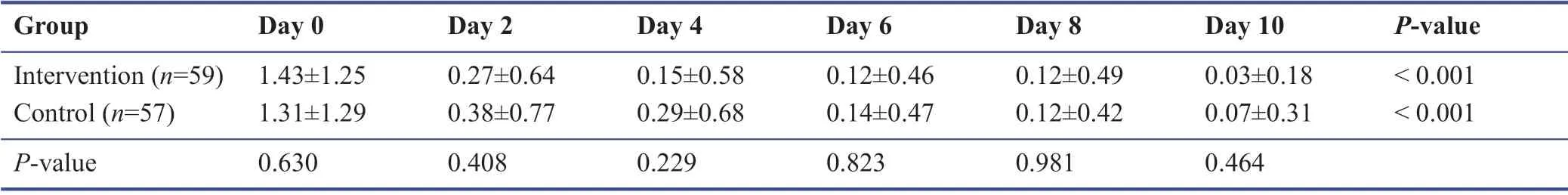
Table 6:Myalgia during the study period
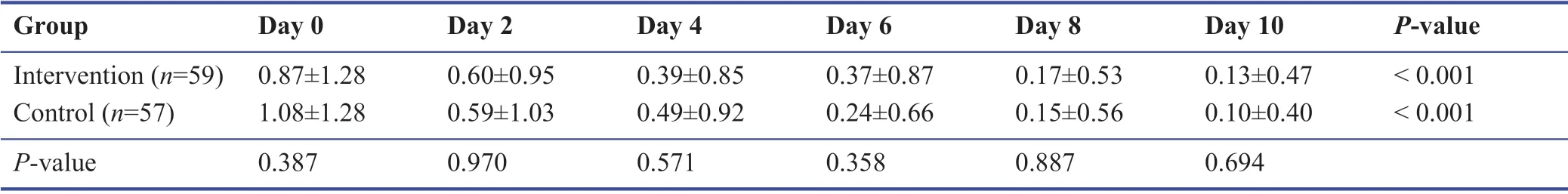
Table 7:Anorexia during the study period
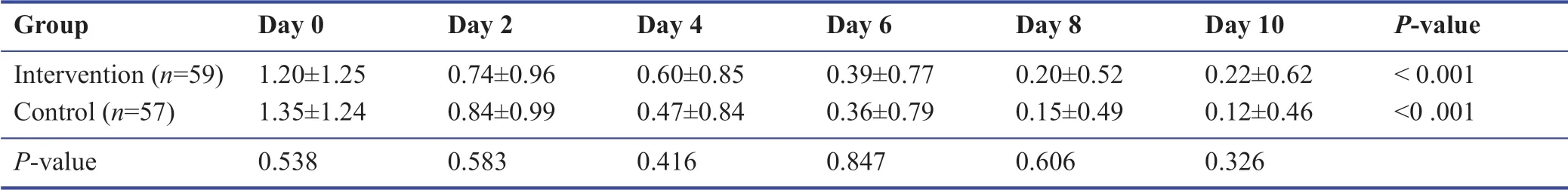
Table 8:Anxiety during the study period
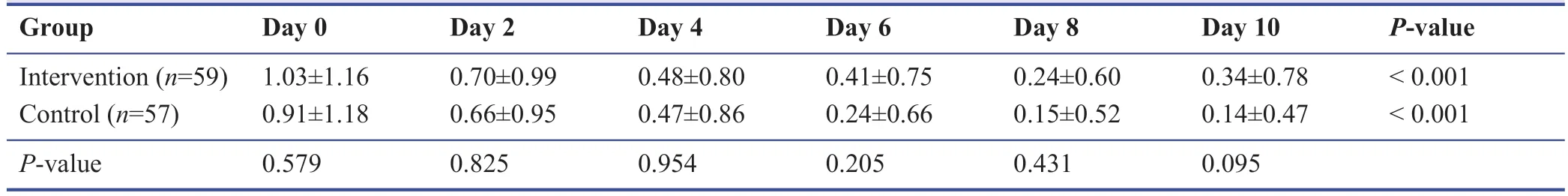
Table 9:Anosmia during the study period
Vital signs
Oxygen saturation and pulse rate were assessed every other day by researchers through home visits.Notably,no patient had a reduced oxygen saturation requiring hospital admission or supplemental oxygen therapy.The overall trend of both saturation and pulse rate were stable in both groups and did not change over time.Essentially,there was no statistically signif icant diff erence in the trends of oxygen saturation and pulse rate between the trial arms.
DISCUSSION
In this study,we aimed to investigate the eff ect of Iranian Polyherbal Syrup by Nepta bractaeta (Zufa syrup),a combination of several plants that have been historically used in traditional Persian medicine for respiratory infections,on the clinical course of patients with mild to moderate symptoms of COVID-19.As compared with placebo,Zufa syrup had no apparent eff ect on the symptomatology of COVID-19;however,the majority of patients improved during the careful follow-up by home visits and phone calls.
The COVID-19 pandemic presents unprecedented challenges.As the scientif ic community continues to search for an eff ective treatment or vaccine,traditional medicine approaches and herbal medicines could provide helpful interventions in this regard.15Taking such potential into account,studies of plant-based medicines for COVID-19 are scarce.Clinical trials of potential treatments for this novel virus have only rarely been successful.Traditional medicine provides researchers with many potential remedies that have been historically used with evidence of benef icial eff ect in similar illnesses.16Several herbal medicines have been studied as potential treatments for respiratory viral infections and alleviation of respiratory symptoms.11,12,17The effi cacy of the components of Zufa syrup has been investigated for respiratory illnesses.Moreover,another study in Iran assessed the eff ect of Nepeta bracteata benth on chronic obstructive pulmonary disease.18
Glycyrrhizaglabra or licorice has been historically used for medicinal purposes in China,India,and the Middle East and has been reported to be used for symptoms caused by viral respiratory diseases.16Glycyrrhizin,a component of Glycyrrhizaglabra has been shown in randomized controlled trials to reduce hepatocellular damage in hepatitis B and C.19In animal studies,glycyrrhizin had antiviral eff ects against herpes simplex virus and inf luenza A.19Moreover,there is evidence of the antiviral activity of glycyrrhizin against human immunodef iciency virus,severe acute respiratory syndrome-related coronavirus,and respiratory syncytial virus.19-21There have been observations of glycyrrhizin and its metabolically active component,glycyrrhetinic acid,to decrease the viral transmission.20Moreover,anti-inf lammatory eff ects of glycyrrhizin have been documented and are exerted through the Toll-like receptor 4.20In 2003,at the time of severe acute respiratory syndrome outbreak,a study from Germany reported that glycyrrhizin was quite potent at inhibiting the replication of severe acute respiratory syndrome coronavirusin vitro,and concluded that glycyrrhizin should be investigated as a potential treatment.22Pharmacologic pathways that have been proposed for the potential of glycyrrhizin in the treatment of COVID-19 include:down regulating inf lammation in the lung and other organs,blockage of the accumulating mechanisms of intracellular reactive oxygen species,decreasing the over-production of airway secretions,decreasing thrombotic activity,inducing interferon activity and reduction of viral entry points.20,23
Nepeta species are a well-known anti-cough remedy in the traditional Percian medicine.7Their eff ects on chronic cough,bronchitis,asthma,and chronic obstructive pulmonary disease has been studied.7,8Such potential could translate into a treatment that safely and eff ectively alleviates symptoms of COVID-19 and reduces the associated anxiety of contracting the virus.24Ficuscarica has been used for centuries as a medicinal plant for a wide variety of ailments,including symptoms of respiratory infections.25Moreover,its extracts,hexanic and hexane-ethyl acetate,have been demonstrated to be potent antivirals which could be used against herpes viruses,echovirus,and adenoviruses.25Viola species are traditional medicinal herbs used for respiratory illnesses and have been demonstrated to reduce the symptoms of asthma and respiratory inf lammation.9,26A study in mice documented the eff ects of viola on asthma to be related to a reduction in the activity of T helper type 2 cells.26The eff ects of viola on respiratory symptoms and immunity could prove benef icial for COVID-19 patients.Plants have antipyretic,antitussive,anti-inf lammatory,antioxidant and antimicrobial properties.It seems that it could be potential candidates for animal studies and clinical trials to prove their specif ic eff ectiveness.27In traditional Persian medicine several herbs have proved their eff ect in alleviation of symptoms associated with common viral respiratory infections.28,29
A major advantage of our study was the close follow-up and observation of patients.This should be emphasized especially during the course of the pandemic.Keeping contact with patients,developing eff ective support systems,and close follow-up of outpatients could reduce the symptomatic burden of disease as well as the anxiety and psychologic eff ects of contracting COVID-19 for patients and their families.Such method could also improve education about methods of social distancing and compliance with respiratory hygiene and ultimately reduce the transmission of the virus in the society.Clinicians should be aware of the benef its of such follow-ups and do their best to change their practice accordingly when feasible.
The results of the current study should be interpreted in light of the study limitations.First,the drug dose was low due to prevention of complication.Second,we did not use the high concentration of extract.Third,a portion of patients have been aff ected to disease a week since the onset of the disease.Fourth,the outpatient follow-up is prone to diff erences in reporting of symptoms of patients.Therefore,a study on admitted patients could have a much more reliable assessment of the COVID-19 course.Due to the unique circumstances of the pandemic,repeating CT scans and conducting laboratory tests were not feasible options.The social distancing policies and the safety of research staff and patients mandated limited exposure.Moreover,we acknowledge that performing a polymerase chain reaction test for detection of COVID-19 virus could have led to a more reliable selection of patients with COVID-19,rather than chest CT alone.Another limitation was that follow-up phone calls and visits were performed by a group of researchers,not a single individual.This was inevitable due to the relatively large sample size and repeated follow-ups;however,it might have led to variations in the reporting of variables.
In this study,we indicated the eff ects of the Zufa syrup,a combination of herbal remedies for respiratory infections,for the treatment of patients with mild to moderate symptoms of COVID-19.Over the 10 days’ follow-up,all symptoms improved gradually,and no patient required hospital admission or supplemental oxygen therapy.The patient-reported severity of cough,headache,myalgia,anorexia,anxiety,and insomnia were not diff erent throughout the follow-up.Dyspnea severity remained the same until the eighth day but was lower on the tenth day follow-up in the placebo arm.Oxygen saturation and pulse rate showed a stable trend throughout the follow-up and was similar between the intervention and control arms.
Acknowledgements
We are very thankful for patients who participated in this study.Moreover,we are grateful from Vic chancellor of Qom University of Medical Sciences for supporting this project as well as managers of Shahid-Beheshti and Forghani Hospital,management of Baghiatallah Clinic.Moreover,we thank from Dr Maryam Nasiri,Dr Atiehsadat Danesh,Dr Rahimeh Dastranj,Dr SeyedReza Vakilinia,Dr Zahra Sarbazhoseini,Rumella Heidar,Dr Mojdeh Poorhoseini,Dr Fatemeh Toiserkani,Dr Mina Atharizadeh,Dr Batool Khaiatzadeh,Dr Fatemeh Jahani,Dr Morteza Aghahasani for all cooperation,such as patient follow up and call them,coordination with hospital and clinic,and booalidaroo company.
Author contributions
AH,HMD,RB:Study design;FA:Sufa syrup production;SHA,AA,HMD,FH:patients visiting;AM:data analysis;MA,SHA,AA:study supervision.All authors approved the f inal version of the manuscript.
Conflicts of interest
The authors declare no actual or potential conf licts of interest regarding this study.
Financial support
This study was funded by Deputy of Research and Technology Qom University of Medical Science.
Institutional review board statement
The study was approved by the Ethics Committee of the Qom University of Medical Science (Ethics committee reference number IR.MUQ.REC.1398.165) on March 10,2020 and was registered in Iranian Clinical Trial Center (approval ID:IRCT20200404046934N1)on April 13,2020.
Declaration of patient consent
The authors certify that they have obtained patients consent forms.In the form,patients have given their consent for their images and other clinical information to be reported in the journal.The patients understand that their names and initials not be published and due eff orts will be made to conceal their identity.
Reporting statement
This study follows the CONsolidated Standards Of Reporting Trials(CONSORT) statement.
Biostatistics statement
The statistical methods of this study were conducted and reviewed by the epidemiologist of Qom University of Medical Sciences,Iran.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file
Additional f le 1:Checklist of demographic information and followup data.
- Medical Gas Research的其它文章
- Can united airway disease be the cause of variable severity experience of COVID-19 in health care workers?
- COVID-19 is more dangerous for older people and its severity is increasing:a case-control study
- COVID-19 incidence and local ozone level:is there any association?
- Acute asthma exacerbation after SARS-CoV-2 vaccine(Sinovac?):a case report
- Prediction of diagnosis and prognosis of COVID-19 disease by blood gas parameters using decision trees machine learning model:a retrospective observational study
- Ozone gas applied through nebulizat?on as adjuvant treatment for lung respiratory d?seases due to COVID-19 infect?ons:a prospective randomized trial

