Study on the antipyretic mechanism of large pushing Tianheshui for young rabbits with endotoxin-induced fever
WANG Huijuan (王慧娟), TANG Wei (湯偉), OU Linglin (歐玲林), CHEN Bichan (陳碧嬋), LIU Mailan (劉邁蘭),YE Yong (葉勇)
1 The First Affiliated Hospital of Hunan University of Chinese Medicine, Changsha 410007, China
2 School of Acupuncture, Moxibustion & Tuina, Hunan University of Chinese Medicine, Changsha 410208, China
Abstract
Keywords: Tuina; Massage; Manual Therapies; Pushing Tianheshui; Fever; Hypothalamus; Rabbits
Fever is a regulatory body temperature increase,caused by the thermoregulation point rising in response to various thermogenic sources.As one of the clinical emergencies in pediatrics, fever may cause neurological generalization in children if it is not controlled in time,leading to convulsions and even death[1].At present,antipyretic and analgesic drugs (such as ibuprofen) are mostly used in the clinical treatment of children with fever for symptomatic management, but repeated use of such drugs will cause digestive and immune dysfunction to a certain extent, and cause liver and kidney damage in severe cases[2].As one of the unique treatments of Chinese medicine, Tuina (Chinese therapeutic massage) effectively treats all kinds of fever in children.As a safe and non-adverse treatment, Tuina has been widely used in clinical practice.The curative effect of Tuina in treating children with fever is remarkable without adverse reactions, and the effective rate is above 90%[3-4].
Previous studies have shown that clearing Tianheshui has a certain antipyretic effect on endotoxin-induced fever in young rabbits, which may be achieved by regulating hypothalamic prostaglandin (PG) E2, arginine vasopressin (AVP), and other mediators[5-6].Large pushing Tianheshui is most frequently used among antipyretic manipulations in LIU’s pediatric Tuina in Western Hunan province.It is developed on the basis of clearing Tianheshui manipulation by adding the air blowing action, that is, blowing while pushing.This manipulation is unique and characterized by concurrent arrival of Qi and hand.At present, experimental studies have been carried out on the antipyretic effect and the related mechanisms of clearing Tianheshui, but the antipyretic mechanism of large pushing Tianheshui manipulation is still unclear.
In this study, endotoxin was used to prepare the fever model, and the antipyretic effect of large pushing Tianheshui manipulation, as well as the content changes of PGE2, cyclic adenosine monophosphate(cAMP), AVP, and α-melanocyte stimulating hormone(α-MSH) in young rabbits were observed.The possible mechanism of large pushing Tianheshui’s antipyretic effect on endotoxin-induced fever in young rabbits was also further explored.
1 Materials and Methods
1.1 Experimental animals and groups
This experiment was approved by the Animal Ethics Committee of Hunan University of Chinese Medicine(Approval No.LLBH-201908120003).Forty 50-day-old ordinary young male New Zealand rabbits with a body mass of 1.5-2.0 kg were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd., China.The rabbits had free access to food and water and were fed under conditions with humidity of (55%±5%), a temperature of (25±2) ℃, and a light/dark cycle of 12 h/12 h in the Experimental Animal Center, Hunan University of Chinese Medicine.
All animals were adaptively fed for 7 d, and the rectal temperature was measured when eating and drinking were normal, once every 30 min, 3 measurements in total.The insertion depth of the thermometer must be ensured to be the same for each measurement, and the duration of each measurement was more than 5 min.The average of the three body temperatures was used as the basal body temperature.Thirty-two qualified young rabbits with a basal body temperature of 38.5-39.5 ℃ and a difference of no more than 0.5 ℃between the highest and the lowest body temperature were recruited.The young rabbits were randomly divided into 4 groups according to the random number table method, including a normal group, a model group,a large pushing Tianheshui group, and an ibuprofen group, with 8 rabbits in each group.
1.2 Main reagents and instruments
Escherichia coliendotoxin (Product No.L2880-10MG,Sigma, USA); ibuprofen suspension (State Food and Drug Administration Approval No.H20000359,Shanghai Johnson Pharmaceutical Co., Ltd., China);rabbit PGE2enzyme-linked immunosorbent assay (ELISA)kit (Lot No.201213R, Jiangsu Feiya Biotechnology Co.,Ltd., China); rabbit cAMP ELISA kit (Lot No.201229R,Jiangsu Feiya Biotechnology Co., Ltd., China); rabbit AVP ELISA kit (Lot No.201206R, Jiangsu Feiya Biotechnology Co., Ltd., China); rabbit α-MSH ELISA kit (Lot No.201245R, Jiangsu Feiya Biotechnology Co., Ltd., China);Tianmu veterinary thermometer (Jiujiang Xinkang Medical Instrument Co., Ltd., China); pipette 20-200 μL(Eppendorf AG, Germany); 450 nm wavelength microplate reader (Raydu Life Science Co., Ltd., China);37 ℃ electric constant temperature incubator (Wuhan Yiheng Sujing Scientific Instrument Co., Ltd., China);plate washer (Beijing Tuopu Analytical Instrument Co.,Ltd., China).
1.3 Preparation before modeling
All rabbits were adaptively fixed and manipulated before modeling.
Adaptive fixation: The experimental young rabbits were adaptively fixed in the supine position on a special rabbit frame for 1 h every day from 3 d before modeling.
Adaptive manipulation: Shaved the left forelimbs of the young rabbits 1 d before modeling.Dipped clean water at room temperature to perform large pushing Tianheshui manipulation on the center of the palmar surface of the left forelimb of the young rabbit equivalent to Tianheshui, at a frequency of 50-100 times/min, 1 min each time, twice.
Body mass measurement: On the day of modeling,young rabbits were captured for body mass measurement, and the doses of modeling drugs and ibuprofen were calculated according to the body mass.
1.4 Model preparation
The refinedEscherichia coliendotoxin was diluted to a concentration of 200 ng/mL using pyrogen-free 0.9%normal saline as the diluents by referring to the relevant literature[5].
Young rabbits in the model group, the large pushing Tianheshui group, and the ibuprofen group were all intravenously injected with 1 mL/(kg·bw)Escherichia coliendotoxin at a concentration of 200 ng/mL from the marginal ear vein.Young rabbits in the normal group were injected with the same amount of normal saline.The degree of body temperature rise (ΔT) in the model group was statistically significantly different compared with that in the normal group 1 h after the injection(P<0.01), indicating that the modeling was successful[7].
1.5 Tianheshui position
The location of Tianheshui in this experiment was referred to theExperimental Acupuncture Science[8]: a straight line from the palm to the bend of elbow of the rabbit’s forelimb.
1.6 Intervention method
Large pushing Tianheshui group: Young rabbits were fixed on a special rabbit frame in a supine position for Tuina intervention[9].The operator performed the straight pushing Tuina manipulation with the index finger and middle finger after dipping in cold water from the palm to the elbow of the young rabbits with fever, blowing and pushing concurrently, 1.5 h and 2.5 h after modeling respectively, at a frequency of 50-100 times/min, 5 min each time.
All Tuina manipulations in this experiment were performed by the same pediatric Tuina doctor.
Ibuprofen group: Ibuprofen suspension (0.01 g/mL)was orally administered [1 mL/(kg·bw)] 1.5 h after modeling.
Normal group and model group: Young rabbits in both groups were fixed on a special rabbit frame in a supine position without Tuina intervention for temperature measurement.
The body temperature of all young rabbits was monitored after modeling.The rectal temperature of the rabbits was measured every 0.5 h for a total of 6 h.
1.7 Detection indicators and methods
1.7.1 General conditions
The spirit, eating, drinking, feces, and activities of the young rabbits in each group were observed and compared after the endotoxin-induced fever model was completed.
1.7.2 Dynamic changes in body temperature
After the model was made, the rectal temperature of each young rabbit was measured every 0.5 h.Calculated the degree of increased body temperature of young rabbits in each group (ΔT = Measured body temperature value - Basal body temperature value), and drew the temperature dynamic change curve to observe the fever time phase of young rabbits with endotoxin fever.
1.7.3 Changes in thermoregulatory mediators in hypothalamic tissue
After the dynamic body temperature measurement,the young rabbits in each group were sacrificed by air embolism.After a quick cervical dislocation, the hypothalamic tissue was identified by using the midpoint between the tuber cinereum and the optic chiasma as the center.About 20 mg of hypothalamic tissue was removed and quickly placed in liquid nitrogen.The hypothalamic tissue was stored at -80°C until testing after being frozen.The specimens were then fully homogenized in buffer after being collected from all rabbits and centrifuged at 3 000 r/min for 15-20 min before supernatant collection.The contents of PGE2and other mediators in the hypothalamus were determined according to the instructions of the ELISA kits.
The specific detection steps of ELISA are as follows.
Setting the standard product wells and sample wells in the antibody-coated 96-well plate: Added standard products of different concentrations into specified standard product wells (50 μL/well).
Setting wells for the blank and samples to be tested in the antibody-coated 96-well plate: Added 40 μL of sample dilution buffer and then 10 μL of sample into each well for the samples to be tested.
Loading samples in the antibody-coated 96-well plate:Samples were added onto the bottom of the wells and shaken to mix.Added enzyme standard reagent into the wells except for the blank wells (100 μL/well).Sealed the plate with a sealing film and incubated at 37 ℃ for 60 min.Diluted the 20-fold concentrated washing solution 20 times with distilled water for later use.Peeled off the sealing film, discarded the liquid, and dried the wells by shaking.Filled each well with washing solution and discarded after 30 s.Repeated 5 times and patted dry.Added 50 μL of chromogenic reagent A into each well, then added 50 μL of chromogenic reagent B and mixed them gently by shaking.Developed color at 37 ℃ in the shade for 15 min.Added 50 μL of stop solution into each well to stop the reaction (blue turned into yellow).The absorbance (optical density value) of each well was measured sequentially at a wavelength of 450 nm.
1.8 Statistical methods
Data were analyzed with SPSS 25.0 statistical software.The results of this study were presented as mean ± standard deviation.The data of body temperature and thermoregulatory mediators in the hypothalamic tissue were analyzed by one-way analysis of variance.The least significant difference method was used for pairwise between-group comparisons when the variances were homogeneous.The analysis of variance Tamhane’s T2 method was used if the variances were not homogeneous.P<0.05 indicated statistically significant.
2 Results
2.1 General information
Compared with the normal group, young rabbits in the model group started fever about 30 min after modeling and reached the first fever peak at about 1.5 h.At this time, the fever was accompanied by curling up, shaking, less eating and more drinking.At about 3.0 h, the respiration was obviously accelerated,the ear vessels were obviously dilated and flushed, and some rabbits had loose stools.Compared with the model group, the auricle blood vessels of the young rabbits were not significantly dilated or flushed in the large pushing Tianheshui group and the ibuprofen group after interventions, and they were more active.
2.2 Body temperature changes of young rabbits
2.2.1 Phase of endotoxin-induced fever in young rabbits
As shown in Figure 1, the ΔT of young rabbits in each group was calculated.The body temperature in the model group increased gradually after modeling and reached the first peak (40.58±0.29) ℃ at 1.5 h, which was statistically different from that in the normal group(P<0.01).Afterward, the body temperature decreased slightly, and the second peak (40.43±0.29) ℃ appeared at 3.0 h, and the difference was statistically significant compared with the normal group (P<0.01).Then the body temperature gradually fell and rose slightly[(39.65±0.35) ℃] at 5.0 h in the model group without statistical significance compared with the normal group(P>0.05), indicating that the endotoxin-induced fever in young rabbits has obvious two-phase characteristics.
2.2.2 Dynamic changes in body temperature of each group
It can be seen from Figure 1 that the body temperature of young rabbits in the model group was significantly higher than that in the normal group during the same period 0.5-4.0 h, 5.0 h, and 5.5 h after modeling (P<0.01).Compared with the model group during the same period within 1.5-5.5 h, the body temperature of young rabbits in the ibuprofen group decreased significantly (P<0.01); the body temperature of young rabbits in the large pushing Tianheshui group significantly decreased at 2.0 h, 2.5 h, 3.0 h, 3.5 h, and 5.0 h (P<0.05).During 0.5-4.5 h, there was no significant difference in the body temperature of young rabbits between the large pushing Tianheshui group and the ibuprofen group (P>0.05).
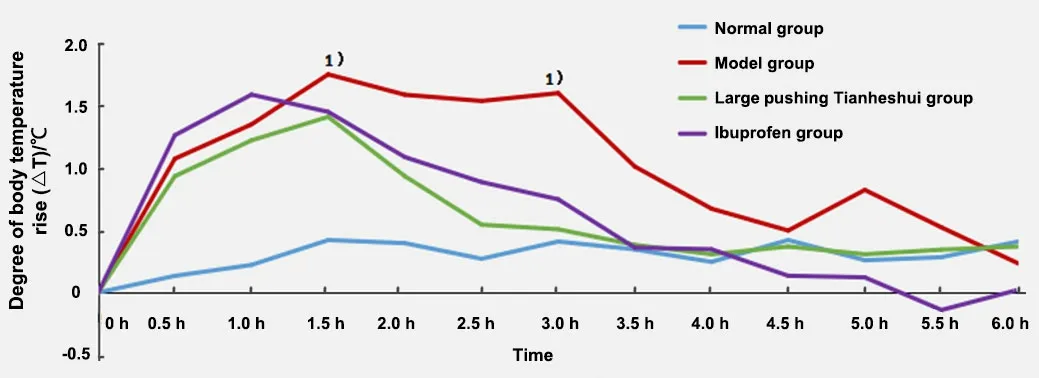
Figure 1 Body temperature changes of young rabbits in each group
2.3 Changes in hypothalamic thermoregulatory mediator contents of young rabbits in each group
2.3.1 PGE2
As shown in Table 1, hypothalamic PGE2in the model group was increased significantly compared with the normal group (P<0.05).Compared with the model group, hypothalamic PGE2in the large pushing Tianheshui group and the ibuprofen group decreased significantly (P<0.01).Compared with the large pushing Tianheshui group, hypothalamic PGE2in the ibuprofen group was insignificantly different (P>0.05).
2.3.2 cAMP
As shown in Table 1, compared with the normal group, the hypothalamic cAMP level in the model group significantly increased (P<0.05).Compared with the model group, the hypothalamic cAMP levels significantly decreased in the large pushing Tianheshui group and the ibuprofen group (P<0.01).There was no significant difference in the hypothalamic cAMP level between the large pushing Tianheshui group and the ibuprofen group (P>0.05).
2.3.3 AVP
As shown in Table 1, the hypothalamic AVP level in the model group was significantly lower compared with the normal group (P<0.01).Compared with the model group, the hypothalamic AVP levels in the large pushing Tianheshui group and the ibuprofen group increased significantly (P<0.01).There was no significant difference in the hypothalamic AVP level between the large pushing Tianheshui group and the ibuprofen group (P>0.05).
2.3.4 α-MSH
As shown in Table 1, the hypothalamic α-MSH level in the model group significantly decreased compared with the normal group (P<0.05).Compared with the model group, the hypothalamic α-MSH levels in the large pushing Tianheshui group and the ibuprofen group significantly increased (P<0.01).There was no significant difference in the hypothalamic AVP level between the large pushing Tianheshui group and the ibuprofen group (P>0.05).
Table 1 Comparison of the hypothalamic mediator levels in young rabbits among groups

Table 1 Comparison of the hypothalamic mediator levels in young rabbits among groups
Note: PGE2=Prostaglandin E2; cAMP=Cyclic adenosine monophosphate; AVP=Arginine vasopressin; α-MSH=α-melanocyte stimulating hormone; compared with the normal group, 1) P<0.05; compared with the model group, 2) P<0.01.
Group nPGE2/(pg·mL-1) cAMP/(nmol·L-1) AVP/(pg·mL-1) α-MSH/(ng·mL-1)Normal 8 286.10±79.05 7.00±1.30 56.01±9.30 60.55±4.68 Model 8 44.20±3.581)Large pushing Tianheshui 8 382.49±79.711)8.94±0.961)30.91±3.771)63.73±6.862)Ibuprofen 8 235.25±72.372)5.08±0.992)56.15±5.452)174.25±84.322)5.23±1.012)63.15±10.612)75.11±7.961)2)
3 Discussion
As an exogenous pyrogen, endotoxin activates endogenous pyrogen cells in the body such as mononuclear macrophages after intravenous injection to generate numerous endogenous pyrogens.This further acts on the hypothalamic thermoregulatory center to elevate the body temperature set point in the hypothalamus and cause fever.The endotoxin-induced fever model can better simulate inflammatory fever and is often used to screen antipyretic drugs and explore their antipyretic mechanisms[10].The fever model in this experiment was prepared by intravenous endotoxin through the marginal ear vein.Our results showed that the body temperature of young rabbits began to rise 0.5 h after endotoxin injection and reached the first peak at 1.5 h, then decreased slightly, and reached the second peak at about 3.0 h.Afterward, the body temperature of the young rabbits dropped steadily and then rose slightly at about 5.0 h without statistical significance.Therefore, we speculate that the third fever peak is due to the stressful increase in body temperature caused by the release of antipyretic mediators from the hypothalamic body temperature control center.Our results show that endotoxin-induced fever in young rabbits has typical biphasic fever characteristics, which is consistent with the fluctuation of body temperature in children with fever caused by exogenous pathogens[11].
Tianheshui is the most commonly used antipyretic point in pediatric Tuina[12].Since modern times, various pediatric Tuina schools have innovated and developed Tianheshui based on the records in ancient books,combined with the characteristics of their respective schools.Therefore, there are certain differences in the names and specific operations of the clearing Tianheshui manipulation in different schools[13].The large pushing Tianheshui manipulation is based on the clearing Tianheshui manipulation but adds blowing action and is obviously different from the clearing Tianheshui manipulation.FANG D S[6]treated pediatric fever caused by exogenous pathogens by clearing Tianheshui; the body temperature of children was successfully reduced and the antipyretic effect was more stable than drugs.WEI L Z,et al[5]proved that clearing Tianheshui manipulation had an obvious antipyretic effect on endotoxin-induced fever in young rabbits, showing evident point specificity.Although preliminary clinical studies have proven the significant efficacy of large pushing Tianheshui manipulation in treating pediatric fever caused by exogenous pathogens[14], research on the antipyretic mechanism has not yet been carried out.Therefore, based on the curative effect of large pushing Tianheshui in clinical work, we conducted experimental research on its antipyretic effect and possible mechanism.The results of this experiment showed that compared with the model group, the body temperature of young rabbits in the large pushing Tianheshui group and the ibuprofen group decreased significantly.The body temperatures of young rabbits in the large pushing Tianheshui group and the ibuprofen group were not significantly different.It can be seen that large pushing Tianheshui manipulation effectively reduces the body temperature of young rabbits with endotoxin-induced fever having no significant difference within 2-3 h after Tuina intervention compared with ibuprofen.However,whether Tuina has the same continuous antipyretic effect as drugs, and whether the stimulating amount of Tuina and the dose of antipyretic drugs will affect the curative effect requires more in-depth observation and research.
PGE2and cAMP are two important fever mediators recognized in the hypothalamic thermoregulatory center.When the endogenous pyrogen acts on the preoptic anterior hypothalamus, it releases central positive regulator mediators (PGE2and cAMP) to raise the thermoregulation point and cause fever in the body.Some studies have found that injecting PGE2or cAMP into different kinds of animals or different parts of animals can produce a fever reaction[15-16].The levels of PGE2and cAMP in the cerebrospinal fluid and plasma of animals significantly increase with infection fever.The levels of PGE2and cAMP are significantly downregulated after the application of fever-reducing drugs[17-18].However, AVP and α-MSH, as important endogenous antipyretic substances in the body, play negative regulatory roles in fever, and their functions are mainly manifested in two aspects: promoting the body to reduce fever and limiting the body’s fever.Studies have shown that when rats with fever are treated with fever-reducing Chinese medicine, the AVP level in the hypothalamus increases, and the body temperature decreases.Some scholars speculate that α-MSH inhibits the fever response in rabbits by inhibiting PGE2synthesis in the plasma[19].HU C F,et al[20]proved that α-MSH inhibited the cAMP level in the hypothalamus.In the research on the antipyretic mechanism related to Tianheshui, various schools speculate that the antipyretic mechanism may be related to the positive and negative mediators of the thermoregulatory center in the hypothalamus.WEI L Z,et al[5]found that the PGE2and cAMP content changes were positively correlated with body temperature, so it is speculated that clearing Tianheshui manipulation can reduce the levels of PGE2and cAMP in the hypothalamus, thereby producing an antipyretic effect.However, FANG D S[6]believes that clearing Tianheshui achieves an antipyretic effect by regulating the hypothalamus and negatively regulating the level of AVP mediator.The results of this experiment showed that large pushing Tianheshui significantly inhibited the hypothalamic PGE2and cAMP levels and promoted the release of AVP and α-MSH, having no significant difference compared with ibuprofen.Therefore, we believe that large pushing Tianheshui may play an antipyretic effect on endotoxin-induced fever in young rabbits by inhibiting the secretion of PGE2and cAMP and promoting the release of AVP and α-MSH in the hypothalamus.This provides a scientific basis for the clinical application of antipyretic Tuina manipulation in children to some extent.However, the specific ways that large pushing Tianheshui reduces the levels of PGE2and cAMP and promotes the release of AVP and α-MSH in the hypothalamus still need to be studied further.
Conflict of Interest
The authors declare that there is no potential conflict of interest in this article.
Acknowledgments
This work was supported by the Project of Hunan Provincial Natural Science Foundation of China (湖南省自然科學(xué)基金項目, No.2021JJ70108); Clinical Innovation Research Project of Science and Technology Department of Hunan Province (湖南省科技廳臨床創(chuàng)新科研項目,No.2018SK51210); Hunan Province “14th Five-Year Plan”Training Program for the First Batch of TCM Leading Talents and Academic Leaders (湖南省“十四五”第一批中醫(yī)藥領(lǐng)軍人才和學(xué)科帶頭人培養(yǎng)項目); High-level Health Talents Training Project of Hunan Province (湖南省高層次衛(wèi)生人才培養(yǎng)項目).
Statement of Human and Animal Rights
The treatment of animals conformed to the ethical criteria in this experiment.
Received: 6 September 2021/Accepted: 9 June 2022
 Journal of Acupuncture and Tuina Science2023年3期
Journal of Acupuncture and Tuina Science2023年3期
- Journal of Acupuncture and Tuina Science的其它文章
- Electroacupuncture stimulation attenuates corpus striatum white matter injury in rats with cerebral ischemia by inhibition of Nogo-A/NgR pathway
- Effects of different moxibustion time on knee cartilage morphology and the expression of TNF-α and IL-10 in rats with knee osteoarthritis
- Effects of horse-riding squat exercise plus Governor Vessel-regulating Tuina therapy on static balance function in patients with stroke
- Observation on the efficacy of traditional Qigong exercise combined with Tuina manipulations in treating lower cervical disc herniation
- Clinical study of treating somatoform pain disorder with the combination of electroacupuncture and duloxetine
- Clinical observation of Tuina combined with Bu Zhong Yi Qi Tang in the treatment of rectocele
