Genitourinary manifestations of Lynch syndrome in the urological practice
Chir Lonti *,Cludio Simeone ,Nzreno Surdi ,Philippe E.Spiess ,Andre Nehi ,Mro Moshini
a Urology Unit,ASST Spedali Civili di Brescia,Department of Medical and Surgical Specialties,Radiological Science and Public Health,University of Brescia,Brescia,Italy
b Department of Urology,Luzerner Kantonsspital,Lucerne,Switzerland
c Department of Genitourinary Oncology,H.Lee Moffitt Cancer Center and Research Institute,Tampla,FL,USA
d University Vita-Salute San Raffaele,Milan,Italy
e Department of Medical Oncology,IRCCS San Raffaele Hospital and Scientific Institute,Milan,Italy
f Division of Experimental Oncology/Unit of Urology,Urological Research Institute,IRCCS Ospedale San Raffaele,Milan,Italy
KEYWORDS Hereditary disease;Hereditary nonpolyposis colorectal cancer;Lynch syndrome;Mismatch repair;Upper tract urothelial carcinoma
Abstract Objective: Lynch syndrome (LS) is an autosomal dominant hereditary disorder resulting from germline mutation in at least one of the four mismatch repair genes or in EPCAM gene.From a clinical perspective,LS patients exhibit an increased predisposition to multiple primary malignancies and early age of onset compared to general population.We aimed to provide a comprehensive overview of all the genitourinary manifestations of LS,focusing on incidence,diagnosis,clinical features,therapeutic strategies,and screening protocols.Methods: Previous literature was assessed through Medline,Scopus,and Google Scholar databases.A narrative review of the most relevant articles from January 1996 to June 2021 on urological manifestations of LS was provided.Results: In the LS tumor spectrum,upper tract urothelial carcinoma(UTUC)represents the third most frequent malignancy,and the first most common cancer in the urological field,with an approximately 14-fold increased risk of developing UTUC compared to general population.LS diagnosis among patients experiencing UTUC as first malignancy is a step-by-step process,including (i) clinical criteria,(ii) molecular testing,and (iii) genetic testing to confirm the hereditary disorder.The current European Association of Urology(EAU)guidelines recommend to perform molecular testing among UTUC patients under 65 years old,or UTUC patients with personal history of LS-related tumor,or UTUC patients with one first-degree relative under the age of 50 years with LS-related tumor,or UTUC patients with two first-degree relatives with LS-related tumor regardless of age of onset.Newly diagnosed LS patients should be referred to a multidisciplinary management,including gastroenterologists and gynecologists.Finally,considering the increased risk of metachronous recurrence,treatments other than radical nephroureterectomy may be a valuable therapeutic alternative.Whether urological malignancies other than UTUC should be included in the LS tumor spectrum is still controversial.Conclusion:Considering the strict association between UTUC and LS,we believe that the urologist should recognize patients at increased risk for hereditary disease according to current EAU clinical criteria and address them to a comprehensive diagnostic algorithm,including molecular evaluation and genetic testing.To date,literature lacks clear evidence regarding the role of LS in developing bladder cancer,prostate cancer,or renal cell carcinoma,and current data are still inconclusive,highlighting the urgent need for further studies.
1.Introduction
First described by Lynch et al.in 1966 [1],Lynch syndrome(LS) is an autosomal dominant hereditary disease,caused by germline mutation of one of the four mismatch repair(MMR) genes (MLH1,MSH2,MSH6,andPMS2) orEPCAMgene (resulting inMSH2inactivation by promoter hypermethylation) [2].Notably,to be clinical manifest,LS requires inactivation of both alleles of one of the four MMR genes;thus,according to the Knudson’s two-hit hypothesis,mutation in the first allele is inherited,predisposing to the acquired mutation on the second allele.The inactivation of MMR genes invalidates the proper correction of single-based nucleotide mismatches and insertion-deletion loops which may occur during DNA replication,resulting in accumulation of somatic mutations in regions of short DNA repeating sequences (microsatellites regions) [3].This phenomenon is known as microsatellite instability (MSI)and increases the risk of multiple primary malignancies and early age of onset [2].From a clinical perspective,although LS is generally knows as hereditary nonpolyposis colorectal cancer (HNPCC),LS is characterized by both colorectal (life-time risk: 30%-73%) and extracolonic malignancies,including endometrial cancer (life-time risk:30%-51%) and upper tract urothelial carcinoma (UTUC)(life-time risk: 2%-20%) (Fig.1) [4,5].Kamiza et al.[6],analyzing the Han Chinese population,estimated an hazard-ratios for colorectal cancer of 23.2 (95% confidence interval [CI]: 9.2-58.2) and 13.2 (95% CI: 5.9-29.6) among male and female LS patients,respectively.
Despite growing interest in LS-related colorectal cancer,evidence regarding the role of LS in the urological field is still sparse and conflicting.In this review,we aimed to summarize the available literature on this topic,focusing on different clinical presentation,incidence,diagnosis,tumor features,and treatment of all potential urological malignancies in the broad LS tumor spectrum.
2.UTUC
2.1.Relative risk(RR)and cumulative life-time risk of developing UTUC among LS patients
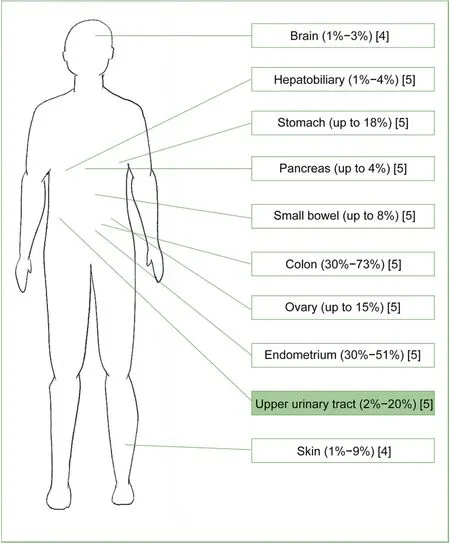
Figure 1 Sites at increased risk of developing primary malignancies among Lynch syndrome patients.
UTUC represents the third most frequent malignancy in the LS tumor spectrum,after colorectal and endometrial cancers(Fig.1),and the most common cancer in the urological field [2].The germline mutation rates among LS patients experiencing UTUC are 63%-100%,0-25%,and 0-15% forMSH2-,MLH1-,andMSH6-mutation carriers,respectively(Supplementary Fig.1)[7].Sijmons et al.[8]reported a RR of developing UTUC among LS patients of 14.0 compared to general population (95% CI: 6.7-29.4).Furthermore,several studies investigated the risk of developing UTUC according to patients’ characteristics,such as gender or pathogenic variants[8-13].Three[8,10,11]out of four[13]studies reported an increased risk of developing UTUC among male LS patients,recording a cumulative life-time risk at 70 years varying from 0.2% [8] to 9.4% [11] and from 0.1% [8] to 6.0% [11] among male and female individuals,respectively.Four studies specifically focused on different pathogenic variants [9,10,12,13]:MSH2-mutation carriers were found to be at increased risk of developing UTUC (cumulative life-time risk at 70 years: 6.9%)compared toMLH1-andMSH6-mutation carriers (2.2% and 2.9%,respectively) [13].Similarly,Barrow et al.[12]observed a RR of 42.7 and 61.9 of developing pyelocaliceal and ureteral tumor,respectively,among LS patients compared to general population.A personalized cumulative life-time risk calculator based on patient age,gender,and involved gene is provided by the Prospective Lynch Syndrome Database (http://www.plsd.edu/).
2.2.LS diagnosis among UTUC patients
LS diagnosis among patients experiencing UTUC as first malignancy is a step-by-step process,starting from clinical suspicion and leading to genetic test to be conclusive [14],as depicted in Fig.2.
2.2.1.Clinical criteria
The first recognition of the association between UTUC and LS dates back to 1999 (Amsterdam II criteria) [15],when renal pelvis and ureter tumors were included into clinical criteria for LS diagnosis among colorectal patients(Table 1).Unfortunately,the Amsterdam II criteria[15]and the subsequent Revised Bethesda guidelines [16] were specifically focused on colorectal patients,considering UTUC only as part of the LS tumor spectrum.
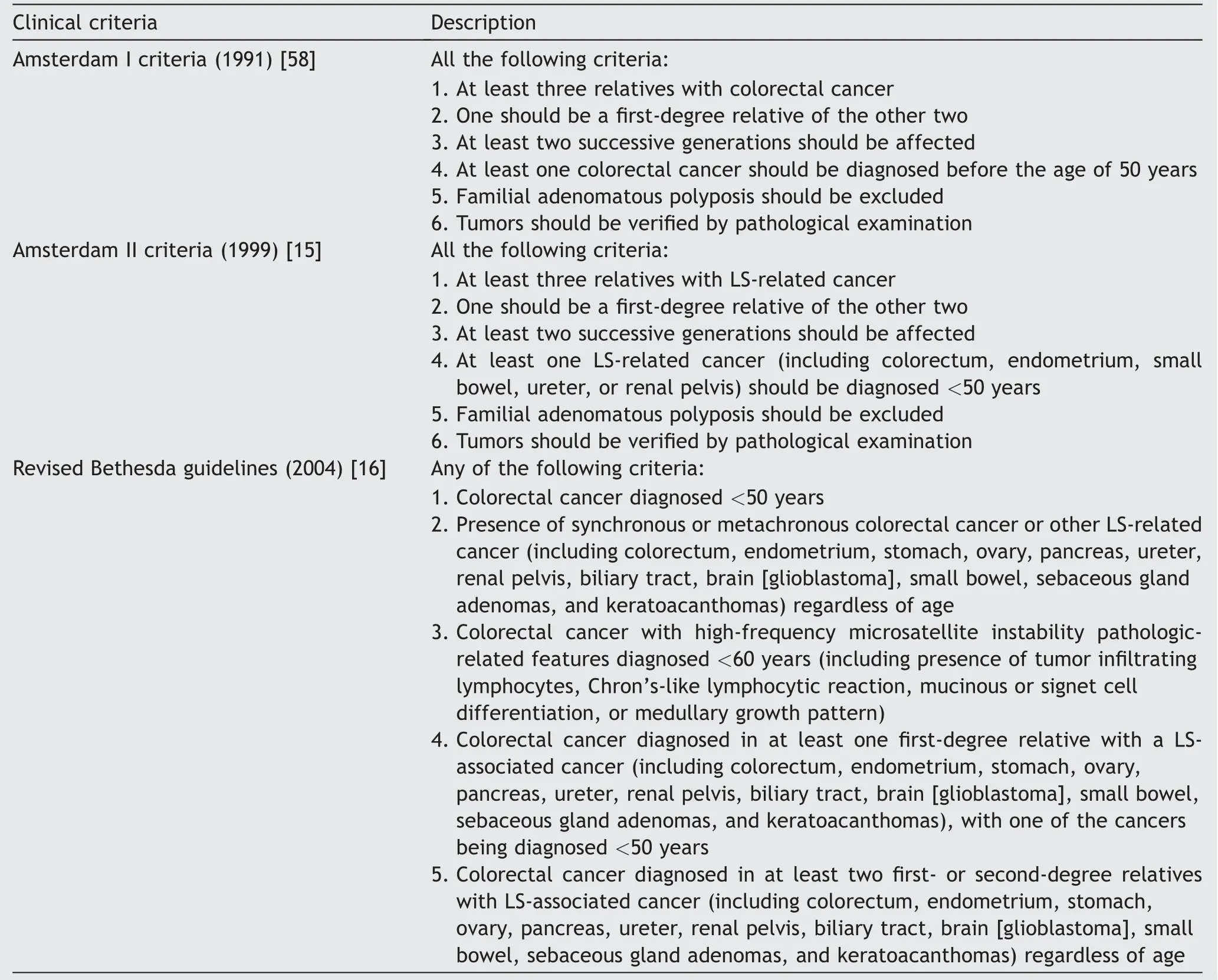
Table 1 Clinical criteria for LS diagnosis.
In 2004,Roupre?t et al.[17] codified clinical criteria specifically focused on UTUC patients for the first time,suggesting to perform further analyses among UTUC individuals<60 years old and/or with personal and/or family history of LS-related tumors.Over the last 15 years,various clinical criteria have been suggested in order to improve hereditary-like UTUC patients’ selection while limiting costs [3,18,19].Lastly,the current European Association of Urology guidelines on UTUC (2021) [20] recommended to perform molecular testing among (i) UTUC patients under the age of 65 years or (ii) UTUC patients with personal history of LS-related tumor,or (iii) UTUC patients with one first-degree relative (FDR) under the age of 50 years with LS-related tumor,or(iv)UTUC patients with two FDRs with LS-related tumor regardless of age of onset.
2.2.2.Molecular evaluation
Patients fulfilling clinical criteria for LS should be referred to molecular testing on tumor tissue specimen,including polymerase chain reaction for MSI status evaluation and immunohistochemistry (IHC) for MMR protein expression detection.
Polymerase chain reaction requires amplification of standardized DNA markers from a tumor tissue specimen and a matched healthy tissue specimen.Over the years,several DNA markers panels have been proposed,differing in type (mono-or di-nucleotide) and number of markers [21,22].Presence of additional peaks in above 30% [2],or at least 2/5 DNA markers [21],or at least 4/15 DNA markers [17] in tumor tissue specimen is defined as high-frequency MSI (H-MSI) and is suspected of LS.However,since approximately 10%-15% of sporadic tumors exhibit H-MSI [23],sporadic mechanisms of MSI should be ruled out with additional investigations,such as IHC[17,24,25].
IHC requires antibodies against MMR proteins to stain tumor tissue specimen and a matched healthy tissue specimen [14].Over the years,several IHC panels have been proposed,differing in combination and number of MMR antibodies [26].Absence or reduction of nuclear staining (<5% [27,28] or <20% [29]) in tumor tissue specimen is defined as loss of MMR proteins and is suspected of LS [2,26];hereditary mechanism behind loss of MMR proteins should be confirmed at subsequent genetic testing [30].
Moreover,since molecular testing failure could be related to technical challenges in DNA extraction or to incomplete tissue fixation [23,26],molecular evaluation should be interpreted in light of clinical judgement[16]and patients with strong clinical suspicion of LS should be referred to genetic testing [18,31].

Figure 2 Flow chart for diagnosis of LS in patients experiencing UTUC without known DNA MMR gene mutation.EAU,European Association of Urology;UTUC,upper tract urothelial carcinoma;LS,Lynch syndrome;FDR,first-degree relative;MSI,microsatellite instability;PCR,polymerase chain reaction;MMR,mismatch repair;IHC,immunohistochemistry.
2.2.3.Histological evaluation
Presence of intratumoral lymphocytes,inverted growth pattern,and pushing borders on tumor tissue specimen has recently been proposed as additional markers in the LS diagnostic pathway [3,17-19,24,25];however,there is no consensus regarding the role of histological evaluation in the LS diagnostic algorithm and further studies analyzing the positive and negative predictive value of histological evaluation on UTUC specimen are urgently needed.
2.2.4.Genetic testing,multidisciplinary management,and genetic counselling
LS diagnosis requires genetic testing on blood sample to be conclusive.The coding exons of MMR gene identified by IHC are amplified and sequenced to confirm germline mutation[3,17-19,24,25].
Considering the life-time risk of other LS-related primary malignancies (Fig.1),newly diagnosed LS patients should be referred to a multidisciplinary management[3,17,24,25],including gastroenterologists and gynecologists.Non-urological screening recommendations for LS patients are depicted in Fig.3.Colonoscopy should be performed every 2 years starting at 25 years old forMLH1-andMSH2-mutation-carriers or 35 years old forMSH6-andPMS2-mutation-carriers [32,33];in all cases,age of colorectal cancer onset in the youngest member of the family should be considered and screening should be started 5 years earlier [5].Esophagogastroduodenoscopy should be considered every 1-3 years starting at 30-35 years old in case of family history of upper digestive tract tumors [5];while screening and treatingHelicobacter pylorishould be performed in all LS patients [5,32,33].Transvaginal ultrasound and endometrial sampling should be performed every 1 year starting at 30-35 years old [5].
Finally,considering the autosomal dominant inheritance,newly diagnosed LS patients’ FDRs should be referred to a genetic counselling[3,17-19,24,25],ensuring an opportunity for the whole family to receive proper screening protocols.
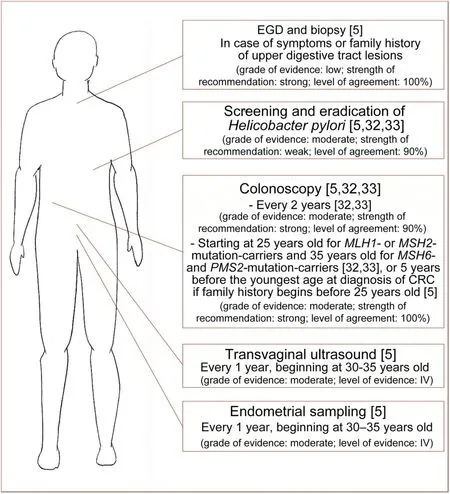
Figure 3 Non-urological screening recommendations for Lynch syndrome patients in a multimodal screening approach.EGD,esophagogastroduodenoscopy;CRC,colorectal cancer.
2.3.Clinical and pathological features of UTUC among LS patients
A clinical and pathological analysis of LS-related UTUC has been performed by several publications [8,11,13,17,27,28,34-42];however,only two studies directly compared hereditary and sporadic UTUC [34,40].When considering LS-related UTUC,patients were generally 10 years younger(p<0.0001 andp=0.005)[34,40]and with no smoking habit(p=0.03) [40] compared to sporadic UTUC patients,with ureteral location being involved in approximately 50% of cases(p=0.001 andp=0.001)[34,40].No differences were found between LS-related and sporadic UTUCs according to tumor size[34],grade[34,40],or stage[40].
2.4.Treatment of UTUC among LS patients
When considering therapeutic approaches,radical nephroureterectomy [17,28,35,39,42] (41 patients) was over twice as common as kidney-sparing surgery,including ureteroscopic laser ablation [38] (13 patients with 15 lesions)and segmental ureterectomy [35] (1 patient).Hubosky et al.[38]performed ureteroscopic laser ablation involving neodymium:yttrium aluminum garnet (YAG) laser and holium:YAG laser for coagulation and ablation or resection,respectively.Successful kidney-sparing procedure was achieved in 13 out of 15 lesions,nine of which requiring only one session.Surveillance scheme included retrograde pyelogram and ureteroscopy every 6 months for 5 years,and annual thereafter,while the contralateral renal unit was evaluated by retrograde pyelogram every 6 months for 5 years,and annual thereafter.Any changes in baseline pyelogram were investigated with additional computed tomography (CT) scan or magnetic resonance imaging.During a mean follow-up of approximately 5 years,three patients experienced metachronous ipsilateral UTUC(within a median time of 64 months) requiring ureteroscopic ablation,while seven patients experienced metachronous bladder cancer(BCa) (within a median time of 10 months) requiring radical cystectomy in two out of seven individuals.Iatrogenic ureteric stricture requiring stenting was recorded in four patients.Considering the potential risk of metachronous contralateral recurrence,the authors concluded that kidney-sparing surgery with endoscopic laser ablation seems feasible among LS patients recording satisfactory oncological outcomes,on the condition that patients undergo a strict surveillance scheme.Taken together,these results should be interpreted as a noteworthy starting point for additional research efforts in this field and further studies focusing on kidney-sparing procedure among LS patients are urgently needed.
2.5.Screening protocol for urothelial carcinoma among LS patients
Considering the increased risk of developing UTUC,LS patients may benefit from a strict screening protocol for early detection of urothelial involvement.
No consensus currently exists regarding the proper screening protocol,and great variability still exists according to methods [31,43-46] and screening starting age[10,44,46],ranging from 25[46]to 50 years[10].Screening methods generally included urinalysis [44-46] and urinary cytology [31,43,45,46],although Myrh?j et al.[43],recording a sensitivity of 29%,stated that urinary cytology should not represent an effective screening method among LS patients.Other suggested screening methods included NMP22 [45],abdominal ultrasound [44,45],CT scan[31,44,45],and cystoscopy [31,44,45].Acher et al.[45]suggested an individualized screening protocol based on presence ofMSH2-mutation and personal and/or family history of prior urothelial cancer.We suggest a screening protocol among all LS patients starting from 45 to 50 years old,involving urinalysis and urinary cytology every year and abdominal ultrasound every 2 years.However,we believe thatMSH2-mutation carriers,as well as patients with family history of UTUC or BCa,could benefit from a more stringent follow-up,alternating abdominal ultrasound and CT scan every year [30].Fig.4 depicts current screening protocols suggested by several authors [30,31,43-46];however,the broad variability in screening recommendations brings to light the urgent need of a consistent screening protocol in terms of methods and starting age,balancing the likely benefit of early diagnosis and the potential risk of overdiagnosis and radiation exposure.
3.BCa
The increased risk of developing urothelial carcinoma among LS patients was first reported in 1990 by Vasen et al.[47].However,although the association between LS and UTUC has been extensively investigated and UTUC has been included in clinical criteria for LS since 1999 (Amsterdam II criteria)[15],little is known regarding the role of LS in BCa development.
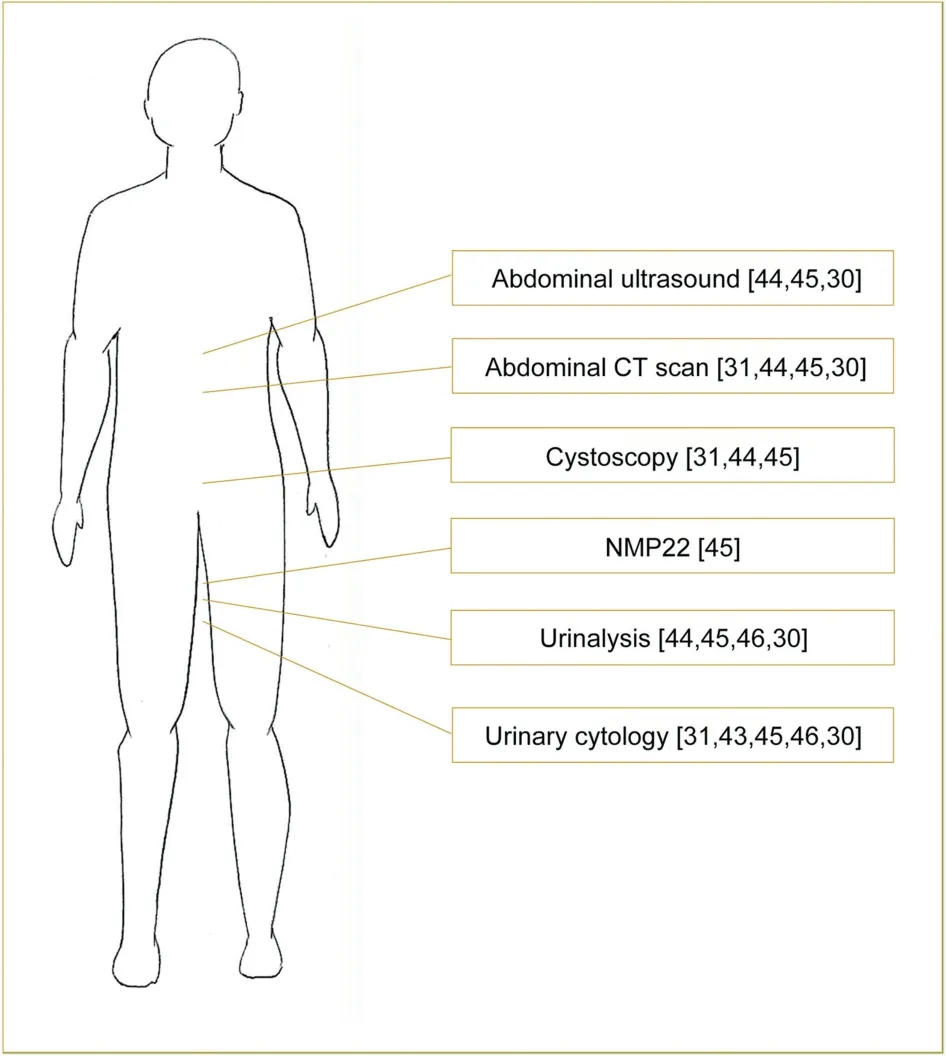
Figure 4 Current urological screening recommendations for Lynch syndrome patients.CT,computed tomography.
Cumulative life-time risk of BCa at 70 years ranged from 4.1%(95%CI:2.6%-5.5%)[13]to 7.5%(95% CI:3.1%-11.9%)[48]among LS male patients and from 1.0%(95%CI:0-2.4%)[48] to 2.6% (95%CI: 1.5%-3.5%) [13] among LS female patients,with a RR of 4.2 and 2.2 for male and female individuals,respectively[48].Overall,MSH2,MLH1,andMSH6gene mutations were involved in 69%-79%,10%-29%,and 0-19%of LS patients experiencing BCa[7].Similarly to UTUC,MSH2-mutation carriers exhibited the highest risk of developing BCa,with cumulative life-time risk of 12.3% (95% CI:4.3%-20.3%)and 2.6%(95%CI:0-3.8%)among male and female patients,respectively[48].Geary et al.[49]analyzing a LS cohort of 982 patients,recorded a RR of 3.6 and 1.0 amongMSH2-andMLH1-mutation carriers,respectively,compared to general population.Conversely,Barrow et al.[12]did not observe a significant predisposition to BCa development for any MMR mutation compared to the general population.
Skeldon et al.[37] identified 14 out of 321 (4%) LS patients experiencing BCa during a median follow-up of 9 years.Overall,one patient had BCa diagnosed first;one patient had UTUC diagnosed first;and four patients experienced BCa and UTUC synchronously.Regarding tumor stage,LS-related BCa was non-invasive (pTa/T1) in 93% of cases,with 71% being high grade [37].
Considering the lack of clear evidence and the well-known association between UTUC and BCa,the role of germline MMR mutation in developing BCa should be further investigated.
4.Prostate cancer (PCa)
Whether PCa should be included in the LS tumor spectrum is still controversial [7],with several studies reporting a statistically significant increased risk of developing PCa among LS patients compared to general population[11,50,51],whereas others authors found no significant evidence for an increased risk [52,53].
A recent meta-analysis from 23 studies [54] recorded a 2.13-fold increased risk of developing PCa among LS patients(95%CI: 1.45-2.80) and a 2.11-fold increased risk among LS patients with a prior diagnosis of colorectal cancer (95% CI:1.27-2.95).Haraldsdottir et al.[51]compared PCa incidence among 188 LS patients and the general population from the Surveillance,Epidemiology,and End Results registry (mean follow-up and standard deviation: 6.2±7.2 years).Overall,11(5.9%)LSpatientsexperienced PCa during the studyperiod,with a median age at diagnosis of 64 years of age.Of whom,5 (45%) individuals were diagnosed with localized disease,1(9%)with locally advanced,and 1(9%)with metastatic disease;in four cases,tumor stage was not reported [51].No significant differences were found according to age at diagnosisand tumorstage[51].Overall,MSH2-,MLH1-,MSH6-,andPMS2-mutation carriers were seven,one,two,and one,respectively [51].Similarly,Barrow et al.[12] estimated a 10-fold increased risk of PCa amongMSH2-mutation carriers(RR:10.41;95%CI:2.80-26.65).Starting from these results,the authors suggested prostate-specific antigen screening amongMSH2-mutation carriers starting from 40 years old[12].
To date,literature lacks clear evidence regarding the association between LS and risk of developing PCa,and current data are still inconclusive.
5.Renal cell carcinoma
The association between LS and kidney cancer has been rarely investigated [7,53].Aarnio et al.[53] reported a significantly increased standardized incidence ratio of 4.7(95% CI: 1.0-14) for renal cell carcinoma among LS patients,with a cumulative life-time risk at 70 years of 3.3%compared to 0.8% among general population.
However,to date the potential association between LS and renal cell carcinoma has not been yet clarified,and further studies are urgently required.
6.Testicular germ cell tumor (TGCT)
TGCT is currently not included in the LS tumor spectrum[16],and evidence is still sparse and limited[7,55].Cárcano et al.[56]evaluated MSI genotype among 133 TGCT patients using five quasi-monomorphic mono-nucleotide markers,finding microsatellite stable and low-frequency MSI genotypes in 126(95%)and 7(5%)individuals,respectively;H-MSI was not detected in the entire cohort.Conversely,Mayer et al.[57]recorded H-MSI in four out of 12 patients (33%) after late recurrence from non-seminoma TGCT.
Considering the lack of current data,this association between LS and TGCT still needs to be extensively investigated.
7.Conclusion
Although literature is still sparse,LS represents a pivotal autosomal dominant disease in the urological practice,especially among individuals experiencing UTUC.Since the prompt diagnosis of LS can lead to a significant oncological benefit both for the patient and the whole family,we believe that the urologist should recognize patients at increased risk for hereditary disease according to current European Association of Urology clinical criteria and address them to a comprehensive diagnostic algorithm,including molecular evaluation and genetic testing.
Author contributions
Study concept and design: Marco Moschini,Philippe E.Spiess,Andrea Necchi.
Data acquisition: Chiara Lonati,Marco Moschini.
Data analysis: Chiara Lonati.
Drafting of manuscript: Chiara Lonati.
Critical revision of the manuscript: Claudio Simeone,Nazareno Suardi,Philippe E.Spiess,Andrea Necchi,Marco Moschini.
Conflicts of interest
The authors declare no conflict of interest.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ajur.2022.05.009.
 Asian Journal of Urology2022年4期
Asian Journal of Urology2022年4期
- Asian Journal of Urology的其它文章
- The first Japanese case of intraductal cancer of the prostate with checkpoint kinase 2 mutation
- The relationship between sexually transmitted microorganisms and seminal quality in asymptomatic men
- Validation of Vesical Imaging Reporting and Data System score for the diagnosis of muscle-invasive bladder cancer: A prospective cross-sectional study
- Phalloplasty following penectomy for penile cancer
- Extramammary Paget’s disease: Updates in the workup and management
- Multidisciplinary management of patients diagnosed with von Hippel-Lindau disease:A practical review of the literature for clinicians
