Quality Changes and Safety Evaluation of Ready-to-Eat Roasted Antarctic Krill (Euphausia superba) During Storage at Room Temperature (25℃)
YANG Xu, SHI Yongfu, CAI Youqiong, and CHI Hai
Quality Changes and Safety Evaluation of Ready-to-Eat Roasted Antarctic Krill () During Storage at Room Temperature (25℃)
YANG Xu, SHI Yongfu, CAI Youqiong, and CHI Hai*
,,200090,
The objective of this study was to determine the quality changes and shelf life of ready-to-eat roasted Antarctic krill, ei- ther untreated (CT) or treated with sodium diacetate (SD) or sodium diacetate and a deoxidizer (SDD), during storage at room tem- perature (25℃) by using sensory, physiochemical, and microbial assessment. Additionally, fluoride accumulation in this food product was used to evaluate its safety. Analysis showed that the addition of SD and SDD resulted in better sensory scores compared of that of CT samples (<0.05). Accordingly, microorganism growth and total basic nitrogen (TVB-N) were maintained at a low level for the duration of storage with SD and SDD treatments. The total viable counts of SD and SDD reached (4.58±0.08)log(CFUg?1) and (4.20±0.11)log(CFUg?1), respectively. The mold was found after 6 and 18 days for SD and SDD treatment, and the numbers reach- ed 5.2×106and 8.5×104respectivelyat the end of shelf life. The TVB-N values from CT continuously increased during the whole storage. While TVB-N values from SD and SDD remained significantly less change (<0.05) during the early 20 days of the storage, and the values reached (12.11±0.07)mg(100g)?1and (10.88±0.15)mg(100g)?1on day 33 and day 70, respectively. Importantly, SDD treatment effectively minimized the oxidation values and retained the color of ready-to-eat roasted Antarctic krill. Our results showed that the shelf life of ready-to-eat roasted Antarctic krill treated with SDD was extended by up to 52 days. Additionally, rats fed ready-to-eat roasted Antarctic krill showed accumulation of fluoride exclusively in the thighbone. The accumulation of fluorideresidues in the thighbone showed concentration-dependent. The concentrations of fluoride residues in rats were (1760.03±38.21),(2371.52±42.15)mgkg?1and (3615.44±30.53)mgkg?1, which were less than sodium fluoride feeding group (4621.01±28.67)mgkg?1. The results suggested that the SD and SDD treatments led to better quality and shelf life extension of ready-to-eat roasted Antarctic krill during storage at room temperature (25℃). Therefore, the ready-to-eat roasted Antarctic krill can be of great interest to the sea- food industry.
Antarctic krill; ready-to-eat product; antioxidant; quality changes; shelf life; safety potential
1 Introduction
Antarctic krill () is a shrimp-like invertebrate widely distributed in the Antarctic Ocean. It playsimportant roles as a basic supplier in the marine ecosystem and provides high-quality proteins with low-fat content to human consumers (Cavan., 2019). Recent studies show that the total capacity of Antarctic krill is approximately equal to the global fishery outputs (Zerbini., 2019). Its abundant availability has the potential to solve the short- age of natural resources for human consumption (Des-camps., 2016;Cavan., 2019). Therefore, the po- tential value of Antarctic krill on commercial and biologi- cal scales have drawn considerable attention. Products andby-products of Antarctic krill were gradually developed. Ini- tially, early products of Antarctic krill were boiled, frozen, and peeled. These low-value products were approved sole-ly for and widely used in aquaculture as food additives (Bao., 2019; Ma., 2019; Xie., 2019). Recently, some attempts on the production of high-end products havebeen successfully applied. Most of these high-value pro- ducts are prepared as bioactive peptides, with 10% of the total production being used for official fishing competitions(Ji., 2017;Lan., 2019; Zhao., 2019). Con- currently, many applications of Antarctic krill for human consumption have been reported by adding this seafood to soup. Many methodological studies are in progress to in- crease consumption and utilization of Antarctic krill as a portion of human food with higher nutritional value (Su- zuki and Shibata, 1990;Kidd, 2007; Parolini., 2017).
Ready-to-eat roasted Antarctic krill, which imitates dried shrimp, has gained popularity with consumers in China onaccount of its taste. The products were made under low wa- ter content (28%). The low water content can lead to bac- terial inhibition, as well as contribute to a crispy texture and colorful appearance with little changes in flavor. However, the low water content caused by thermal treatment may al- so lead to the growth of mold and lipid oxidation, which consequently leads to off-flavor development and rancidi- ty, thereby directly impacting the shelf life of these products(Wang., 2019). Traditionally, adding antioxidants andacid, such as deoxidizers, citric acid, and sodium diacetate, during food processing are common strategies for inhibit- ing mold and overcoming lipid oxidation, particularly inready-to-eat heat-treated products (Hidalgo and Zamora, 2017; Butts-Wilmsmeyer., 2018).
A high level of fluoride in Antarctic krill is a major con-cern for its utilization. The fluoride is a component in theexoskeleton of krill. The contents of it in Antarctic krillrange between 500–2400mgkg?1 in the whole body, 5–570mgkg?1 in the muscle, and 1800–4260mgkg?1 in the cuticle(Sands., 1998; Camargo, 2003; Peng., 2019). Fluoride is accumulated in the body of animals, al- though the toxic effects of accumulation are unknown. Con- sidering that a large amount of Antarctic krill is consumed, and the harmful effects of fluoride is still uncertain, studies are required to address this controversy.
At present, the evaluation of shelf life and safety of ready-to-eat roasted Antarctic krill, particularly stored at 25℃, is limited. The aims of this study were 1) to investigate the quality changes of ready-to-eat roasted Antarctic krill dur- ing storage, 2) to analyze the advantage of using sodium diacetate and antioxidants during food processing on thequality of the products, and 3) to evaluate the safety of fluo- ride residue in ready-to-eat roasted Antarctic krill.
2 Materials and Methods
2.1 Sample Collection
Antarctic krill were captured at the FAO48.2 area in the Antarctic Ocean in March 2019, and rapidly frozen and stored at ?20℃ in the polar research vessel. The Antarctic krill was delivered to the laboratory in May and stored at ?80℃ before use.
2.2 Preparation of Ready-to-Eat Roasted Antarctic Krill
Frozen Antarctic krill (1kg) were thawed under runningtap water at 25℃ until it completely separated. The An-tarctic krill were manually selected and then cooked in boil- ing water for 10min. Boiled Antarctic krill were cooled in ice-water for 15min. The water from the boiled Antarctickrill was removed naturally at 4℃ for 2h and Antarctic krill were subsequently divided into two groups. Each group was seasoned with sugar (12.0%), salt (2.0%) and monosodium glutamate (0.5%) correspondingly to the sample weights. One group with adding sodium diacetate (0.05%) was term- ed as SD group. Another group without sodium diacetate was employed as control group and was termed as CT group. Both of them were roasted at 155℃ until the water content of Antarctic krill reached 28%. All roasted Antarctic krill were sorted individually into 50g portions, and immediate- ly vacuum-sealed in a sterile package after cooling down at 25℃ for 30min. The packaged samples were sterilized at 90℃ for 50min and then cooled in running water for 20min. Food grade iron powder organic deoxidizer oxygen absor- ber packets (20mg) (Shenzhen Topcod Packing Materials Co., Ltd., China ) was added into one-half of the SD sam- ples, and this group was termed as SDD group. Three groups of ready-to-eat roasted Antarctic krill were stored at 25℃ for further study.
2.3 Microbiological Analysis
Total viable count (TVC) and number of mold formed on ready-to-eat roasted Antarctic krill were determined dur- ing the storage at 25℃. In brief, the ready-to-eat roasted Antarctic krill (25g) were aseptically transferred into ste- rile stomacher bags, diluted in 225mL saline (0.85%) and homogenized in a Stomacher Masticator (IUL, Spain) for 30s. A serial dilution (1:10) was prepared with saline and 100μL of the solution was spread in triplicate onto the fol- lowing agar plates: Plate count agar (PCA) (Oxoid, Ger-many) for TVC, and Rose Bengal Medium (RBM) (Solar- bio, China) for mold detection. The TVC was recorded af- ter 48h of incubation at 30℃, and the number of mold on RBM was counted after 5 days of incubation at 28℃.
2.4 Total Volatile Basic Nitrogen (TVB-N) Determination
Approximately 10g of roasted Antarctic krill were blend- ed with 100mL distilled water and equilibrated at 25℃ for 30min. The solution was filtered using filter paper (?11 cm, Hangzhou Xinhua Paper Industry Co., Ltd., China). The filtrate was used for TVB-N content measurement accord- ing to Xia. (2016). The amount of TVB-N was mea- sured in duplicate and expressed as mg(100g)?1.
2.5 Thiobarbituric Acid Reactive Substances (TBARS) Determination
Lipid oxidation of ready-to-eat roasted Antarctic krill was determined by analyzing the amount of TBARS described previously by Ghani. (2017). Approximately 10g of roasted Antarctic krill were chopped and homogenized in 50mL trichloroacetic acid (TCA) (7.5% with 0.1% EDTA) for 30min. Then the solution was filtered twice by using fil- ter paper (?11cm, Hangzhou Xinhua Paper Industry Co., Ltd., China). The filtrate (5mL) was blended with 5mL of thiobarbituric acid (TBA) solution (0.02molL?1) and incu- bated at 90℃ for 40min. The solution was centrifuged at 3000rmin?1for 5min after incubation. The supernatant (5mL) was blended with 5mL of chloroform and placed at room temperature for 10min. The optical density of the su- pernatant was measured at 532nm (532) and 600nm (600). The value of TBA was calculated by using the following formulation:
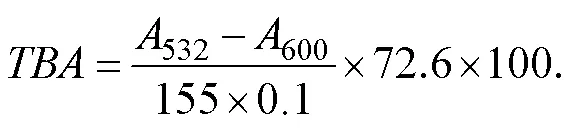
The value of TBA was expressed as mg(100g)?1.532and600represent the optical densities at 532nm and 600nm, respectively. The TBARS value converted the TBA va-lues into the amounts of malondialdehyde (mgMA) per kg of roasted Antarctic krill.
2.6 Color Properties
Roasted Antarctic krill (20g) were cut and measured by using a colorimeter (Microptix Corporation S560, USA). The color parameter of roasted Antarctic krill was express-ed by the(yellowness) values. Each sample was analyzed in triplicate.
2.7 Fluoride Content
Fluoride contents for all samples were detected by ionchromatography. Samples (0.5g) were dissolved with 8mL of deionized water containing hydrochloric acid (v/v, 11:1), and subsequently digested for 1h. Deionized water was add- ed to the digestion solution to make up 100mL. The mix- ture was kept at 25℃ for 10min and then centrifuged at 4000rmin?1for 15min. The whole supernatant was collect- ed successively via the aqueous phase membrane (0.22μm) and IC-Ag10 phase (Pu’an Co., Ltd., Shanghai). Fluoride contents of all samples from the supernatant were detect- ed following the method described by Li. (2018). The values of the fluoride content were expressed as mgkg?1.
2.8 Animal Experiment
A total of 50 female KunMing rats (15–21g) were ob- tained from Fudan University (Shanghai, China). All ani- mals were individually housed at 25℃. Rats were starved for at least 12h prior to the commencement of the experi- ment, and they were randomly assigned into 5 groups (=10 per group). All groups were fed identically with fluoride- free rat food. Among all groups, one was intragastrically fedwith 1mL of sodium fluoride-containing deionization wa- ter (300mgmL?1) daily, denoted as sodium fluoride group. Three groups of rats were intragastrically fed with different amounts of roasted Antarctic krill (converted as 100, 300, and 500mgL?1of fluoride, marked as T1, T2 and T3, res- pectively) in the deionization water. The rats fed with fluo- ride-free deionization water were considered as a control group. All rats were sacrificed following ethical protocols and procedures 10 days after fluoride-feeding treatment (Jech, 1979). Terminal blood samples from all rats were col-lected by heart puncture according to Sun. (2017) and the serum samples were stored at ?80℃ before the inves- tigation. The small intestine, liver, kidney, and thighbone of all rats were collected and stored at ?20℃ before use. Ap- proval was obtained from the Fudan University Institutional Review Board.All experiments with rats were approved bythe Fudan University’s Animal Care and Use Committee,and were performed following the Fudan University guide- lines on animal care.
2.9 Sensory Evaluation
Seven well-trained panelists volunteered to evaluate the ready-to-eat roasted Antarctic krill during the whole stor- age period. The samples with different treatments were ran- domly labeled and served to the panelists. All panelists were asked to score texture, color, odor, and flavor using a ten- point scale, where point 10 was the highest quality score, 0 was the lowest, and point 5 was acceptable level accord- ing to Haouet. (2018).
2.10 Statistical Analysis
All experiments were performed a minimum of three times. For each experiment, the results were recorded as mean±standard deviation. A two-way analysis of variance (ANOVA) was carried out by using SPSS 24.0 (SPSSInc., Chicago, IL, USA). Differences in the mean values of all re- sults were calculated by using Duncan’s multiple comparisons test. Statistical significance was characterized by<0.05.
3 Results
3.1 Shelf Life of Ready-to-Eat Roasted Antarctic Krill with Different Treatments
Sensory evaluation is one of the intuitive indicators of consumer acceptance of products. It can directly reflect con- sumers’ demand and the quality changes of the products du- ring storage. Therefore, sensory evaluation can be one of the effective indicators to evaluate the shelf life of products.Table 1 shows the sensory scores of the ready-to-eat roast- ed Antarctic krill with different treatments. In general, com- pared to the control group, the shelf life of the krill sam- ples were extended by the treatments. The sensory score ofCT decreased rapidly during the storage at 25℃, and it was close to the sensory rejection point at day 18. The sensory rejection point of the SD group could be extended to 40 days. A similar trend was found in the SDD group, while the sensory scores of SDD groups reached rejection point after approximately 70 days.
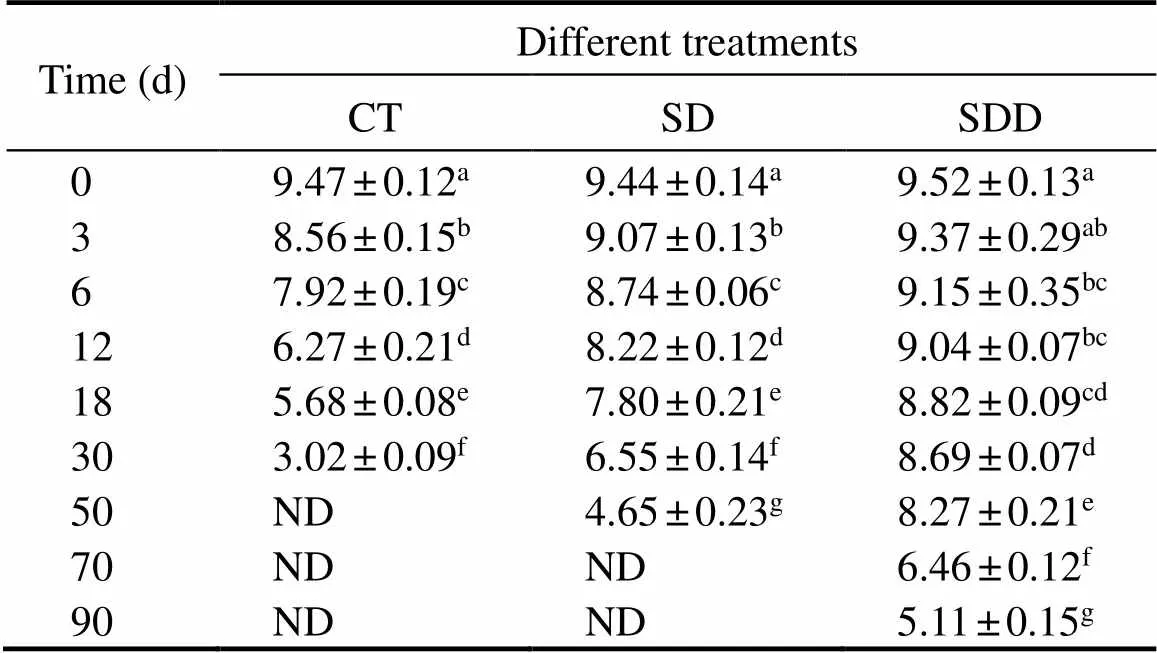
Table 1 Sensory evaluation of ready-to-eat roasted Antarctic krill with different treatments during storage
Notes: ND means not detected. Different letters indicate significant differences between groups (<0.05).
3.2 Total Viable Count and Number of Mold on Ready-to-Eat Roasted Antarctic Krill
Counts of the total bacterial population in ready-to-eat roasted Antarctic krill with different treatments are shown in Fig.1. While a significant difference between the control group (CT) and treated groups (SD and SDD) was noted (<0.05), the difference between the SD and SDD groups was not significant (>0.05). The bacterial residues were approximately (1.78±0.13)log(CFUg?1) initially, indicat- ing that the procedures were performed in a highly hygie- nic manner. The TVCs of all samples increased at similar rates, while the CT group increased faster compared to treat- ed groups. The TVC of the CT group reached (4.62±0.19)lg(CFUg?1) after 18 days. The SD and SDD groups de-monstrated a slow logarithmic phase at approximately days 6 and 10, respectively. The TVCs of SD and SDD groups reached (4.58±0.08)lg(CFUg?1) at day 33 and (4.52±0.06)lg(CFUg?1) at day 85, respectively. According to the Chi- nese standard for roasted shrimp (SC/T 3305-2003), they were considered unacceptable once the TVC reaches 4.5lg(CFUg?1). Hence, when they were stored at 25℃, the shelf life of CT, SD, and SDD groups were approximately 18, 33 and 70 days, respectively.
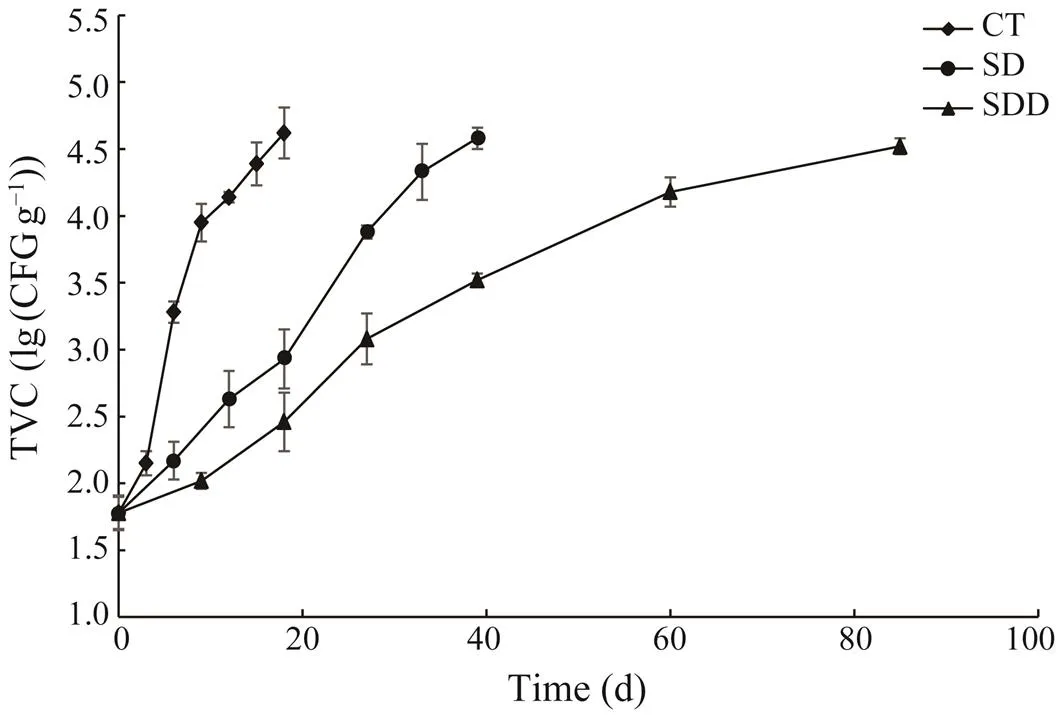
Fig.1 Total viable count changes of ready-to-eat roasted An- tarctic krill with different treatments during storage.
Counts of mold in ready-to-eat roasted Antarctic krill with different treatments are shown in Table 2. Mold counts in CT showed a significant difference between SD and SDD groups (<0.05). No mold was found on all samples at the commencement of storage. The mold counts of CT increased from 1.9×102to 4.7×105on day 18, whereas mold on SD plates was found after 6 days of storage and increased to 5.2×106by the end of its shelf life. Additionally, the mold of SDD was found after 12 days, and the number reached 1.6×106on day 90.
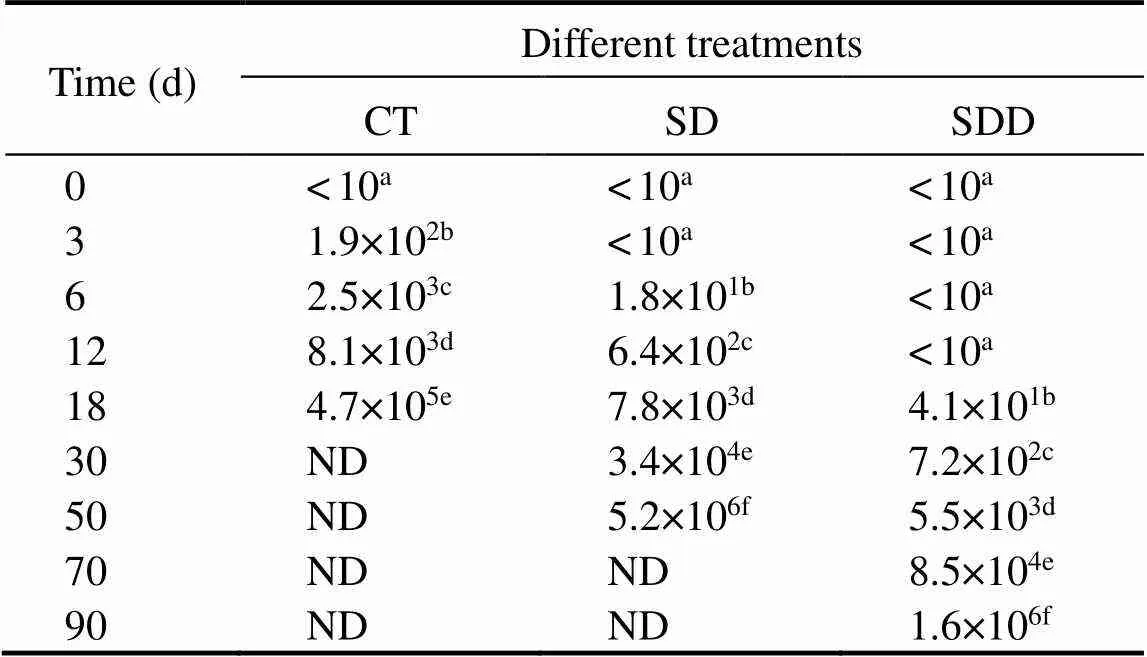
Table 2 Numeral changes in mold of ready-to-eat roastedAntarctic krill with different treatments during storage
Notes: ND means not detected. Different letters indicate significant differences between groups (<0.05).
In our study, Fig.2 shows the TVB-N values of ready-to- eat roasted Antarctic krill with different treatments during the storage. The initial TVB-N value was approximately(5.76±0.09)mg(100g)?1. The values of TVB-N in the con- trol group increased rapidly during the storage period, and reached (10.96±0.33)mg(100g)?1at the end of its shelf life, while the TVB-N values in SD and SDD groups were main- tained below 6.50mg(100g)?1. The TVB-N values from the SD group increased after 20 days and reached (12.11±0.07)mg(100g)?1on day 33. TVB-N values from SDD groups in-creased linearly to (10.88±0.15)mg(100g)?1at the end of its shelf life.
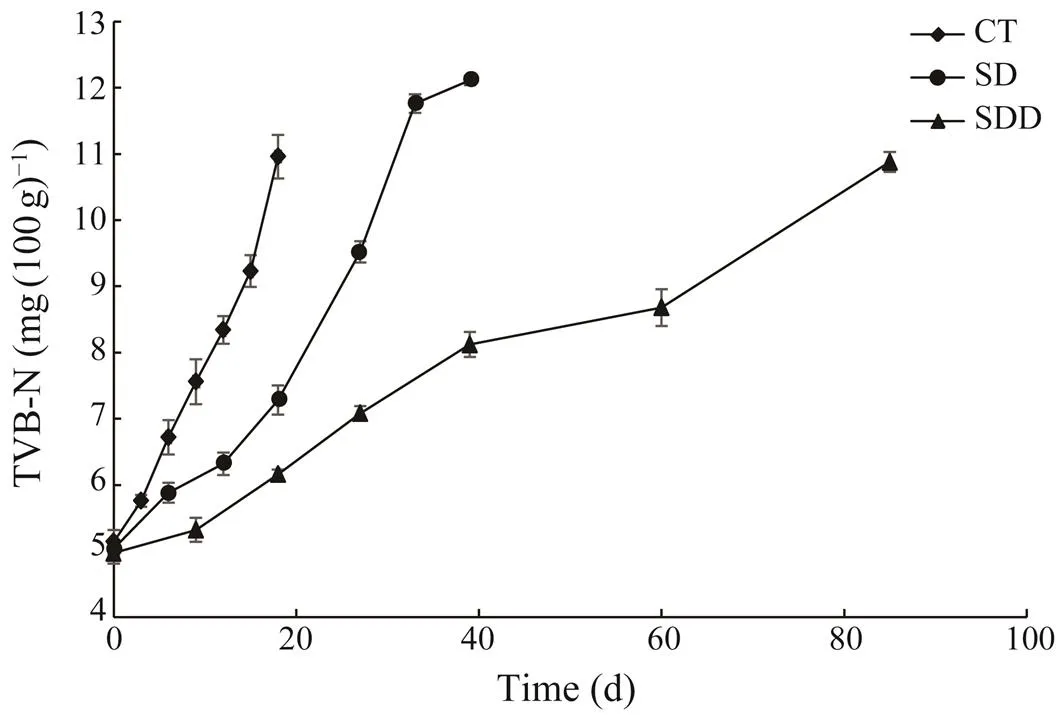
Fig.2 Changes in TVB-N of ready-to-eat roasted Antarctic krill with different treatments during storage.
Thiobarbituric acid reactive substances determination changes of TBARS values of ready-to-eat roasted Antarc- tic krill are shown in Fig.3. Thiobarbituric acid reactive sub-stances values of CT and SD groups showed an increasingtrend, changing from (22.85±0.23)mgMAkg?1(initial po- int) to approximately 95mgMAkg?1for the first 20 days. The TBARS values from CT and SD groups increased to approximately 120mgMAkg?1at 33 and 40 days, respec- tively. The TBARS values of SDD group changed slowly during the whole storage period. They increased from (22.85±0.23)mgMAkg?1to (43.30±0.14)mgMAkg?1by day 40,and eventually reached (91.88±0.85)mgMAkg?1at the end of its shelf life.
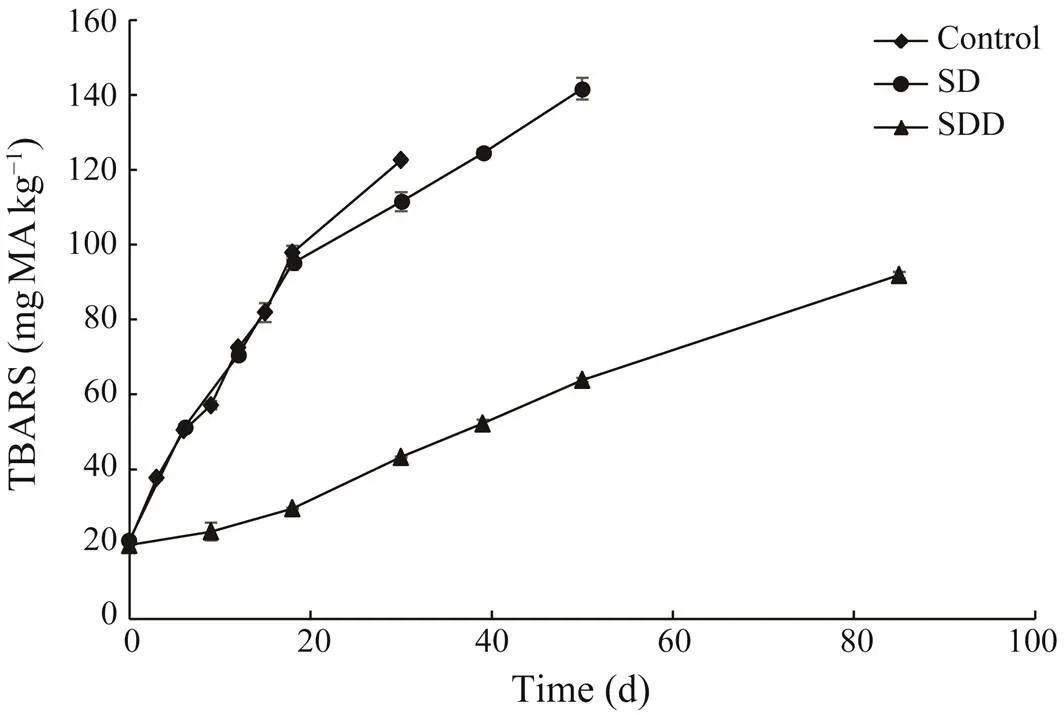
Fig.3 Changes in TBARS of ready-to-eat roasted Antarc- tic krill with different treatments during storage.
3.3 Color Profiles
Color in seafood products is a critical characteristic that provokes consumer purchasing decisions. The enhancement of* values lead to the stimulation of acceptability of the products’ appearance (Shaviklo., 2011). The* values were used to determine the color properties and qualitychanges of ready-to-eat roasted Antarctic krill in this study(Table 3). The* values demonstrated increasing trend du-ring storage, and varied from 11.63 to 27.48 for the CT groups. The* values for SD and SDD groups increased gradually, and reached 29.23 and 28.60 at the end of their shelf life, respectively.
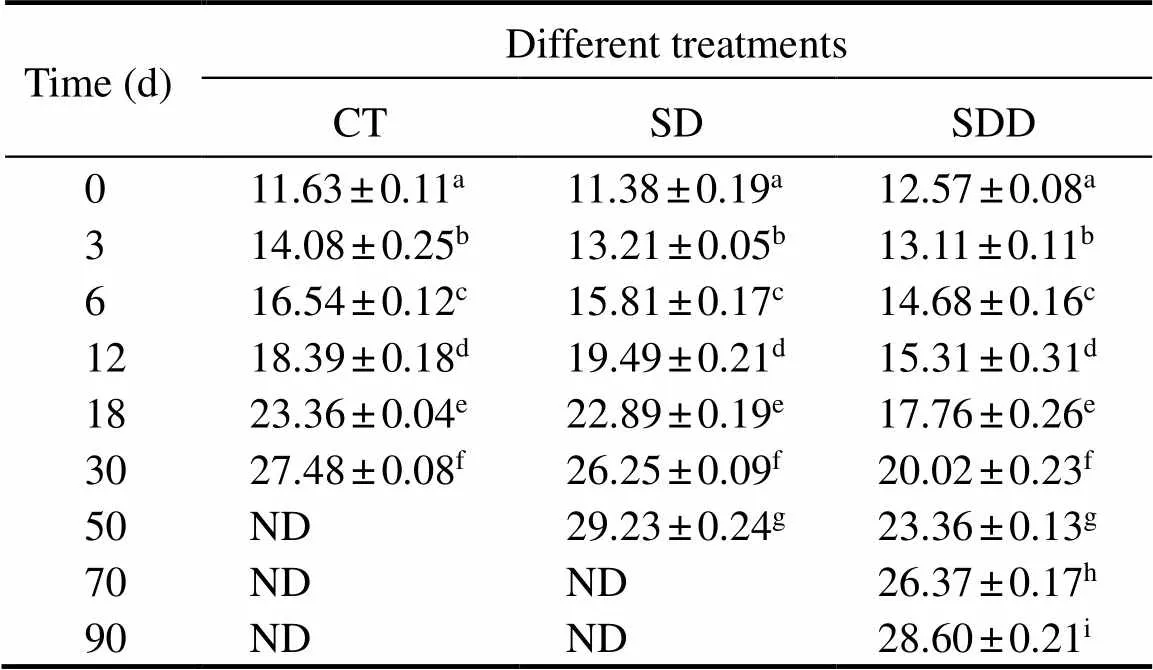
Table 3 Changes in b* value of ready-to-eat roasted Antarctic krill with different treatments during storage
Notes: ND means not detected. Different letters indicate significant differences between groups (<0.05).
3.4 Fluoride Residues Determination in Different Tissues
Table 4 shows the concentrations of fluoride residues re- maining in different tissues of rats. The concentrations of fluoride residues in rats fed with ready-to-eat roasted An- tarctic krill shows a concentration-dependent trend. The amounts of fluoride residues in the kidney, liver, blood and small intestine maintained a low dose (less than 1mgkg?1) and showed no significant difference to the control group (>0.05). Conversely, the concentration of fluoride in the thighbone remained high level, while the fluoride content of the control group was (500.13±5.02)mgkg?1. The test- ed groups showed a significantly higher concentration than the control group (<0.05). The concentration of fluoride in the sodium fluoride group reached (4621.01±28.67)mgkg?1, and the concentrations of fluoride from Antarctic krillfeeding groups were (1760.03±38.21), (2371.52±42.15), and (3615.44±30.53)mgkg?1, respectively.
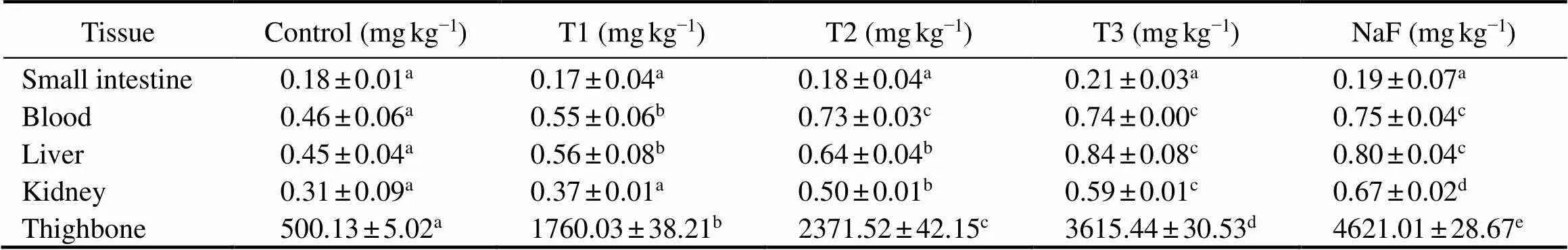
Table 4 Concentrations of fluoride residues in different tissue (blood, small intestine, liver, kidney and thighbone) of rats
4 Discussion
Ready-to-eat roasted Antarctic krill is a new approach in an attempt to increase the utilization and processing of thisfood source. The product was designed at low water content (28%), which can provide a crispy texture and inhibit bac-terial proliferation. Concurrently, the usage of preservatives, such as sodium diacetate and food-graded deoxidizer, has been suggested as an antibacterial and anti-oxidation strate- gy. Particularly, the use of diacetate is an effective means to prevent the growth of bacteria in ready-to-eat foods. Fur- thermore, diacetate used in the processing of ready-to-eat products is active immediately and is effective while theproduct is at its freshest situation, which considerably hin- ders bacterial growth. Jiang. (2011) reported the inhi- bitory effect of diacetate for reducing the bacteria in ready- to-eat smoked salmon. The results suggested that diacetate treatment at consumption level reduced the TVC through- out the shelf-life period, providing an important economic advantage of antimicrobial effect in the food industry. Al- ternatively, a high amount of lipid in Antarctic krill is vul- nerable to high temperatures. Both flavor and color indica-tive of lipid oxidation are critical for the quality of ready- to-eat roasted Antarctic krill. The TBARS values were sig- nificantly decreased in the SDD group, even at 25℃. Low temperatures are predominantly used to restrain the lipidoxidation from seafood products. Since cost is essential for companies to consider, the use of preservatives are recom- mended to improve the quality of the products. Notably, combining sodium diacetate and food-graded deoxidizer of-fers potential for increasing the quality and prolonging the shelf life of ready-to-eat roasted Antarctic krill, and this combination is recommended in the treatment of ready-to- eat products.
A positive effect of deoxidizer on color changes of ready- to-eat roasted Antarctic krill was observed(Table 3). The effect indicates that deoxidizer slowed the oxidation pro- cess of ready-to-eat roasted Antarctic krill during the stor- age. Importantly, this effect maintains the red color of An- tarctic krill. The color evaluated by* value reflects the quality changes of ready-to-eat roasted Antarctic krill, showing quality changes are not subject to the* (light- ness) and* (redness) values. Similar results showing a po- sitive correlation between* values and product quality were found by Wang. (2018).
It has been reported that consumption of Antarctic krill can cause the accumulation of fluoride in the body (Zuo., 2018). Some reports pertaining to krill-based dietary uptake by fish found fluoride accumulation in the muscle and bone of the fish (Yoshitomi and Nagano, 2012; Chen., 2013). Fluoride concentrations in vertebral bone of krill-eating fish increased due to the increasing proportion of Antarctic krill. The fluoride concentrations in vertebral bone from those fish remained as 33000mgkg?1for, 15000mgkg?1for,and 2150mgkg?1for(Yoshitomi and Nagano, 2012)The results indicate that the fluoride in fish bone likely depends on fish species and daily intake. The high concentration of fluoride was exclusively observ-ed in the thighbone and rare concentrations in other organs in our study. The fluoride may be accumulated in the mus- clea long-term dietary uptake to reach high values. Si-milar results have been found in Adélie penguins (), whose femur possess a high concentration of accumulated fluoride (Chen., 2013). Simultaneous- ly, fluoride concentration in the sodium fluoride treatment group was higher than that in all other tested groups, indi- cating the chemical form of fluoride in ready-to-eat roast- ed Antarctic krill is different from sodium fluoride. Tenuta-Filho and Alvarenga (1999) analyzed the distribution and chemical forms of fluoride in Antarctic krill. They noted that the exchangeable form of fluoride was a fundamental form in Antarctic krill. Importantly, the concentration offluoride in all tested groups showed a linear relationship withingested concentration. The results indicate that the con-centration of fluoride is possibly predictable by monitor-ing the dietary uptake of Antarctic krill. FDA recommend- ed an upper limit of 100mgkg?1as sodium fluoride for hu- mans (Turner, 1996). Accordingly, 500mgkg?1of fluoride or less from ready-to-eat roasted Antarctic krill would be safe for human. Considering that Antarctic krill is not the primary food source for humans, the consumption of ready- to-eat roasted Antarctic krill may show less hazardous ef- fects caused by fluoride accumulation. Further investigation is required to provide accurate limitations on the quantity of ready-to-eat roasted Antarctic krill for use.
5 Conclusions
In this study, ready-to-eat roasted Antarctic krill () with SD and SDD treatment preserves the quality of this product by reducing microorganisms and inhibiting lipid oxidation. Compared with that of the CT groups, The usage of SD and SDD, in a meanwhile, allowedthe products to maintain an attractive color and pleasingsensory quality during storage at 25℃, the TVC and num-ber of mold maintain low values (<0.05) and TVB-Nand TBARS values increased slowly (<0.05). The improvement in the quality of ready-to-eat roasted Antarctic krill extended its shelf life. Accordingly, the shelf life ofready-to-eat roasted Antarctic krill prolonged up to 15 days for SD treatment and 52 days for SDD treatment, respec- tively.
The fluoride residues from ready-to-eat roasted Antarc- tic krill were substantially accumulated in the thighbone of rats compared to other organs. The concentrations of fluo- ride residues remained less than 1mgkg?1in small intes- tine, blood, live and kidney. While the fluoride concentra- tions exclusively in thighbone showed a fluoride concen- tration-dependent phenomenon and to be less than sodium fluoride feeding group. The chemical form of fluoride re- sidues from ready-to-eat roasted Antarctic krill is uncertain,although it is different from sodium fluoride commonly de- limited by the FDA. Hence, further studies are required to evaluate the limitations on human consumption of ready- to-eat roasted Antarctic krill.
Acknowledgements
This work was supported by the National Key R&D Pro-gram of China (Nos. 2020YFD0901204, 2017YFC1600706), and the Natural Science Foundation of Shanghai (No. 22ZR1478500). We highly appreciated that the 36th Antarctic ex- pedition of China supported the samples of Antarctic krill.
Bao, J., Chen, L.,and Liu, T., 2019. Dandelion polysaccharide suppresses lipid oxidation in Antarctic krill ()., 133: 1164-1167.
Butts-Wilmsmeyer, C. J., Mumm,R. H., Rausch, K. D., Kand- hola,G., Yana,N. A., Happ, M. M.,., 2018. Changes in phenolic acid content in maize during food product process- ing., 66:3378-3385.
Camargo, J. A., 2003. Fluoride toxicity to aquatic organisms: A review., 50:251-264.
Cavan, E. L., Belcher, A., Atkinson, A., Hill, S. L., Kawaguchi, S., McCormack, S.,., 2019. The importance of Antarctic krill in biogeochemical cycles., 10: 4742- 4754.
Chen, J., Cao, J., Wang, J., Jia, R., Xue, R., Li, Y.,., 2013.Effects of fluoride on growth, body composition, and serum biochemical profile in a freshwater teleost,., 32:2315-2321.
Descamps, S., Tarroux, A., Cherel, Y., Delord, K., God?, O. R., Kato, A.,., 2016. At-sea distribution and prey selection of Antarctic petrels and commercial krill fisheries., 11: 1-18.
Ghani, M. A., Barril, C., Bedgood, D. R.,and Prenzler, P. D., 2017.Measurement of antioxidant activity with the thiobarbituric acid reactive substances assay., 230:195-207.
Haouet, M. N., Tommasino, M., Mercuri, M. L., Benedetti, F., Bel-la,S. D., Framboas, M.,., 2018. Experimental accelerat- ed shelf life determination of a ready-to-eat processed food., 7:189-192..
Hidalgo, F. J., and Zamora, R., 2017. Food processing antioxi- dants., 81:31-64.
Jech, J. A., 1979. Comparative uptake of fluoride from sodium fluoride, ammonium fluoride, and barium fluoride in rat teeth when predominantly administered in the pre-eruptive stage of development., 27: 117-119.
Ji, W., Zhang, C., and Ji, L., 2017. Two novel bioactive peptides from Antarctic krill with dual angiotensin converting enzyme and dipeptidyl peptidase IV inhibitory activities., 82:1742-1749.
Jiang, Z., Neetoo, H.,and Chen, H., 2011. Control ofon cold-smoked salmon using chitosan-based anti-microbial coatings and films., 76:22- 26.
Kidd, P. M., 2007. Omega-3 DHA and EPA for cognition, beha- vior, and mood: Clinical findings and structural-functional sy- nergies with cell membrane phospholipids., 12: 207-227.
Lan, C., Zhao, Y. Q., Li, X. R.,and Wang, B., 2019. High Fischer ratio oligopeptides determination from Antartic krill: Prepara- tion, peptides profiles, andantioxidant activity., 43: 1-12.
Li, X., Zhang, M., Wang, Y., Wang,X., Ma, H., Li, P.,., 2018. Direct detection of fluoride ions in aquatic samples by surface-enhanced Raman scattering., 178: 9-14.
Ma, X., Liu, C., Wang, C., Ma, X., Che, S., Feng, X.,., 2019. Effects of three products from Antarctic krill on the nitrogen balance, growth, and antioxidation status of rats., 7: 2760-2768.
Parolini, C., Bjorndal, B., Busnelli, M., Manzini, S., Ganzetti,G. S., Dellera, F.,., 2017. Effect of dietary components from Antarctic krill on atherosclerosis in apoE-deficient mice., 61:1700098-1700108.
Peng, Y., Ji, W., Zhang, D., Ji, H., and Liu, S., 2019. Composi- tion and content analysis of fluoride in inorganic salts of the integument of Antarctic krill ()., 9: 7853.
Sands, M., Nicol, S.,and McMinn, A., 1998. Fluoride in Antarc- tic marine crustaceans., 132: 591-598.
Shaviklo, G. R., Olafsdottir, A., Sveinsdottir, K., Thorkelsson, G., and Rafipour, F., 2011. Quality characteristics and consumer acceptance of a high fish protein puffed corn-fish snack., 48: 668-676.
Sun, D., Zhang, L., Chen, H., Feng, R., Cao,P., and Liu, Y., 2017. Effects of Antarctic krill oil on lipid and glucose metabolism in C57BL/6J mice fed with high fat diet., 16: 218-225.
Suzuki, T., and Shibata, N., 1990. The utilization of Antarctic krill for human food., 6: 119-147.
Tenuta-Filho, A., and Alvarenga, R. C., 1999. Reduction of the bioavailability of fluoride from Antarctic krill by calcium., 50: 297- 302.
Turner, C. H., 1996. Fluoride and the FDA: A curious case.,11:1369-1371.
Wang, H., Wu, Y., Wang, N., Yang, L.,and Zhou, Y., 2019. Effectof water content of high-amylose corn starch and glutinous rice starch combined with lipids on formation of starch-lipid com- plexes during deep-fat frying., 278: 515-522.
Wang, Z. C., Yan, Y., Nisar, T., Sun, L., Su, P., Chen, D. W.,.,2018. Influence of postmortem treatment with nitric oxide on the muscle color and color stability of tilapia () fillets., 76: 122-128.
Xia, Z., Zhai, X., Liu, B., and Mo, Y., 2016. Conductometric ti- tration to determine total volatile basic nitrogen (TVB-N) for post-mortem interval (PMI)., 44: 133-137.
Xie, D., Gong, M., Wei, W., Jin, J., Wang,X., Wang, X.,., 2019. Antarctic krill () oil: A comprehen- sive review of chemical composition, extraction technologies, health benefits, and current applications., 18: 514-534.
Yoshitomi, B., and Nagano, I., 2012. Effect of dietary fluoride derived from Antarctic krill () meal on growth of yellowtail ()., 86: 891-897.
Zerbini, A. N., Adams,G., Best,J., Clapham, P. J., JacksonJ. A.,and Punt, A. E., 2019. Assessing the recovery of an Antarctic predator from historical exploitation., 6:190368-190389.
Zhao, Y. Q., Zhang, L., Tao, J., Chi, C. F., and Wang, B., 2019.Eight antihypertensive peptides from the protein hydrolysate of Antarctic krill (): Isolation, identification, and activity evaluation on human umbilical vein endothelial cells (HUVECs).,121:197-204.
Zuo, H., Chen, L., Kong, M., Qiu, L., Lü,P., Wu, P.,., 2018. Toxic effects of fluoride on organisms., 198: 18-24.
(August 16, 2021; revised November 29, 2021; accepted May 10, 2022)
? Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2023
Corresponding author. E-mail: chih@ecsf.ac.cn
(Edited by Qiu Yantao)
 Journal of Ocean University of China2023年1期
Journal of Ocean University of China2023年1期
- Journal of Ocean University of China的其它文章
- The Influence of Sea Sprays on Drag Coefficient at High Wind Speed
- Highly Efficient Heavy-Metal-Ion Removal from Shellfish Processing Liquid with Low Protein and Polysaccharide Loss by Hybrid Mesoporous Silica Diol-APDC-SBA15
- Ship Weather Routing Based on Hybrid Genetic Algorithm Under Complicated Sea Conditions
- L-Band Analysis of the Effects of Oil Slicks on Sea Wave Characteristics
- A Method for Reducing Ocean Wave-Induced Magnetic Noises in Shallow-Water MT Data Using a Complex Adaptive Filter
- Wave Force on the Crown Wall of Rubble Mound Breakwaters at Intermediate Depths
