Ozone gas applied through nebulizat?on as adjuvant treatment for lung respiratory d?seases due to COVID-19 infect?ons:a prospective randomized trial
Erhan Dengiz,?a?r? ?zcan,Yusuf ?zzettin Güven,Selcen U?ar,Beh?et Kemal Ener,Semih S?zen,Buket Ya?c?,?nal Albek Güzel,Betül Yi?it,Asl?nur Anda?,Beyza Güne?,Emire Bor,U?ur Karabudak,Ali Kaya
1 Edis Pharma Pharmaceutical Industry,Istanbul,Turkey
2 Health Science Univercity,Umraniye Training and Research Hospital,Istanbul,Turkey
3 Dr.Feriha ?z Emergency Hospital,?stanbul Turkey
4 Pendik State Hospital,?sttanbul,Turkey
5 Health Science Univercity,Haydarpa?a Training and Research Hospital,Istanbul,Turkey
Abstract The objective of this study was to provide lung disinfection by nebulizing ozone gas with distilled water and olive oil for patients who have clinical symptoms due to coronavirus disease 2019 (COVID-19).The study attempted to reduce the viral load of COVID-19 in the lungs of patients,to provide a faster response to medical treatment.Between August 2020 and September 2020,30 patients who met the study criteria were prospectively evaluated.There were 2 groups with 15 patients in each group:patients in control group were not treated with ozone and only received standard COVID-19 treatment;patients in ozone group received lung disinfection technique with ozone and standard COVID-19 treatment.A statistically signif icant diff erence was found in the length of stay in hospital,change in C-reactive protein,polymerase chain reaction results after 5 days,and computed tomography scores between two groups.There was no statistically signif icant diff erence in D-dimer,urea,lactate dehydrogenase,lymphocyte,leukocyte,and platelet between two groups.According to the data,we think that the lung disinfection technique applied with ozone inhalation reduces the rate of pneumonia in COVID-19 patients and makes the patients respond faster to the treatment and become negative according to the polymerase chain reaction tests.The study was approved by the Ethical Committee of the ?stanbul Medipol University Clinical Trials (approval No.0011) on July 2,2020.
Key words: coronavirus;COVID-19;lung disinfection;medical ozone gas;ozone gas;ozone inhalation;ozone therapy;pulmonary disease;viral puenmonia
INTRODUCTION
Coronavirus are causing an epidemic that threatens global health,which has a very fast and serious respiratory contagion that causes severe acute respiratory syndrome in the lung.1Currently there is no def initive treatment for the new severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2).The use of steroids in the stage of lung inf lammation suggests that it reduces parenchymal involvement and destruction.2Hydroxychloroquine has been used as tumor necrosis factor and interleukin 6 suppressor since the beginning of the epidemic.However,there are still doubts in the literature about its use due to its serious side eff ects.3Many antiviral agents such as remdesivir,lopinavir,and ritonavir are the drugs recommended for the treatment of coronavirus-induced diseases.
Ozone therapy has been applied in the treatment of coronavirus disease 2019 (COVID-19),generally using major auto-hemotherapy technique.4These treatments have also been reported by the World Health Organization.5When ozone is inhaled by living things at the concentration present in the atmosphere,it chemically reacts with many biological molecules in the respiratory system,causing a number of adverse pathologies in the lungs.It reacts with the surfactant in the lung and disrupts the lung epithelization.Thus,it causes serious lung parenchymal damage.6This pathological f inding usually occurs at high ozone concentration.It has antiviral and antibacterial properties without damaging the parenchyma at low ozone concentration.Especially this eff ect is used in most places for air disinfection today.
After contamination of COVID-19,infection causes severe morbidity and mortality in the patient with pulmonary phase and systemic hyper inflammation stages.7In the pulmonary phase,both contagiousness and symptoms increase signif icantly.Lung disinfection technique with ozone inhalation in the pulmonary phase can provide a faster recovery in these patients.
In order to benef it from the therapeutic eff ect of ozone gas in an optimum way and to avoid potentially harmful eff ects on living things,daily ozone exposure determined by the U.S.Food and Drug Administration was taken as a basis.According to this,it has been noted that industrial workers’ozone exposure of 0.10 parts per million (0.2 mg/m3) of 8 hours does not aff ect the current threshold limit value (American State Conference of Industrial Hygienists).8,9
Ozone,distilled water and olive oil,with the help of a specially designed new nebulizer device,is sent to the lungs by cold steam inhalation,isolation of the lung from the virus and providing comfortable breathing to support the recovery of patients in a short time.It can also be used as a prophylactic against all viral and bacterial pneumonias in the lungs.Especially in viral epidemic,we aim to reduce the viral load in the respiratory system,thus reducing the contagiousness of the coronavirus and accelerating the recovery of patients.
SUBJECTS AND METHODS
Ethical approval
This prospective randomized study was approved by the Ethical Committee of the ?stanbul Medipol University Clinical Trials (approval No.0011) on July 2,2020 and written informed consent was obtained from all the participants.This study follows the CONsolidated Standards Of Reporting Trials(CONSORT) statement (Additional f ile 1).
Subjects
Patients who were treated in Dr.Feriha ?z Emergency Hospital for coronavirus were examined prospectively between August and September 2020.
Diagnostic criteria of COVID-19:Common symptoms of COVID-19 are respiratory symptoms,fever,cough,dyspnoea,headache,sore throat,runny nose,muscle and joint pain,extreme weakness,emerging loss of sense of smell and taste,and diarrhea.Polymerase chain reaction (PCR) test was routinely applied in patients with such symptoms.
Inclusion criteria:Patients with a positive PCR test regardless of sex between the ages of 18-80 years.
Exclusion criteria:Patients with glucose-6-phosphate dehydrogenase def iciency (favizm allergy),previous lung cancer or suspected lung mass in lung tomography,and patients with previous surgery in their lungs were excluded from the study.The patients did not complete treatment.
Out of 72 patients,30 patients who met the study criteria who were admitted to the emergency department due to coronavirus were included and prospectively evaluated.
Treatment
After admitted to hospital from the emergency department,the treatments,either ozone or placebo,were applied for every patient.In placebo group we use only standart COVID-19 treatment.In ozone group,ozone inhalation treatment was added in standart COVID-19 treatment.Diff erent doctors were responsible for treatment and follow-up.Only two doctors used ozone inhalation for patients to be a blind study.The doctors who made this application were unaware of the clinical and laboratory results of the patients until the end of the study.The doctors who followed the patients and applied other treatments followed the patients until the end of the study and examined their clinical and laboratory parameters.
Patients were divided into control (n= 15) and ozone (n= 15)groups.All the patients were treated with routine COVID-19 treatment (Table 1).10

Table 1:COVID-19 treatment protocol released by Turkey Ministry of Health
In our study,a device that has never been produced before,and that is not available in the market has been designed and the prototype of this device has been used.The device enables the nebulization of ozone gas with distilled water and olive oil,allowing it to be delivered directly to humans by cold steam inhalation (Figure 1A).The olive oil mechanism added to the device is designed to prevent the irritating eff ect of ozone and to transfer it to a wider surface area with distilled water,providing a soft and moist (Figure 1B),thus comfortable breathing,and applying it to patients without any side eff ects,inf lammation or pain (Figure 1C).
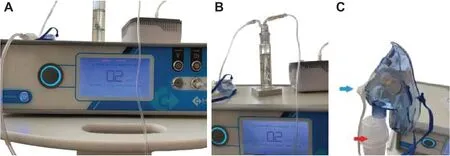
Figure 1:Nebulization device.
Ozone treatment protocol:consists of three sessions applied for 10 minutes at intervals of 5 minutes daily for 5 days.In each session;0.2 ppm ozone gas was given as cold vapor using lung disinfection technique inhalation device.In clinical practice,ozone gas is connected from the ozone generator equipped with an oxygen tube to a glass reservoir with olive oil inlet from the bottom and exit from the top 0.2 ppm ozone gas coming from the lower inlet passes through the olive oil and is connected to the mask via a silicone hose attached to the outlet at the top of the glass chamber.The mask is connected to the nebulizer drug reservoir to which 5 mL of distilled water has been added with its hose.In this way,cold water vapor and 0.2 ppm olive oil ozone gas are given directly to the patient in the mask.In this way,ozone gas at a concentration of 0.2 ppm/second is applied directly for one session for 10 minutes.The purpose of the study in this way is to ensure that the ozone gas obtained is spread to a wider area homogeneously in the lung and respiratory system with water vapor and to eliminate its irritating feature with the eff ect of olive oil.With this application,it is aimed to enable patients to take ozone gas at a dose of 0.2 ppm without any inf lammation or inf lammation in the lungs,to reduce their symptoms and to relieve the respiratory system.
Measurement
Patients diagnosed with COVID-19,before starting the study and after the study was applied for 5 days;hemogram,Creactive protein (CRP),D-dimer,urea,lactate dehydrogenase,PCR,chest X-ray or thoracic computed tomography (CT) tests were performed.
Thorax CT scans were made with a 64-channel CT (GE,Boston,MA,USA).Images were taken during a single breathholding,with the hands raised and the hand in the supine position.Tube voltage is standard 100 kV,current 110 mA,pitch 1.375,section thickness 5 mm.CT scoring system11has been developed to more clearly express the severity and prevalence of COVID-19 pneumonia.Opacities in each segment were scored according to how much of the segment was visually covered.Each segment involved was scored as 0,1,and 2 (0 point if there is no opacity in the segment parenchyma,1 point if the opacities cover 0-50% of the segment,and 2 points if the opacities cover more than 50%).
Statistical analysis
Number Cruncher Statistical System software (NCSS version 5.0,LLC,Kaysville,UT,USA) was used for statistical analysis.Descriptive statistical methods (mean,standard deviation,median,frequency,ratio,minimum,maximum)were used while evaluating the study data.The suitability of the quantitative data to normal distribution was tested by Kolmogorov-Smirnov,Shapiro-Wilk test and graphical evaluations.In the comparison of the normally distributed age variable between two groups,Student’st-test was used.Mann-WhitneyUtest was used for comparing variables not showing normal distribution between two groups.Pearson chi-square test and Fisher Exact test were used to compare qualitative data.Wilcoxon Signed Ranks test was used for in-group comparisons before and after the application.Signif icance was assessed at least at theP< 0.05 level.
RESULTS
Inpatient clinic with 30 patients,43% of them were female and 57% were male.The ages of the cases ranged from 21 to 80 years,with a mean of 51.56 ± 16.91 years.Chronic disease (e.g.,hypertension,diabetes mellitus,peripheral arterial disease,rheumatological diseases,Chronic obstructive pulmonary disease) is seen in 43% of the cases.The length of stay varies between 12 and 25 days,with an average of 19.96 ± 2.74 days.There is no statistically signif icant diff erence in the rates of chronic disease in the cases according to the groups (P> 0.05).The length of stay of the ozone group cases was lower than the control group (P< 0.001;Table 2).
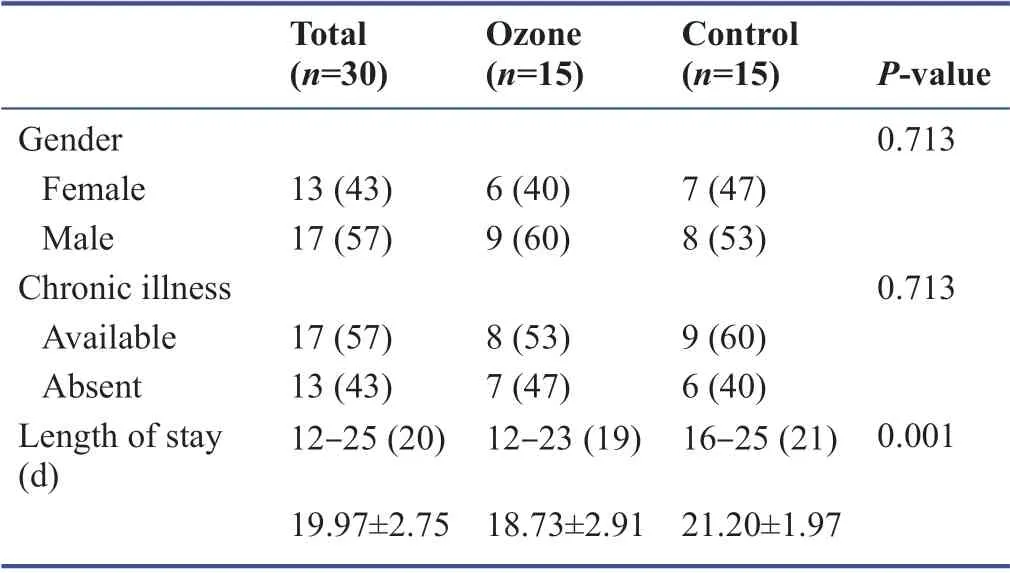
Table 2:Evaluation of demographic characteristics
There was no statistically signif icant diff erence in the CRP measurements of the cases before and after the application according to the groups (P> 0.05).The change in postapplication CRP measurements of the ozone group cases compared with the baseline was not statistically signif icant (P> 0.05).When the change in CRP measurements after treatment compared with the baseline was examined,a decrease was detected in 75% of the cases in the ozone group,which was statistically signif icantly higher than that (25%) in the control group (P< 0.05;Table 3).Before the application,PCR was found to be positive in all cases in both groups.While the rate of positive cases which turned negative was 93% in the ozone group,and 20% in the control group,and the rate of negativity was statistically signif icantly higher in the ozone group (P< 0.01;Table 3).A statistically signif icant diff erence was found between the groups in terms of CT severity scores (P< 0.05).Biochemistry results of the patients in the control group and ozone group before and after treatment are listed in Table 4.
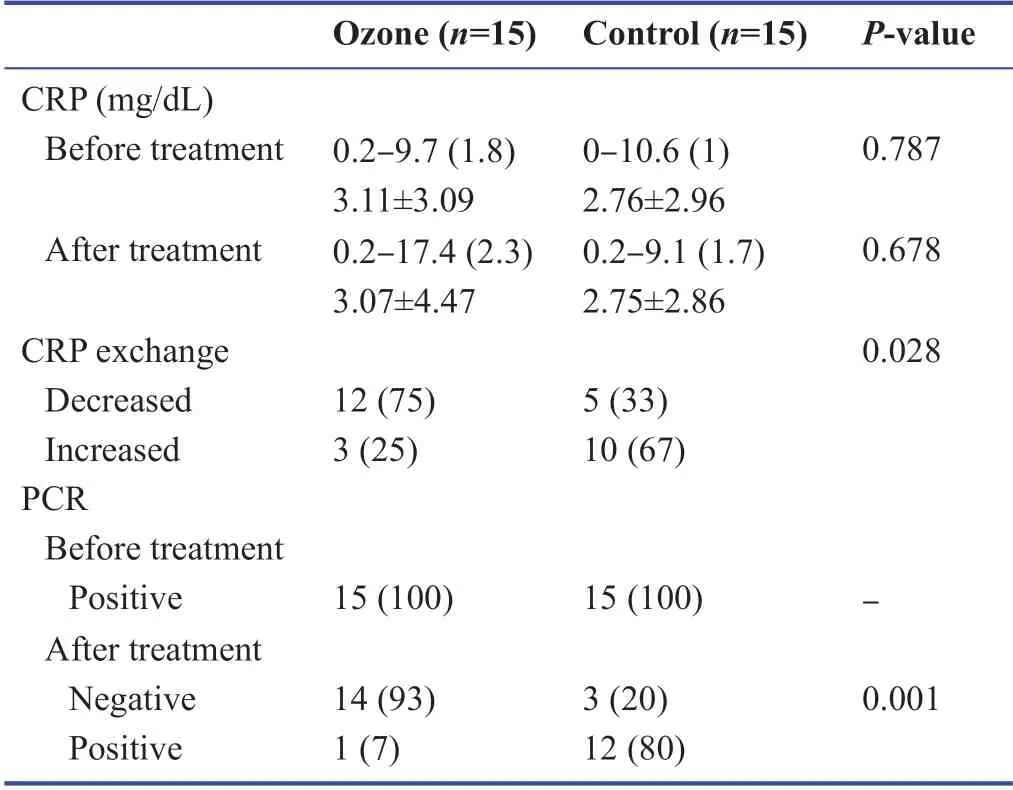
Table 3:Evaluation of PCR and CRP results before and after ozone treatment in coronavirus disease 2019(COVID-19) patients
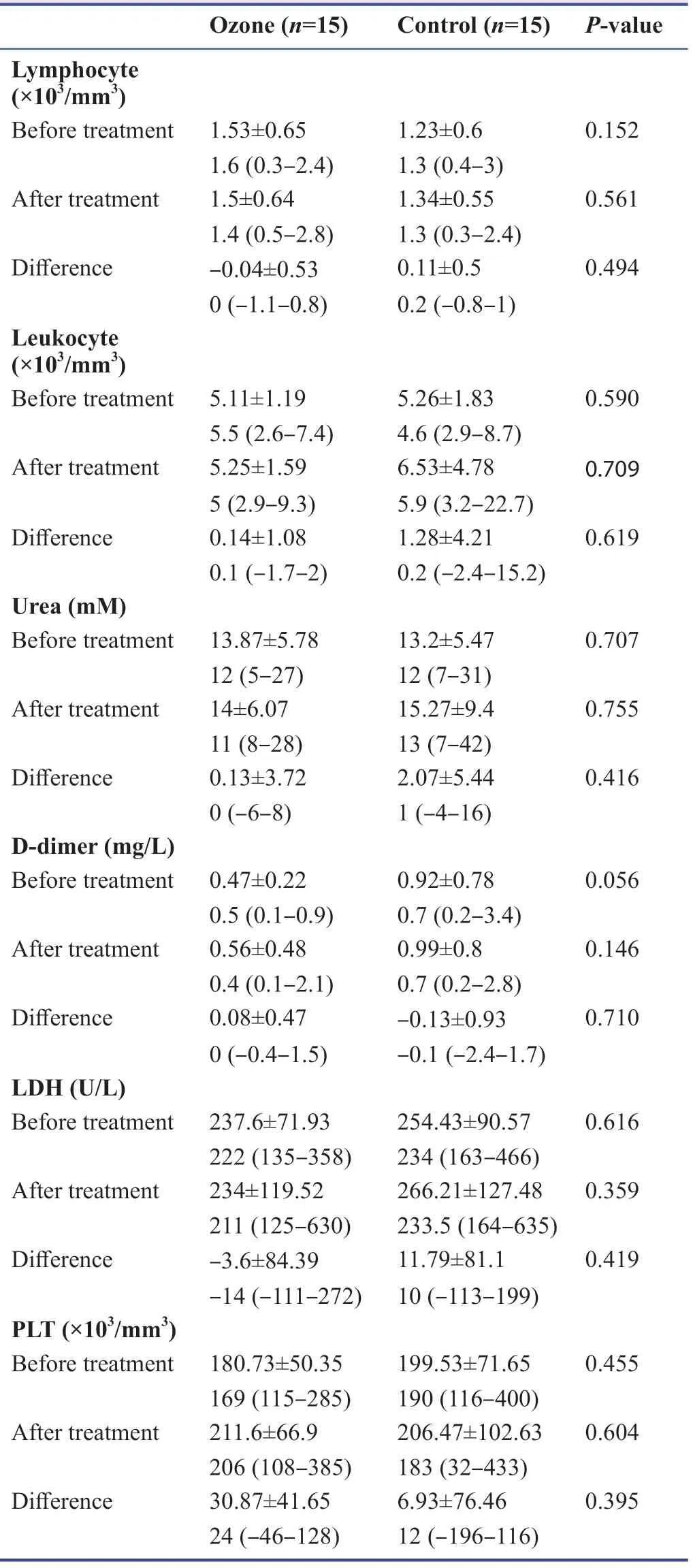
Table 4:Biochemical variables before and after ozone treatment in patients with coronavirus disease 2019
DISCUSSION
As we all know,no drug or vaccine studies have been completed so far for the full treatment of the COVID-19 virus,a type of coronavirus that is extremely dangerous and deadly,causing a pandemic both in Turkey and in the world.According to the current data of the World Health Organization,more than14,971,036 cumulative conf irmed cases and 618,017 deaths have been reported globally till July 23,2020.5There are many bacteria and viruses on the earth.Apart from the good bacteria and viruses that are benef icial for our body.12,13there are also many bacteria and viruses that weaken our immune system,therefore inducing diseases.14,15These organisms cause various diseases in humans and animals.Among them,it appears as faced allergic reactions,upper respiratory tract (chronic obstructive pulmonary disease-bronchitis,asthma,etc.),and digestive system diseases (ref lux,constipation,diarrhea,etc.).
Bacterial and viral infections cause a decrease in body system resistance,severe illness,and damage to other vital organs in the body due to the side eff ects of the drugs used in the treatment process.It is possible to treat existing diseases more quickly and easily without any side eff ects,by killing even these deadly viruses and bacteria.Bacteria and viruses can only be killed by disinfection methods.
Remondino et al.16used the maximum use dose of ozone for disinfection,released by the U.S.Food and Drug Administration in order to treat lung respiratory diseases(for 8 hours,5 days a week,0.1 ppm ambient air application).A clinical study was conducted on 40 randomly selected patients and the therapeutic eff ect was examined.17
Ozone is a highly reactive gas composed of three oxygen atoms.It is a compound that occurs naturally in the upper atmosphere and lower atmosphere of the Earth or can be produced man-made.Stratospheric ozone occurs naturally through the interaction of solar ultraviolet radiation with molecular oxygen.The “ozone layer” approximately 10 to 50 km above the Earth’s surface reduces the amount of harmful ultraviolet radiation reaching the Earth’s surface.
In addition,studies on ozone have shown that ozone has an antiviral,antibacterial and disinfectant eff ect depending on the concentration,environment and application method.18,19Ozone therapy refers to the process of applying ozone gas to the body to treat a disease or wound.It can be used to treat medical conditions by stimulating the immune system.20It has been stated in studies that it can also be used to disinfect and treat the disease.1Medical ozone has been used to disinfect medical supplies and treat diff erent conditions for more than 150 years.For example,ozone therapy can stop its spread if you have an infection in your body.21
Ozone therapy shows its eff ect by destroying bacteria,viruses,yeast,fungi and unicellular animals.It also helps cleanse the infected cells.When the body escapes from these infected cells,it produces new and healthy cells.21Ozone therapy can be used for a variety of conditions such as respiratory disorders,22diabetes,immune system diseases.23-25This treatment can be applied in three ways (directly to the tissue,intravenously and intramuscularly).26,27
Despite the existence of anatomical diff erences between human,nonhuman primate and canine lungs and common experimental rodent lungs,the anatomical lesion of ozone inhalation occurs at the functionally equivalent site of the region between the conductive airway and the respiratory zone.The ciliary cells of the upper respiratory tract and the type 1 cells of the centriacinar region are most aff ected.Type 2 cell proliferation is the hallmark of ozone toxicity.A study with ozone has noted various biochemical and physiological changes in various animal species and humans.28In a scientif ic study conducted in 2017,a drug intended to be used for ozone therapy may have a therapeutic eff ect on human diseases such as chronic obstructive pulmonary disease and cystic f ibrosis (NCT00551811).Ozone therapy is currently being studied in patients with knee arthritis and other inf lammatory diseases,but results are not yet available.Patients with back pain from herniated discs can also benef it from ozone therapy(NCT00832312).
It is a well-known fact that ozone has toxic eff ects on lungs at high doses.However,inhalation of low doses of distilled water and olive oil into the lungs does not have a toxic effect.Low-dose ozone improves blood circulation and tissue delivery to ischemic as a result of the concerted eff ect of nitric oxide.7To prevent cytokine storm,ozone also has potent anti-inf lammatory properties through the modulation of the nucleotide-binding domain-like receptor protein 3 inf lammasome which is recognized to play a crucial role in the initiation and continuance of inf lammation in various diseases.29
The limited number of cases in the study is due to the lack of power analysis before starting the study.It is diffi cult to make a safe power analysis because there are as yet no longterm studies on COVID-19.Therefore,we did not need to do power analysis.
According to the data we obtained in our study,we think that the lung disinfection technique applied with ozone inhalation can reduce the rate of pneumonia in COVID-19 patients and make the patients respond faster to the treatment and become negative according to the PCR tests.
Author contributions
Conceptualization:ED,AK,UK;?nvestigation:BG;methodology:SS;project administration:ED;resources:Y?G;data collection:??,BY;data analysis:BKE;visualization:ED,EB;Writing - original draft:??,ED;writing - review &editing:??,SU;supervision:AA,BY,?AG,BY.
Conflicts of interest
Ozone generator was produced by Edis Pharma Pharmaceutical ?ndustry,and the patent of this generator belongs to Edis Pharma Pharmaceutical ?ndustry.
Financial support
None.
Institutional review board statement
The study was approved by the Ethical Committee of the ?stanbul Medipol University Clinical Trials (approval No.0011) on July 2,2020.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms from the conscious patients.In the forms,the patients have given their consent for the images and other clinical information to be reported in the journal.The patients have understand that their names and initials will not be published and due eff orts will be made to conceal their identity.
Reporting statement
This study follows the CONsolidated Standards Of Reporting Trials(CONSORT) statement.
Biostatistics statement
The statistical methods of this study were reviewed by the biostatistician of Health Science Univercity,Umraniye Training and Research Hospital.
Copyright license agreement
The Copyright License Agreement has been signed by all authors before publication.
Data sharing statement
Datasets analyzed during the current study are available from the corresponding author on reasonable request.
Plagiarism check
Checked twice by iThenticate.
Peer review
Externally peer reviewed.
Open access statement
This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file
Additional f ile 1:CONSORT checklist.
- Medical Gas Research的其它文章
- Can united airway disease be the cause of variable severity experience of COVID-19 in health care workers?
- COVID-19 is more dangerous for older people and its severity is increasing:a case-control study
- COVID-19 incidence and local ozone level:is there any association?
- Acute asthma exacerbation after SARS-CoV-2 vaccine(Sinovac?):a case report
- Prediction of diagnosis and prognosis of COVID-19 disease by blood gas parameters using decision trees machine learning model:a retrospective observational study
- Effects of Iranian Polyherbal Syrup (Zufa syrup)on oxygen saturation and clinical symptoms in suspected patients with COVID-19:a triple-blinded,randomized,placebo-controlled trial

