Association between homologous recombination deficiency and outcomes with platinum and platinum-free chemotherapy in patients with triple-negative breast cancer
Yimeng Chen*, Xue Wang*, Feng Du, Jian Yue, Yiran Si, Xiaochen Zhao, Lina Cui, Bei Zhang, Ting Bei,Binghe Xu, Peng Yuan
1Department of Medical Oncology and Clinical Trial Center, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China;2Department of VIP Medical Services, National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing 100021, China; 3The Medical Department,3D Medicines Inc., Shanghai 201114, China
ABSTRACT Objective: The choice of chemotherapeutic regimen for triple-negative breast cancer (TNBC) remains controversial.Homologous recombination deficiency (HRD) has attracted increasing attention in informing chemotherapy treatment.This study was aimed at investigating the feasibility of HRD as a clinically actionable biomarker for platinum-containing and platinum-free therapy.
KEYWORDS Homologous recombination deficiency; triple-negative breast cancer; platinum; survival; BRCA
Introduction
Triple-negative breast cancer (TNBC) is a tumor type that does not express estrogen receptor, progesterone receptor, or human epidermal growth factor receptor 2 (HER2)1-3.Effective therapeutic strategies are lacking for TNBC4.In recent years,immune checkpoint inhibitors and anti-angiogenic drugs have shown efficacy in TNBC treatment5-8.However, chemotherapy remains the main treatment for TNBC9, and the optimum chemotherapeutic regimen has remained undefined,partially because of the high degree of TNBC heterogeneity.Platinum-based chemotherapy, a first-line treatment option for advanced TNBC, and the neoadjuvant regimen for resectable TNBC9have shown modest advantages over platinum-free regimens.A considerable proportion of patients do not respond to platinum10-18.Biomarkers are needed to inform patient selection and allow effective therapy to be provided to defined responding patients while enabling nonresponders to avoid the severe toxicity of ineffective chemotherapeutic regimens.
Previous studies have shown that patients withBRCAmutations are platinum-sensitive19-21; on this basis,BRCAmutations have been clinically used as predictors of platinum therapy efficacy.BRCA1/2mutation-associated-signatures overlap with abnormalities in homologous recombination repair (HRR) genes, which are deficient in a substantial subset of TNBC termed sporadic basal TNBC22,23.The hallmark aberrations in theBRCAand HR gene pathways are sensitive to DNA crosslinking induced by platinum24-28.Therefore, studies have been conducted to develop measures including HR deficiency (HRD) detection for identifying bothBRCA1/2-intact andBRCA1/2-deleterious HR-deficient patients, and predicting sensitivity to platinum29-31.The Myriad HRD assay was established to assess HRD by measuring loss of heterozygosity (LOH), telomeric allelic imbalance (TAI), and large-scale state transition (LST)29,32-34.This assay was initially approved as a companion diagnostic test to identify patients with HRDpositive advanced ovarian cancer for niraparib treatment35.In patients with resectable TNBC, the Myriad-HRD score is associated with the response to platinum neoadjuvant therapy10,36-42, whereas its predictive value for adjuvant therapy, a controversial platinum treatment for TNBC, remains unknown9,43,44.Patients with recurrent or metastatic TNBC experience a similar plight.An observational study has reported that patients with advanced breast cancer treated with platinum and with HRDetect scores ≥ 0.7 have greater 3-month overall survival, thus suggesting the value of HRD in guiding platinum treatment of advanced breast cancer45.The TNT trial has compared the response between carboplatin and docetaxel, and biomarker subgroup analyses have revealed that among the Myriad-HRD status,BRCAmutation,BRCAmRNA level,BRCA1promoter DNA methylation15, and structural chromosomal instability (CIN)30, onlyBRCAmutation and CIN predict a greater benefit from carboplatin over docetaxel in patients with metastatic TNBC,thus indicating a need for optimizing the HRD detection method.The question of whether platinum is an optimum treatment option forBRCA1/2-intact patients with HRD remains to be answered.
In this study, we analyzed the association between HRD status and the response to platinum-based treatment compared with platinum-free chemotherapy in patients with advanced TNBC in the first-line setting, and in patients with resectable TNBC in the adjuvant setting.Our aim was to explore the potential of HRD to guide personalized chemotherapy.This study used a Chinese population-based 3D-HRD detection assay incorporating LOH, TAI, and LST with next-generation sequencing (NGS).The associations of HRD with genomic aberrations and clinicopathological features were also explored.
Materials and methods
Study design and participants
This study analyzed HRD in Chinese patients with TNBC who received chemotherapy between May 1, 2008 and February 9, 2021 at the National Cancer Center.The study design and sample used during HRD algorithm development and the exploration of HRD’s clinical relevance in each step are shown in Supplementary Figure S1.For the assessment of HRD’s clinical relevance, 386 chemotherapy-treated patients with TNBC were screened from a surgical cohort(NCT01150513)46and a metastatic cohort11,14,47administered chemotherapy in the adjuvant and first-line metastatic setting, respectively.In the metastatic cohort, 495 patients with metastatic TNBC were screened from more than 10,000 patients with breast cancer, thus resulting in the selection of 40 patients with qualified samples.Finally, 189 patients with TNBC with available clinical and tumor sequencing data were included in this study.All tumor samples were collected during surgical operation and were subjected to NGS to evaluate HRD and genomic mutations.The primary aim of this study was to explore the association between HRD status and clinical outcomes.The associations of HRD with genomic mutations and clinicopathological features were also investigated.Data were analyzed from July 1 to September 1,2021.As of March 22, 2021, the median follow-up times of the metastatic and adjuvant cohorts were 27.7 [interquartile range (IQR): 15.9–44.5] and 86.8 (IQR: 75.8–101.7) months,respectively.This study was reviewed and approved by the ethics committee of the Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College(Approval No.19/147-1931) and was conducted in accordance with the Declaration of Helsinki (as revised in 2013).Exemption from written informed consent was approved by the above ethics committee.This study is presented according to the STROBE reporting checklist.
NGS and 3D-HRD algorithm development
NGS and HRD detection were conducted at 3D Medicines,Inc.(Shanghai, China), a College of American Pathologistsaccredited and Clinical Laboratory Improvement Amendmentscertified laboratory.Genomic DNA was extracted from formalin-fixed paraffin-embedded tumor and paired normal tissue samples.Eligible tumor tissue with ≥ 20% tumor cells was retained for subsequent analyses.Indexed libraries were subjected to probe-based hybridization with a customized NGS panel targeting 733 cancer-associated genes(Supplementary Table S1), including HRR-associated genes.The 3D-HRD algorithm was developed to estimate HRD with a training cohort of 594 tissue samples from Chinese patients with breast or ovarian cancer, on the basis of more than 10,000 single-nucleotide polymorphisms (SNPs) in the human genome.The HRD score was calculated as the sum of the LOH, TAI, and LST.All 3 measures predict benefits from neoadjuvant platinum therapy in patients with TNBC36.The threshold score for HRD was determined to be 30 to achieve 95% sensitivity in identifying patients withBRAC1/2-deficient mutations, through analysis of the HRD status of the training cohort of 106 breast and 488 ovarian tumors with knownBRCA1/2mutation status36,48.BRCAdeficiency was defined as having pathogenic or likely pathogenic deleteriousBRCA1/2mutations, with LOH in the wild-type copy.A tumor was defined as HRD-positive if it had an HRD score of ≥ 30 and/or a deleterious mutation inBRCA1/2.BRCA1/2-intact tumors with an HRD score of < 30 were defined as HRD-negative.Technical validation of the HRD assay was performed with 2 HCC cell lines and 75 tumor samples from an independent cohort of patients with breast or ovarian cancer.The HRD assay exhibited a sensitivity of 95.7% and a limit of detection of ≥ 20% tumor cells in tumor tissue (Supplementary Methods, Supplementary Figures S2 and S3).
Statistical analysis
Continuous variables were compared with Student’s t-test or the Wilcoxon test, and categorical variables were compared with the chi-square test or Fisher’s exact test, as appropriate.Simple linear regression analysis was performed to determine the relationship between the HRD score and the Ki67 proliferation index (%).Survival curves and median survival times for all groups were depicted with Kaplan–Meier survival curves and analyzed with the log-rank test.The hazard ratio (HR)and 95% confidence interval (CI) were estimated with the Cox proportional hazards model.Median follow-up was analyzed with the reverse Kaplan–Meier method.Univariate survival analyses were performed with the Cox proportional-hazards model.InteractionPvalues (P-interaction) are reported for the joint effects of biomarkers and treatment.P< 0.05 was considered statistically significant.Statistical analyses were performed in R software (version 3.6.1).
Results
Correlation between HRD and clinicopathological features
The mean age of the 189 included patients was 48.3 years(standard deviation, 9.5), and all patients were women.The remaining 197 patients were excluded because of incomplete clinical information (n= 1), or a lack of sufficient or qualified tumor samples for sequencing (n= 196) (no tumor sample,n= 159; unqualified tissue sample,n= 23; DNA extraction failure,n= 4; and sequencing library construction failure,n= 10).Among the 189 included patients, 149 patients received platinum-containing or platinum-free chemotherapy in the adjuvant setting (surgical cohort), and 40 patients with metastatic disease received chemotherapy in the first-line metastatic setting.The major pathological type was ductal carcinoma, accounting for 96.3% (182/189) of the patients in the entire cohort.Of the 149 patients in the surgical cohort, more than half (91/149, 61.1%) had stage II disease (Table 1).
Among the entire metastatic and surgical TNBC cohort(n= 189), 49.2% (93/189) of patients were classified as HRDpositive.The baseline characteristics indicated younger age(P= 0.01) and a higher Ki67 proliferation index (%) (P< 0.001)in the HRD-positive patients than the HRD-negative patients(Table 1).A high HRD score was positively associated with the levels of Ki67 expression (Spearman’s correlation coefficient, 0.32;P< 0.001), histologic grade (grade IIIvs.II: median 27.0vs.13.0;P= 0.02), and T stage (cT2vs.cT1: median 29.0vs.14.0;P= 0.001).The HRD score was not significantly correlated with lymph node status, TNM stage, N stage, menopausal status, or PD-L1 expression (Figure 1A, Supplementary Figures S4 and S5).
Genomic association with HRD
BRCA1/2mutations were identified in 21.2% (40/189) of patients from the entire metastatic and surgical TNBC cohort.Forty-three percent (40/93) of HRD-positive patients were classified as having deleteriousBRCA1/2-mutation, and 57.0%(53/93) were classified as havingBRCA1/2intact (Table 1 and Supplementary Table S2).The median HRD scores ofBRCAmutandBRCAwtpatients were 35.5 (range 0–90) and 20.0 (range 0–91), respectively.Among the 40 patients carryingBRCA1/2 mutations, 85.0% (34/40) had germlineBRCA1/2mutations (gBRCA1/2), and 27.5% (11/40) had somaticBRCA1/2mutations (sBRCA1/2).Two patients carried both gBRCA1and sBRCA1, one patient had concurrent gBRCA2and sBRCA2, and 2 carried both gBRCA1and sBRCA2(Supplementary Figure S6 and Table S2).
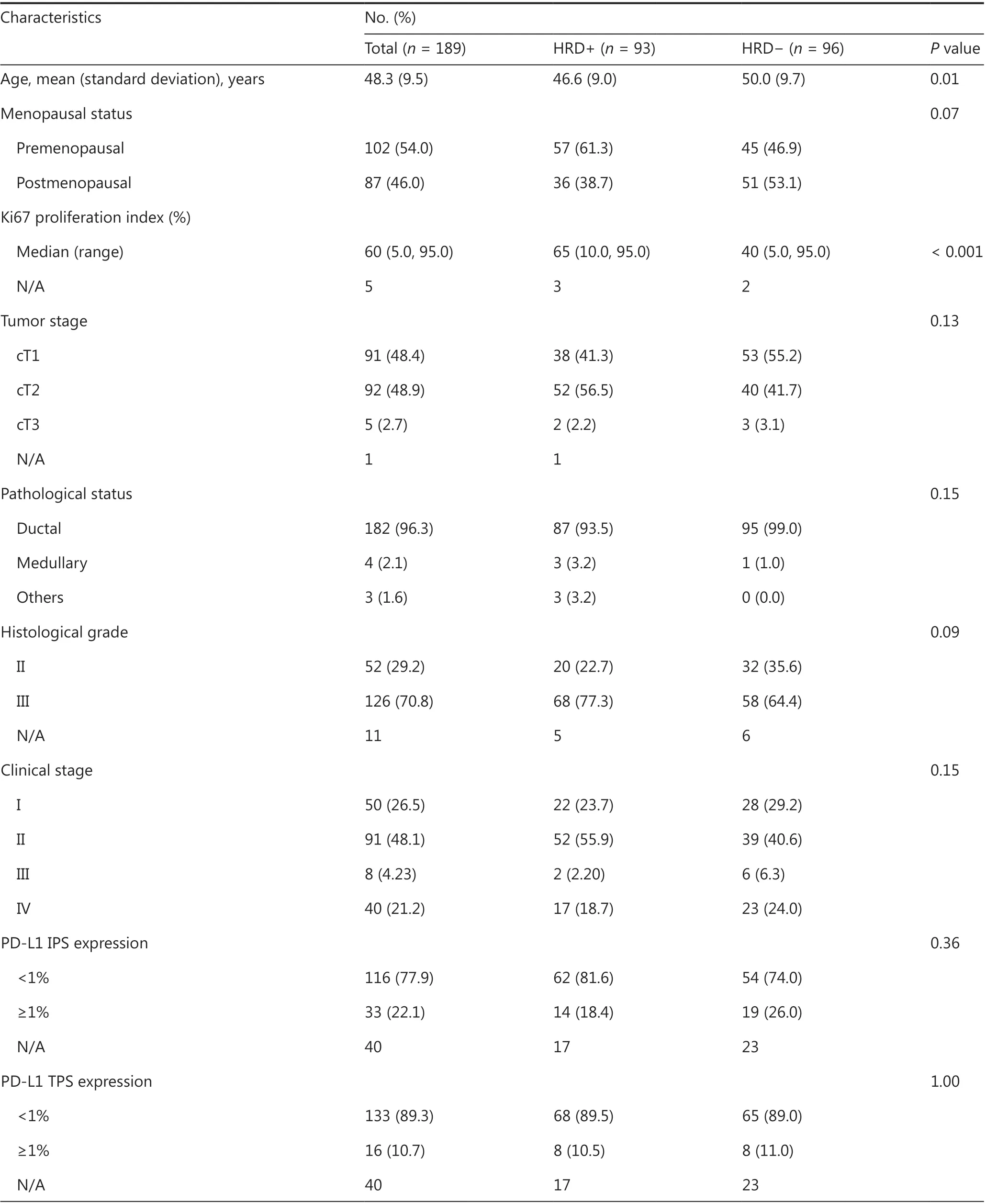
Table 1 Correlation between clinicopathological features and HRD status in 189 triple-negative breast cancers
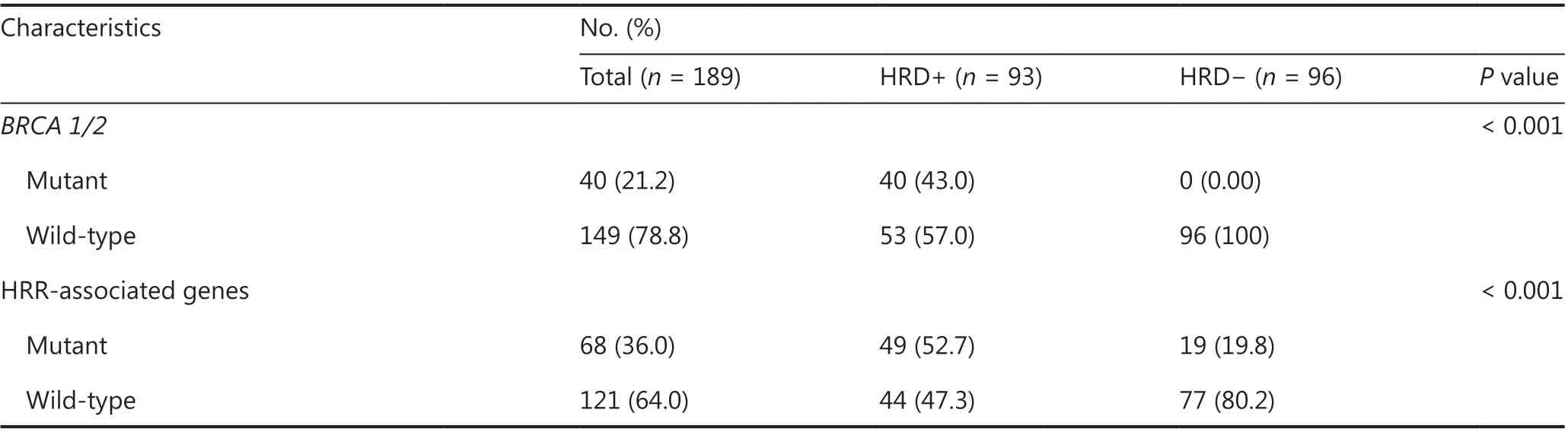
Table 1 Continued
Compared withBRCA1/2-intact patients, those carryingBRCA1/2mutations had a significantly higher HRD score(median, 35.5vs.20.0;P= 0.02, Supplementary Figure S7A).Because theBRCA1/2mutation subtypes may exert distinct effects on genomic instability49, we further examined HRD status among patients with gBRCA1/2and sBRCA1/2, and found that gBRCAmutpatients had a numerically higher HRD score (median, 48.0vs.16.0;P= 0.05) than their sBRCAmutcounterparts (Supplementary Figures S7B and S8).Further analysis revealed that only gBRCA1mutpatients had a significantly higher HRD score thanBRCA1/2-intact patients(median: 55.0vs.20.0;P< 0.001, Figure 1B), thus suggesting a critical role of germlineBRCA1mutation in genomic instability in TNBC.
Because LOH was incorporated into the algorithm used to calculate the HRD score, we analyzed the HRD scores amongBRCAmutpatients on the basis of theBRCALOH status.BRCALOHwas observed in 82.1% of patients withBRCA1mutation, compared with 35.7% ofBRCA2-mutated patients(P= 0.005).The HRD score ofBRCA1LOHpatients was significantly higher than that of theBRCA1non-LOHpopulation(median: 53.0vs.1.5,P= 0.008).Further analysis revealed that, among theBRCA1LOH,BRCA1non-LOH,BRCA2LOH, andBRCA2non-LOHsubgroups, theBRCA1LOHgroup achieved the highest HRD score and was the only subgroup showing a significantly higher HRD score than theBRCAwtgroup(P< 0.001), thus suggesting that LOH ofBRCA1had a relatively greater contribution to HRD (Supplementary Figure S7C and S7D).Notably, a patient withgBRCA1LOH had an HRD score of 90.
Because deficiencies in other HRR-associated genes may also confer genomic instability, we analyzed the association of the HRD score with deleterious variants, defined as pathogenic/likely pathogenic mutations, in 15 HRR-associated genes (Supplementary Table S3).Deleterious HRR-associated gene mutations were identified in 36.0% of patients from the entire 189-patient cohort (Supplementary Figure S9).Patients with HRR gene mutations had HRD scores similar to those of HRRwtpatients (median, 28.0vs.21.0,P= 0.83,Supplementary Figure S10A and S10B).Serendipitously, all 5 patients with ATM mutations were HRD-negative and had HRD scores less than 18 (Figure 1C and Supplementary Figure S10C).
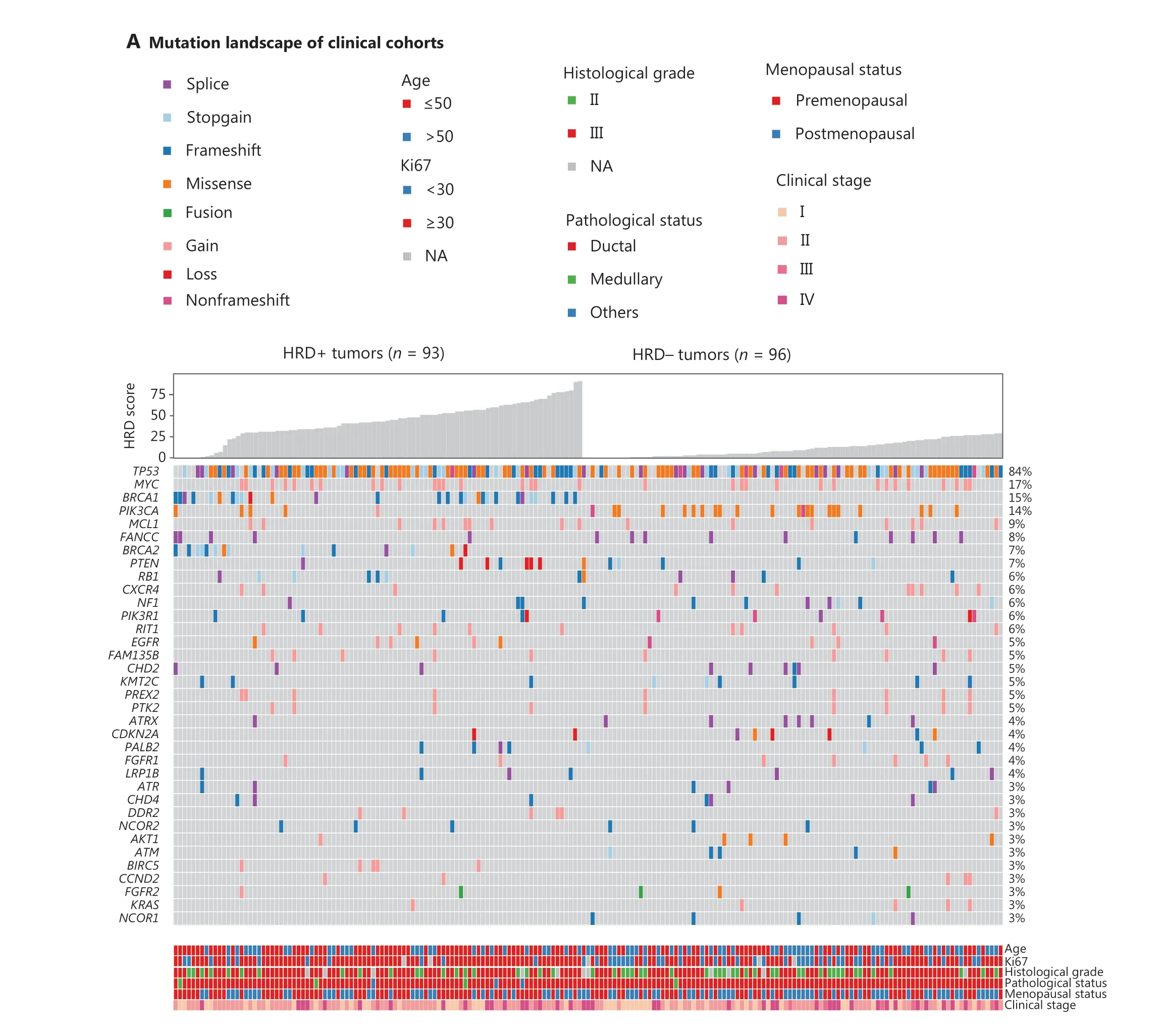
Figure 1 Continued
In addition to HRR gene mutations, deleterious mutations in other cancer-associated genes were investigated for HRD relevance.PIK3CA(P= 0.001) andBIRC5(P= 0.03) mutations were strongly associated with HRD status.ThePIK3CAmutation frequency was 22.9% (22/96) in HRD-negative patients, compared with 5.4% (5/93) in HRD-positive patients.FANCC(10.4%vs.5.4%),NF1(8.3%vs.3.2%),ATRX(7.3%vs.1.1%), andNCOR1(5.2%vs.0%) were more commonly mutated in HRD-negative than HRDpositive patients.BIRC5,SOX2, andPDCD1LG2mutations appeared in only HRD-positive patients (Figure 1A and Supplementary Table S4).Interestingly, patients withPIK3CAmutations was correlated with shorter disease-free survival(DFS) thanPIK3CA-intact patients in patients treated with platinum-containing adjuvant therapy (HR, 0.25; 95% CI 0.08–0.80;P= 0.01, Supplementary Figure S11).
Utility of HRD in guiding first-line chemotherapeutic treatment for metastatic TNBC
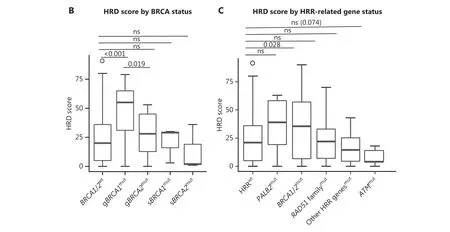
Figure 1 Correlation of HRD with molecular and clinical features.(A) Pathogenic alterations in tumor-associated genes and HRD scores, detected by next-generation sequencing of tumor DNA from 149 patients with TNBC.Patients with an HRD score ≥ 30 and/or deleterious mutations in BRCA1/2 were classified as HRD-positive, whereas patients with an HRD score < 30 and BRCA1/2-intact status were defined as HRD-negative.Mutated genes are listed in descending order from most to least frequently altered.The clinical features include, from top to bottom, patient age, Ki67 expression, disease stage, histologic grade, and pathological subtype.B, HRD scores of BRCA-wild-type tumors and tumors with either germline or somatic BRCA1/2 mutations.C, HRD scores of tumors with HRR gene mutations.HRD scores were determined with 3D-HRD assays.Differences in groups were calculated with either Wilcoxon or t-tests, as appropriate.P < 0.05 was considered statistically significant.
We analyzed the clinical outcomes of 40 patients with TNBC with metastatic TNBC treated with first-line platinum-based treatment, compared with patients receiving platinum-free chemotherapy (21vs.19; mean cycles of treatment, 5vs.4), to elucidate the utility of HRD in guiding first-line chemotherapeutic treatment for metastatic TNBC.Eight (8/40, 20%)patients had received neoadjuvant chemotherapy (5 patients received 6 cycles, and 3 patients received 8 cycles).The remaining 32 patients had no exposure to cancer drugs before tumor resection.Of the 8 patients treated with neoadjuvant chemotherapy, 5 and 3 were administered platinum and platinum-free drugs, respectively.No patients had prior exposure to radiation before surgery.Thirty-four patients (34/40, 85%) received adjuvant chemotherapy, and 22 (22/40, 55%) were administered radiotherapy postoperatively.At the data cutoff, disease progression or death had occurred in 38 patients.Among the entire metastatic TNBC cohort, 17 patients were HRD-positive,and 23 were HRD-negative.The baseline clinical characteristics were balanced (Supplementary Table S5).
The progression-free survival (PFS) of patients receiving platinum-containing treatment was significantly longer than that of patients receiving platinum-free therapy [median PFS (mPFS), 9.1vs.3.0 months; HR, 0.43; 95% CI 0.22–0.84;P= 0.01].The difference was more significant in HRD-positive patients (platinumvs.platinum-free, mPFS, 13.6vs.2.0 months; HR, 0.11; 95% CI 0.02–0.51;P=0.001, Figure 2A).For HRD-negative patients, no significant difference in PFS was observed between the platinum and platinum-free groups(mPFS, 6.8vs.4.5 months; HR, 0.88; 95% CI 0.70–2.10;P= 0.77).For patients treated with platinum, a longer PFS was observed in the HRD-positive population than in the HRDnegative population (mPFS, 13.6vs.6.8 months; HR, 0.35;95% CI 0.12–1.00;P< 0.05).Among patients who received platinum-free therapy, HRD-negative patients experienced a longer PFS than their HRD-positive counterparts (mPFS,4.5vs.2.0 months; HR, 0.30; 95% CI 0.10–0.88;P= 0.02,
Figure 2A and Supplementary Table S6).No significant differences in the objective response rate or disease control rate was observed in the unselected group or subgroups stratified by HRD status (Supplementary Figure S12).
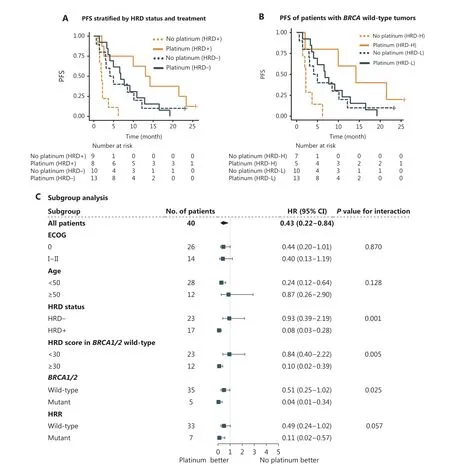
Figure 2 Association of progression-free survival (PFS) with HRD in metastatic TNBC.(A) Kaplan–Meier curves showing the PFS of metastatic patients with TNBC (n = 40) in each group, by chemotherapeutic regimen and HRD status.HRD+ was defined by an HRD score ≥ 30 and/or deleterious mutations in BRCA1/2, whereas BRCA1/2-intact tumors with HRD scores < 30 were defined as HRD?.(B) PFS of BRCA1/2-wildtype TNBC treated with first-line platinum-containing or platinum-free chemotherapy (n = 35).Patients with HRD scores ≥ 30 were defined as HRD score high (HRD-H), whereas those with an HRD score < 30 were defined as HRD score low (HRD-L).(C) Subgroup analyses according to baseline characteristics including ECOG, age, HRD score, and mutations in BRCA1/2 and HRR genes.Forest plots showing the estimated hazard ratios (HR) for progression with a univariable Cox proportional hazards model; 95% confidence intervals (CI) of HRs are represented by horizontal bars.HRR, homologous recombination repair.HRD, homologous recombination deficiency.ECOG, Eastern Cooperative Oncology Group performance score.
Consistently, inBRCA1/2-intact patients, PFS also favored platinum therapy (mPFS, 8.4vs.3.1 months; HR, 0.51; 95% CI 0.25–1.00;P= 0.05), particularly in HRD score-high patients(HRD score ≥ 30vs.HRD score < 30, mPFS, 14.2vs.2.2 months;HR, 0.10; 95% CI 0.01–0.84;P= 0.01).AmongBRCA1/2-intact patients treated with platinum, HRD score-high patients had a PFS twice that of HRD score-low patients (mPFS, 14.2vs.6.8 months; HR, 0.32; 95% CI 0.09–1.20;P= 0.07), but in patients administered a platinum-free regimen, HRD score-high was associated with a shorter PFS (mPFS, 2.2vs.4.5 months; HR,3.00; 95% CI 0.96–9.10;P= 0.05, Figure 2B).
Univariable analysis revealed that among age, ECOG score,HRD status,BRCAstatus, and mutation in HRR genes, only HRD status showed a statistically marginally significant association with a PFS benefit in the platinum-containing group(Supplementary Table S7).Forest plots indicated a significant benefit from platinum in HRD-positive patients compared with HRD-negative patients (interactionP= 0.001), thus indicating that HRD-positive patients may benefit more from platinum (Figure 2C).We observed a significantly prolonged PFS from platinum inBRCA1/2mutated patients compared withBRCA1/2-wildtype patients (interactionP= 0.025), thus indicating thatBRCA1/2-mutated patients may benefit more from platinum therapy.
The median OS was 36.8 and 18.9 months for patients with metastatic TNBC who were treated with platinum and platinum-free chemotherapy, respectively.No difference was observed in overall survival between the treatment groups(Supplementary Figure S13).
Association of HRD with clinical outcomes of patients with TNBC receiving adjuvant chemotherapy
In the surgical cohort of 149 patients with TNBC, no patients received neoadjuvant therapy.After surgery, 74 patients were treated with 6 cycles of TP (docetaxel: 75 mg/m2or paclitaxel 175 mg/m2day 1; carboplatin AUC = 5, day 1), and 75 received 4 cycles of EC (epirubicin: 90 mg/m2; cyclophosphamide: 600 mg/m2, day 1) followed by 4 cycles of T (docetaxel: 75 mg/m2or paclitaxel 175 mg/m2, day 1).Approximately 26.1%(39/149) of patients had received postoperative radiation.At the data cutoff, 28 (18.8%) patients had experienced disease recurrence or died.Seventy-six patients were HRD-positive,and 73 were HRD-negative (Supplementary Table S8).The HRD-positive patients in the platinum group tended to benefit more than those in the platinum-free group (HR, 0.33;95% CI 0.11–1.10;P= 0.05) and did not reach mDFS, and the interaction between HRD status and treatment was significant(P= 0.02).In addition, for HRD-positive patients, the 5-year DFS rate after treatment with platinum was 91.7%, which was numerically better than the 82.4% value for those treated with platinum-free chemotherapy (Figure 3A and Supplementary Table S9).Among the patients treated with platinum-free chemotherapy, no significant difference in DFS was observed between HRD-positive and HRD-negative patients (HR, 0.39;95% CI 0.12–1.20;P= 0.10, Figure 3A).Among patients treated with platinum, HRD-positive patients tended to benefit more than HRD-negative patients, but the difference was not significant (HR, 0.35; 95% CI 0.10–1.20;P= 0.08,
Supplementary Tables S9 and S10).BRCA1/2mutation (24%,35/149) was associated with poor prognosis (HR, 2.1; 95% CI 1.0–4.6;P< 0.05) in the unstratified surgical cohort but not in the platinum-treated subgroup (Figure 3B, Supplementary Tables S9 and S10).In addition,BRCA-intact patients exhibited longer DFS thanBRCA1/2-mutated patients in the platinum-free group (mDFS not reachedvs.116.8 months; HR,0.30; 95% CI 0.11–0.85;P= 0.02), whereas no significant difference was observed in the patients treated with platinum(Figure 3).In theBRCA-intact subset, no difference was found in DFS between the different treatment or HRD status groups(Supplementary Figure S14).
Discussion
This study provides novel evidence of the utility of HRD in guiding chemotherapeutic decision-making in a metastatic cohort and a surgical cohort treated with platinum-containing and platinum-free chemotherapy.Our findings revealed that among HRD-positive patients with metastatic disease,the mPFS was significantly better in patients administered first-line platinum-based treatment than in patients receiving platinum-free treatment (mPFS, 13.6vs.2.0 months;P= 0.001; treatment-biomarkerP-interaction = 0.001).In patients with metastatic disease who were treated with a firstline platinum-free regimen, HRD-negative status was associated with a longer PFS (mPFS, HRD?vs.HRD+, 4.5vs.2.0 months;P= 0.02).However, for patients with metastasis who were treated with first-line platinum regimens, HRD-positive patients benefited more than HRD-negative patients (mPFS 13.6vs.6.80 months,P< 0.05).In theBRCA1/2-intact subgroup, PFS also favored platinum therapy in HRD-high patients (mPFS, 14.2vs.2.2 months;P= 0.01), thus informing the first-line platinum treatment choice for metastatic TNBC.In the surgical cohort of patients with TNBC treated with adjuvant chemotherapy, HRD-positive patients treated with platinum tended to experience a greater benefit than those treated with platinum-free therapy (P= 0.05,P-interaction = 0.02).
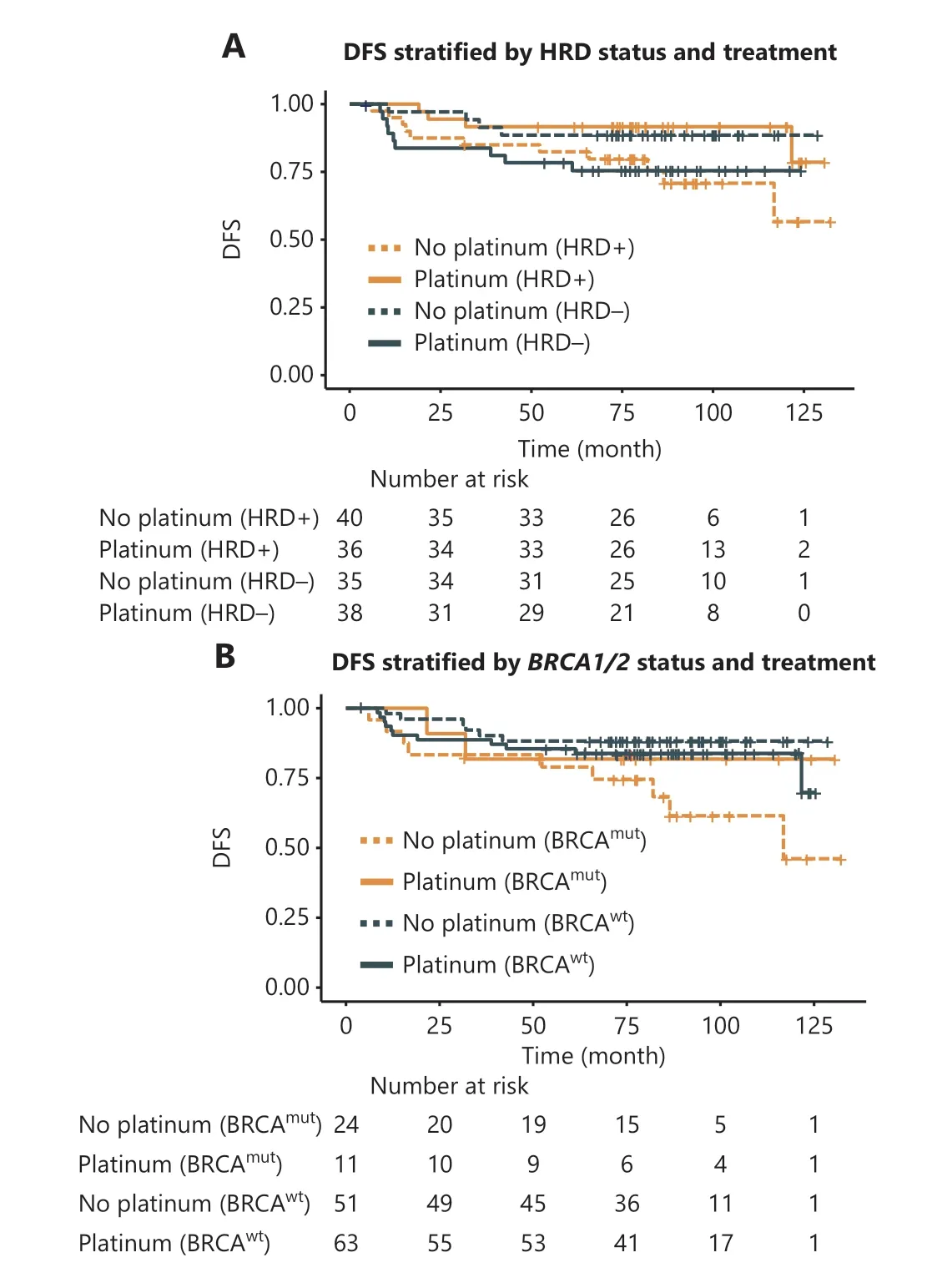
Figure 3 Association of HRD with disease-free survival (DFS)among patients with TNBC receiving adjuvant chemotherapy in the surgical cohort.(A) DFS of HRD+/? patients with TNBC receiving platinum-containing in comparison to platinum-free chemotherapy.HRD+ was defined by an HRD score ≥ 30 and/or deleterious mutations in BRCA1/2, whereas BRCA1/2-intact tumors with HRD scores < 30 were defined as HRD?.(B) DFS of BRCA1/2mut and BRCA1/2wt patients with TNBC treated with platinum-containing or platinum-free regimens.
Several studies have investigated the efficacy of platinum-containing regimens in treating patients with metastatic TNBC.In a randomized phase II trial comparing first-line docetaxel-cisplatin (TP) and docetaxel-capecitabine (TX) in patients with metastatic TNBC, the mPFS of the TP group was significantly longer than that of the TX group (10.9vs.4.8 months)14.Consistently with these findings, an open-label, multicenter randomized crossover trial (CBCSG006) has indicated significantly better mPFS for the platinum-containing group than the paclitaxel-containing group (7.7vs.6.1 months)13.A multicenter, real-world retrospective study of 495 Chinese patients with metastatic TNBC has also reported a longer PFS elicited by first-line platinum-based chemotherapy than platinum-free chemotherapy (8.4vs.6.0 months)11.Although previous reports have shown that patients with metastatic TNBC may benefit more from platinum than platinum-free therapy, 36.4%–68.6% of patients with TNBC are insensitive to platinum11,14-16.In addition, owing to the toxicity-associated adverse effects induced by platinum and the physical limitations of patients with advanced tumors,clinicians remain hesitant in deciding on treatment options,particularly for patients carrying noBRCA1/2mutations.Biomarkers guiding treatment are urgently required beyond the context ofBRCA1/2mutations.In our patients with metastatic TNBC who were treated with first-line platinum and platinum-free chemotherapy, mPFS favored platinum in the HRD-positive group but not in the HRD-negative group,thus showing a significant treatment-biomarker interaction(P= 0.001).The results from theBRCA1/2-intact subpopulation were consistent with that in the population not stratified byBRCA1/2mutations.These results differed from those of the TNT trial, in which no interaction was observed between HRD and the clinical outcomes of patients with metastatic TNBC receiving platinum and platinum-free therapy, according to the report published in 201815.Potential explanations for the difference in results between the TNT trial and our study include that the 2 HRD methods differed (3D-HRDvs.myChoice HRD), the study population was diverse (100%Chinesevs.87.2% White/6.1% Black/2.9% Asian/3.5% not stated/0.3% mixed), the onset age differed (mean of 48 yvs.55 y), and the disease status varied (metastatic 100%vs.90.2%).Moreover, another post hoc analysis of the TNT trial reported in 2021 has revealed that patients with TNBC tumors lacking high-level amplifications and displaying intermediate CIN benefit more from carboplatin than docetaxel.These data indicate a complex association of the degree of genomic instability with treatment response in patients with metastatic TNBC30.
In contrast to the recommendation of platinum therapy for metastatic TNBC, platinum use in adjuvant therapy remains controversial.In a recent randomized, multicenter phase II noninferiority clinical trial investigating platinum-containing adjuvant chemotherapy in patients with operable TNBC, the activity of taxanes combined with carboplatin chemotherapy was similar to the standard regimen of epirubicin and cyclophosphamide followed by taxanes (5-year DFS, 84.4%vs.85.8%,Pnoninferiority=0.03)46.In another retrospective study of platinum efficacy in patients with early stage TNBC,addition of carboplatin to standard adjuvant chemotherapy was not associated with improved relapse-free survival or OS43.However, a phase III randomized PATTERN trial has suggested a significantly longer DFS in the platinum treatment group than the platinum-free treatment group (5-year DFS, 86.5%vs.80.3%)18,44.Therefore, biomarkers must be screened to predict platinum efficacy in the adjuvant setting.The patients in the surgical TNBC cohort in this study were recruited from our noninferiority phase II trial46.The 5-year DFS rate of patients receiving adjuvant platinum therapy was 91.7%, which was numerically better than the value of 82.4%in those receiving platinum-free therapy.Although platinum-containing therapy did not provide a significant benefit in the adjuvant setting, HRD-positive patients treated with platinum tended to experience a greater benefit than those who received platinum-free therapy (P= 0.05), and the interaction between HRD status and treatment was significant(P= 0.02).
In this study, we also analyzed the correlations of HRD with HRR pathway genes and clinicopathological characteristics.Patients with TNBC with germlineBRCA1/2mutations showed higher HRD scores than those with somaticBRCA1/2mutations (median: 48.0vs.16.0), and a subsequent analysis indicated that this result might have been due to a higher frequency of LOH in patients withBRCA1/2pathogenic germline mutations than in those with somatic variants.Our results also suggested that the contributions ofBRCA1andBRCA2to genomic scarring was distinct, given the significantly higher HRD score observed in gBRCA1mutpatients than in those carrying gBRCA2mut(P= 0.02), thus potentially affecting the efficacy of DNA-damaging agents.In 2020,Sokol’s group has reported thatBRCA1mutations result in more intensive HR deficiency thanBRCA2mutations49.Moreover, a study published in Nature in 2019 has associatedBRCA1mutations with higher HRD scores than those withBRCA2mutations, and associated gBRCA1biallelic mutations with higher HRD scores than those with gBRCA2biallelic mutations50.Although that study did not directly compare the effects of gBRCA1and gBRCA2mutations on HRD, as was performed herein, their results partially support our finding that gBRCA1might be associated with more intensive HR deficiency than gBRCA2.Notably, mutations in HRR pathway genes (excludingBRCA) had no effect on the HRD scores of patients with TNBC.A high HRD score was significantly correlated with malignant phenotypes, including Ki67 expression, histological grade, and T stage, in agreement with a previous report51.
Although both 3D-HRD and the well-known Myriad myChoice HRD assay incorporate LOH, TAI, and LST to evaluate HRD, the 3D-HRD assay might be more cost-effective and applicable and more suitable than the Myriad myChoice HRD assay to evaluate HRD in Chinese patients with cancer.Our study used a well-designed HRD algorithm developed on the basis of sequencing data from Chinese patients with ovarian and breast cancer, with a minimized panel size of 10,000 SNPs.In contrast the Myriad myChoice HRD assay was trained on White patients and included more than 50,000 SNPs29.Moreover, the 3D-HRD assay did not includeBRCA1promoter DNA methylation, which was considered in the Myriad HRD assay.A recent study has found thatBRCA1promoter DNA methylation is a functionally plastic state that is depleted rapidly after exposure to chemotherapy, andBRCA1methylation status was not associated with a survival advantage52.
Although our study used a retrospective design, it included 2 clinical cohorts, whose data were obtained in a randomized clinical trial in a highly homogeneous population, which had highly consistent treatment exposures and in a real-world study, thus providing some assurance of data quality and clinical significance.However, prospective studies with larger sample sizes are warranted.
Conclusions
In HRD-positive patients with TNBC, a platinum-containing regimen may be preferred, and the platinum-free regimen may be more beneficial for HRD negative patients.The HRD status determined according to the HRD score andBRCA1/2mutation status has the potential to screen the population suitable for platinum-containing therapy.Notably, this cohort study suggested that HRD may be a more robust predictor of platinum efficacy in the metastatic setting than the adjuvant setting.Further studies with larger cohorts and prospective designs are needed to validate our findings.
Acknowledgements
We greatly appreciate all participating patients, and their families and caregivers.We particularly thank Msc.Yuezong Bai,Dr.Hao Chen, Dr.Jie Wang, Msc.Bin Li, and Msc.Kai Cao(3D Medicines Inc., Shanghai) for assistance in study design and data interpretation.
Grant support
This study was granted by Capital’s Funds for Health Improvement and Research (Grant No.2018-2-4023) and the National Natural Science Foundation of China (Grant No.82001559).
Conflict of interest statement
Dr.Xu reports grants from Hengrui Pharmaceutical, personal fees from AstraZeneca, personal fees from Pfizer, personal fees from Roche, and personal fees from Eisai outside the submitted work.X.Zhao, L.Cui, B.Zhang, and T.Bei report that they are employees of 3D Medicines Inc.The remaining authors declare no conflicts of interest.
Author contributions
Conceived and designed the analysis: Peng Yuan, Binghe Xu.Collected the data: Yimeng Chen, Xue Wang, Feng Du,Jian Yue, Yiran Si.
Contributed data or analysis tools: Xiaochen Zhao, Lina Cui,Bei Zhang.
Performed the analysis: Bei Zhang, Lina Cui, Xiaochen Zhao,Ting Bei.
Wrote the paper: Yimeng Chen, Xue Wang, Ting Bei, Lina Cui.
 Cancer Biology & Medicine2023年2期
Cancer Biology & Medicine2023年2期
- Cancer Biology & Medicine的其它文章
- Optimization of regional nodal irradiation in the era of sentinel lymph node biopsy
- Cancer risk in relatives of BRCA1/2 pathogenic variant carriers in a large series of unselected patients with breast cancer
- Circular RNAs: implications of signaling pathways and bioinformatics in human cancer
- Conceptualizing the complexity of ferroptosis to treat triplenegative breast cancer: theory-to-practice
- Intercellular transmission of cGAS-STING signaling in cancer
- 2022 Chinese expert consensus and guidelines on clinical management of toxicity in anti-CD19 chimeric antigen receptor T-cell therapy for B-cell non-Hodgkin lymphoma
