The Response of Four Energetic Compound Molecules to Ultraviolet Light Using TDDFT
SUO Cheng-xiang,GUAN Meng-xue, WANG Feng, LIU Rui-bin, GUO Wei,3,
(1.School of Physics, Beijing Institute of Technology, Beijing 100081, China; 2.Key Laboratory of Advanced Optoelectronic Quantum Architecture and Measurement (MOE), Beijing Institute of Technology, Beijing 100081, China; 3.Frontiers Science Center for High Energy Materials (MOS), Beijing Institute of Technology, Beijing 100081, China; 4.State Key Laboratory of Explosion Mechanics, Beijing Institute of Technology, Beijing 100081, China)
Abstract:The time-dependent density functional theory (TDDFT) was used to study the light absorption spectra of 3-nitro-1,2,4-triazol-5-one (NTO), 1,3,5-trinitroperhydro-1,3,5-triazine (RDX), 1,3,5,7-tetranitrooctahydro-1,3,5,7-tetrazocine (HMX) and 2,4,6,8,10,12-hexanitro-2,4,6,8,10,12-hexaazatetracyclo[5.5.0.0(3,11).0(5,9)]dodecane (CL-20) molecules, the evolution of molecular structure and differential charge density with time under Ultraviolet (UV) light. The sensitivity trends of TDDFT compared with the simulation results show that the number of excited-state electrons under UV light may serve as descriptors of electronic stability, where the electronic stability rank is NTO>CL-20>HMX>RDX. The ease of nitro group detachment under ultraviolet excitation may serve as descriptors of irradiation sensitivity in molecular level, where the irradiation sensitivity rank is CL-20—RDX>HMX>NTO. And NTO molecule exhibits the lowest irradiation sensitivity. Under UV light, these four explosive molecules undergo several main reactions, including dehydrogenation, ring opening, nitro group separation, and nitro group deoxygenation, to form nitrogen gas. The excited nitro group, known for its strong electron-withdrawing properties, plays a crucial role in electron transfer to the surrounding environment. The extent of charge transfer on the nitro group varies with light intensity, leading to the different decomposition products. The simulation results demonstrate that under weak light fields, nitro groups dissociate to form nitrogen dioxide, with the main decomposition products being nitrogen dioxide and hydrogen radicals. However, some nitro groups undergo deoxygenation to form nitrous groups when exposed to intense light. If the dissociation energy of the nitro group is sufficiently high, complete deoxygenation can occur, causing the nitro group to detach from the ring structure and form nitrogen. The intermediate products of the reaction include hydrogen, oxygen free radicals, nitrogen dioxide, nitroso, and nitrogen molecules.
Keywords:quantum chemistry; TDDFT;ultraviolet light;explosive molecule;irradiation sensitivity; NTO; RDX; HMX; CL-20
Introduction
Sensitivity is an important index to measure the performance and safety of explosives, thus the sensitivity of an explosive should be considered as one of the key points in the design and application[1-2]. Irradiation sensitivity of explosive is a special sensitivity issue that has been widely concerned in experiments, and a large number of studies about explosives under X-ray irradiation, electron beam and ultraviolet light have been carried out[3-7]. In recent years, with the continuous improvement of explosive properties, laser initiation has become a safer and more reliable way of initiation[8-11], ultraviolet (UV) light can detonate explosives with applicable catalyst[12-14]. The process of detonating explosives involves the absorption of energy to overcome the barriers of chemical bonds. Hence, researchers investigate the impact of various laser frequencies on explosive detonation[12-14]. Among them, ultraviolet light shows a splendid detonation effect, and people focus on the mechanisms of UV laser-induced detonation of explosives[13]. However, laser may bring high energy electromagnetic irradiation, leading to excitations of electrons in the energetic compound[13-14]. In the excited states, properties of materials will change dramatically, for example, UV light may break chemical bonds indirectly or directly. Therefore, investigation of the responses of explosive molecules to UV illumination can help to better control and improve the performance of high-energy materials for combustion and explosion, and also provide a reference for the detonation of explosives under UV laser.
The effects of UV light on explosives have been explored mostly in experiment. Beard, B.C. et al. used XPS technology to show that damage to NTO caused by UV irradiation is manifested by the loss of the nitro functional group, and then transferred to a more reductive chemical state, forming nitroso products of TO and nitroso products of NTO[3]. GUO Y. Q. et al. used both time-of-flight mass spectroscopy and laser-induced fluorescence spectroscopy to show that NO molecule is one of the initial dissociation products of HMX, RDX and nitro-nitrite isomerization is suggested to be part of the decomposition mechanism[15-16]. HAWARI J. et al. found that initial photo-denitration of the monocyclic nitramine RDX leads to ring cleavage and decomposition and CL-20 degraded via at least two initial routes[17]. YI SUN et al. demonstrated the formation of NO2free radicals and nitroso derivatives of 2,2′,4,4′,6,6′-hexanitrostilbene (HNS) upon UV irradiation by the UV-Vis spectra, X-ray photoelectron spectroscopy (XPS) and electron paramagnetic resonance spectra (EPR)[18]. The decomposition products of explosive under UV light have been observed and the formation path has been speculated, but the specific reaction process and reaction mechanism are still elusive.
Excitation of molecules caused by light pulses is a complicated physical process involving the coupling of electrons and ions in electromagnetic fields within multiscale in time and space. Nowadays, one of the most popular and widely used theories to describe the excited states of matter under light is the time-dependent density functional theory (TDDFT). In this study, we performed calculations based on TDDFT to analyze the reaction process and reaction mechanism of various excited explosive molecules under UV light. We provide insights on the sensitivity of explosives under UV ignition by analyzing the initial detonation reactions through non-adiabatic molecular dynamics simulations, and shed light on the possible intermediate of laser-induced detonation of NTO, RDX, HMX and CL-20 molecules.
1 Calculation Methods
Within TDDFT, two regimes can be distinguished. The first regime is mainly applied to matter exposed to a weak light field by the use of linear response theory, such as the calculation of absorption spectra. The second solves the full time-dependent Kohn-Sham (TDKS), equations to describe the extremely nonlinear effects in the light-matter interaction under external intense light fields, e.g., chemical reactions[19-21].
The TDKS equation satisfying the single particle wave function can be written as eq.(1):
(1)
The electronic density of the system is expressed as:
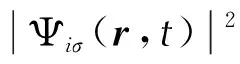
(2)
Whereσrepresents electronic spin direction. Here,VKSrepresents Kohn-Sham effective potential and consists of the following four parts:
VKS(r,t)=Vion(r,t)+Vext(r,t)+VHartree(r,t)+Vxcσ(r,t)
(3)
Vionrepresents ionic potential, which describes the action potential of an ionic core on a valence electron, andVextrepresents external potential.
Vext=Elight·r
(4)
Elight=Emaxsin(ωt)
(5)
Emaxrepresents the maximum field strength.VHartreeis the classical electro-static interaction between the electrons:
(6)
Vxcσrepresents the exchange correction potential.
In order to obtain the absorption spectra of the four explosive molecules, we give the electron a small momentum in x-direction at the initial moment and excite all the frequencies of the system, such as:
Ψi(r,δt)=e-ikxφi(r,0)
(7)
The time-dependent evolution of the dipole moment in x-direction is obtained as:
(8)
The dipole moment is transformed into the frequency domain by Fourier transformation, yielding the dipole strength functional:
(9)
Tis the evolution time andF(t) is a damping function:
(10)
The cross-section for optical absorption is proportional to the dipole strength function, the dipole strength function can reflect degree of absorption. Thus, the absorption spectra of x-direction can be plotted according to the intensity function. We can use similar methods to get absorption spectra in theyandzdirections[19, 22-24].
Excited states calculations were carried out through TDDFT code OCTOPUS[25-27]. We chose the local density approximation (LDA) for the exchange correlation functional and used Hartwigsen-Goedecker-Hutter pseudopotentials[28]. The simulation zone was defined by assigning a sphere around each atom with a radius of 6.5? and a spacing of 0.18? between grid points. We set the initial temperature to 300K according to the Boltzmann distribution with temperature. The ground-state geometry structure optimizations of explosive molecules were performed by DFT calculations as implemented in Vienna Ab initio simulation package (VASP)[29]. PBE (Perdew-Burke-Ernzerhof) was used to describe the exchange-correlation effects[30]. Pseudopotentials generated with projector-augmented wave (PAW) method were used in treating the valence electron-ion interaction with energy cutoff at 500eV for the plane-wave basis sets[31]. The convergence criteria of self-consistent electronic and ionic loops were set to 10-6eV and 0.01eV/?, respectively. The optimized molecules are shown in Fig.1. There are two types of N—NO2bonds in HMX, we call the vertical one as type-A, and the horizontal one as type-B. The red, grey, brown and light pink spheres are denoting oxygen, nitrogen, carbon, and hydrogen atoms, respectively. Combining the bonds length changes with visualization software VESTA[32], we define that when the distance between atoms is larger than twice the original length of the bond, the chemical bonds are considered to be broken.
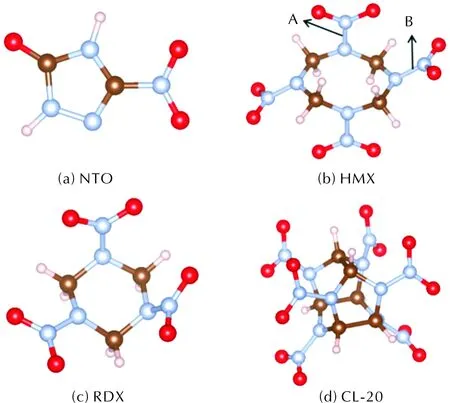
Fig.1 Molecular structures of the four explosive molecules
There are two widely used semi-classical methods in treating the non-adiabatic dynamical processes, which are the surface hopping approaches (SH) and the Ehrenfest dynamics[33-34]. Due to the fact that the accuracy of SH can be reliable only when both the potential energy surface and force are around the ground state, it fails to describe the strong-field driven phenomena when the system is excited to high-energy excited states. Therefore, we choose the Ehrenfest scheme to treat the non-adiabatic molecular dynamic processes during the simulating detonation reactions, where the electronic part evolves by propagating the TDKS equations, while the ion part evolves according to the classical dynamics equation:
(11)
Here,M,RandZrepresent the mass, position and charge of ion, respectively.
This method can accurately describe the charge transfer and electron-hole interaction in excited states.
2 Results and Discussion
2.1 The absorption spectra of the four explosive molecules
We first calculate the absorption spectra of the geometrically optimized explosive molecules as shown in Fig.2. The absorption intensity of molecules increases first and then decreases with the increase of energy and is positively correlated with the number of atoms. The absorption peaks of NTO, HMX, RDX and CL-20 respectively are distributed in 19.8, 19.5, 20.4, and 20.2eV when the polarization is in the x direction. The absorption peaks of the four explosive molecules correspond to the energy gap of the highest occupied molecular orbitals (HOMO) and the lowest unoccupied molecular orbitals (LUMO),which is a resonant process[35]. The absorption spectra indicate that the four explosive molecules have similar absorption peak in photon energy. Thus, we set the photon energy to 20eV, which is located in UV light range. The polarization direction is selected in thexdirection, which represents the maximum absorption.
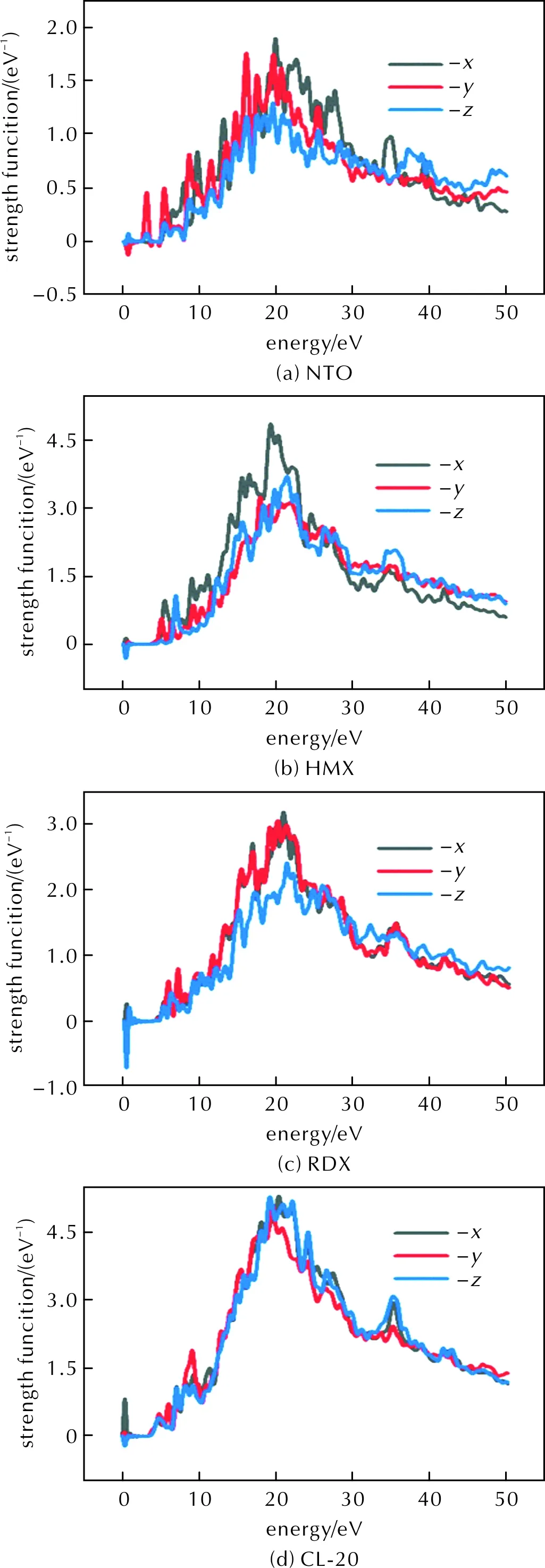
Fig.2 The absorption spectra of the four explosive molecules
2.2 Electronic stability of the four explosive molecules
The susceptibility to irradiation damage reflects the electronic stability of a molecule, the higher the irradiation stability of an explosive molecule, the harder it is for electrons to be excited to arrive the excitation saturation. We then calculate the number of the photo-excited electrons of the four explosive molecules under 20eV UV light based on the TDDFT to estimate the relative irradiation sensitivity of NTO, HMX, RDX, and CL-20.
The number of photo-excited electrons of the four explosives molecules accumulates with the increase of irradiation time, but the difference of excited electrons of them indicates the relative electronic stability among them. If the electronic stability of one molecule is low, it is easier to arrive an electron-excitation saturation state under UV light. For NTO molecule, even if theEmaxreaches 1.0V/?, there is no obvious saturation tendency in electron excitation at 65fs and electrons are still constantly excited rapidly [Fig.3(a)]. Therefore, NTO does not arrive saturation within 65fs under UV light of 1.0V/? in our calculation, indicating that and the electronic stability of NTO molecules is the highest among the four calculated molecules.
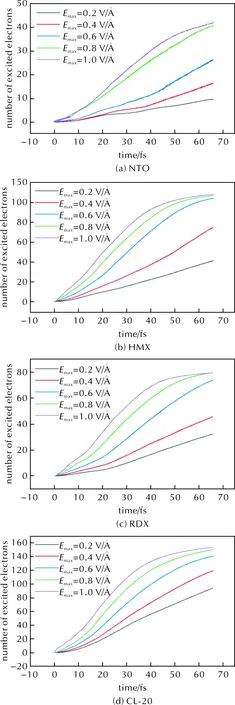
Fig.3 The number of photo-excited electrons of the four explosive molecules
For HMX and RDX, the rate of generating photo-excited electron slows down and tends to reach saturation under UV light of 0.8V/? after 60fs [Fig.3(b) and Fig.3(c)]. As a result, HMX and RDX molecules arrive saturation state of the electron excitation more easily than NTO, hence they have lower electronic stability than NTO. Fig.3(b) illustrates that the green line (0.8V/?) coincides exactly with the purple line (1.0V/?) at 65fs, which means that HMX reaches saturation at 65fs under light of 0.8V/?. Similarly in Fig.3(c), the green line (0.8V/?) coincides exactly with the purple line (1.0V/?) after 60fs, which means that RDX reaches saturation at 60fs under light of 0.8V/?. The difference indicates that HMX is a slightly more stable than RDX.
Fig.3(d) illustrates that there is obvious difference in the number of excited electrons between 0.8V/? and 1.0V/? for CL-20 comparing with HMX and RDX, but the rate of generating photo-excited electrons decreases after 40fs when theEmaxreach 0.8V/?. Thus, we can infer that the resulting rank of electronic stability among the four molecules is NTO>CL-20>HMX>RDX. The trend based on our calculation is consistent with the irradiation stability observed in experiments for HMX, RDX and NTO[3]. However, the irradiation stability of CL-20 was not compared with HMX, RDX and NTO in experiment, and we predict that the electronic stability under laser irradiation of CL-20 is in between RDX and NTO, and possibly in between HMX and NTO.
2.3 Decomposition reactions of the four explosive molecules under UV light
In order to study the decomposition reaction mechanism of explosive molecules under irradiation, we focus on the breaking of chemical bonds and decomposition products of NTO, HMX, RDX and CL-20 molecules. The ring structure can reflect the integrity of explosive molecules, and nitro is a key functional group for explosive properties, so we focus on the reaction of the nitro group and ring structure of the four explosive molecules under UV light.
2.3.1 NTO
Our simulations indicate that the molecular structure of NTO keeps stable within tens of fs untilEmaxreaches 0.6V/?. The initial chemical bond breaking of NTO is N—H bond under 0.6V/? or 0.8V/? (Fig.4), the red dotted lines indicate broken bonds. After the complete fracture of N—H bonds at 45fs, the nitro group begins to separate from NTO at 65fs to form NO2molecule caused by C—NO2bond fracture under UV light ofEmax=0.6V/? [Fig.4(a)]. After the complete fracture of N—H bonds at 27fs, NTO occurs ring-opening at 60fs [Fig.4(b)]. The dispersed hydrogen atoms are unstable and they easily combine to form hydrogen molecules. The existence of hydrogen was also confirmed in explosive irradiation experiment[7]. The five-member ring of NTO keeps stable within 60fs under UV light ofEmax=0.8V/? and completely break after 65fs, meanwhile, the nitro group attaching to the ring decomposes to nitroso group [Fig.4(b)]. According to the order in which the bonds break under UV excitation, we speculate that the N—H bonds of NTO are more vulnerable than the nitro group and the five-member ring. The C—NO2bond of NTO only occurs fracture to from NO2molecules under the UV light ofEmax=0.6V/?, and C—NO2bond deoxidizes to form nitroso group under the UV light ofEmax=0.8V/?.

Fig.4 The decomposition process of NTO molecule under UV light
In order to analyze the cause of the different reactions about nitro group under UV light of different intensity, we calculate the time-dependent charge density difference (as shown in Fig.5). Blue and yellow denote loss and gain of charge respectively in Fig.5. Fig.5(a) illustrates that the charge transfer around the C—NO2bond is obvious under UV light ofEmax=0.6V/?, and the stability of the C—NO2bond is reduced because of electronic charge transfer. When theEmaxincreases to 0.8V/?, the center of charge loss is around the five-member ring until the five-member ring breaks [Fig.5(b)]. So, UV light with different intensity causes different electron transfer on the same molecule, generating different decomposition pathway of NTO.
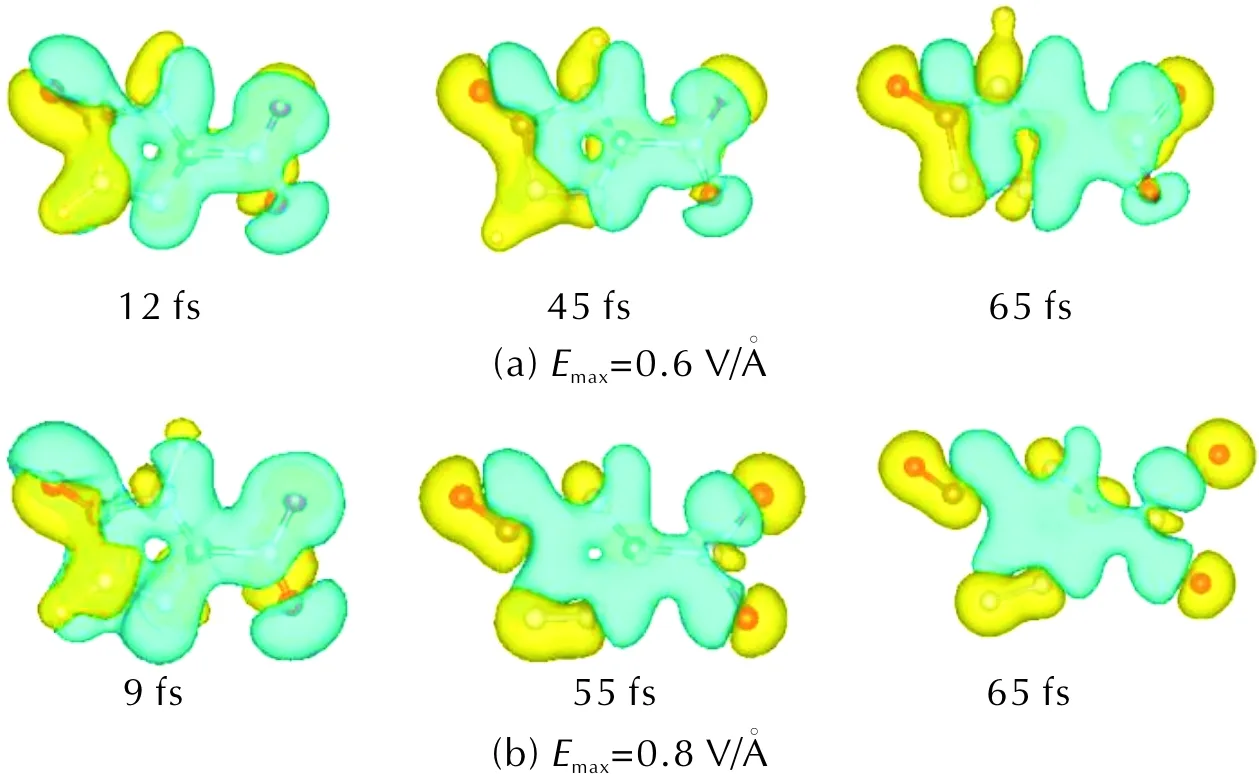
Fig.5 Time-dependent charge density difference between the density at the present time and that at t=0 under UV light
2.3.2 HMX
The calculation results indicate that the molecular structure of HMX keep stable within tens of fs untilEmaxreaches 0.4V/?. The initial broken bond of HMX is C—N bond from the eight-member ring under 0.4V/? or 0.6V/? (Fig.6), the red dotted lines indicate broken bonds. HMX occurs ring-opening at 60fs under UV light ofEmax=0.4V/? without fracture of C—H and N—NO2bonds within 65fs [Fig.6(a)]. HMX gradually begins to dehydrogenate accompanying ring-opening at 44fs under UV ofEmax=0.6V/?, and N—NO2bond occurs fracture to form NO2molecule at 47fs [Fig.6(b)]. HMX completely dehydrogenates within 28fs followed by ring opening at 41fs under UV light ofEmax=0.8V/?, meanwhile,some N—NO2bonds occur fracture to form NO2molecule at 41fs and some N—NO2bonds deoxidize to form N2molecule at 57fs [Fig.6(c)]. The C—H bonds of HMX are more vulnerable to intense UV lights and N—NO2bonds of HMX have two decomposition paths under UV excitation.
N—NO2with large dissociation energy is likely to cause the destruction of the ring structure. Considering the direction of electric field, there are two types of N—NO2bonds in HMX, as shown in Fig.1(b). Two N—NO2bonds of type-B in HMX do not break in our simulation atEmax=0.6V/?. When increasing toEmax=0.8V/?, we observe deoxidation of the nitro groups and N2molecule is found during the simulation. The type-B N—NO2is harder to break, this can be explained by the dissociation energies from previous simulation of A and B types of N—NO2bonds, which is 178 and 192kJ/mol, respectively[7]. This could explain the reason that HMX have two decomposition paths. Besides, we also observe ring-opening occurs around the type-B N—NO2under ultraviolet excitation, the type-B N—NO2reduces the stability of the eight-member ring and aggravate the destruction of the ring.
2.3.3 RDX
RDX keeps stable within tens of fs untilEmaxreaches 0.4V/?. The initial broken bond of RDX is N—NO2under 0.4V/? (Fig.7), the red dotted lines indicate broken bonds. As shown in Fig.7(a), only N—NO2bond fracture is observed at 58fs under UV light ofEmax=0.4V/?. RDX dehydrogenates within 48fs and N—NO2bonds occur fracture and release NO2molecule at 48fs under UV light ofEmax=0.6V/?, and meanwhile RDX occurs ring-opening [Fig.7(b)]. Under UV light ofEmax=0.8V/? as shown in Fig.7(c), after complete C—H bond fracture of at 27fs, the ring structure breaks at 44fs and N—NO2group releases NO2molecule, then NO2molecules deoxidize to form nitroso group at 47fs.
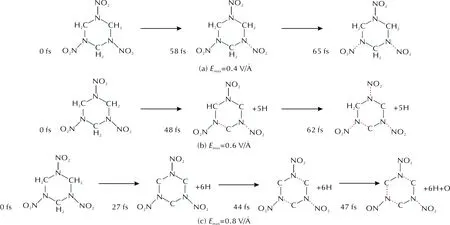
Fig.7 The decomposition process of RDX molecule under UV light
N—NO2bonds of RDX have two reaction paths under UV excitation. Under UV light ofEmax≤0.6V/?, they occur fracture to generate NO2molecules; UnderEmax=0.8V/?, some of the generated NO2molecules deoxidize to form nitroso group. Comparing the field intensity of the nitro group decomposition and ring-opening, the nitro groups of RDX are easier to react under the UV light than HMX and the six-member ring in RDX is more stable than the eight-member ring in HMX.
2.3.4 CL-20
CL-20 molecule has a rigid polycyclic cage consisting of two five-member rings and one six-member ring with six nitro groups attached to six bridging nitrogen atoms in the cage [shown in Fig.1(d)]. In our simulation, the molecular structure of CL-20 keeps stable within tens of fs untilEmaxreaches 0.4V/?. The initial broken bond of CL-20 is N—NO2under 0.4V/? (Fig.8), the red dotted lines indicate broken bonds. The C—H bonds of CL-20 keep stable and the N—NO2bonds of CL-20 occur fracture to form NO2molecules after 57fs under the UV light ofEmax=0.4V/? [Fig.8(a)]. CL-20 dehydrogenates accompanying the fracture of N—NO2bond to form NO2molecule at 47fs under UV light ofEmax=0.6V/?, and the C—N bond, which is part of the six-member ring, breaks at 60 fs [Fig.8(b)]. According to our simulation, the five-member rings of CL-20 keep stable when UV light ofEmax≤0.6V/?, and they are more stable than the six-member ring which completely breaks after 65fs under UV light ofEmax=0.6V/? [Fig.8(b)]. After complete fracture of N—H bonds at 32fs, the five-member rings open and completely break at 50fs, and the N—NO2bonds deoxidize to form nitroso group at 50fs under UV light ofEmax=0.8V/? [Fig.8(c)]. As with HMX and RDX, the C—H bonds of CL-20 are more vulnerable to intense UV light.
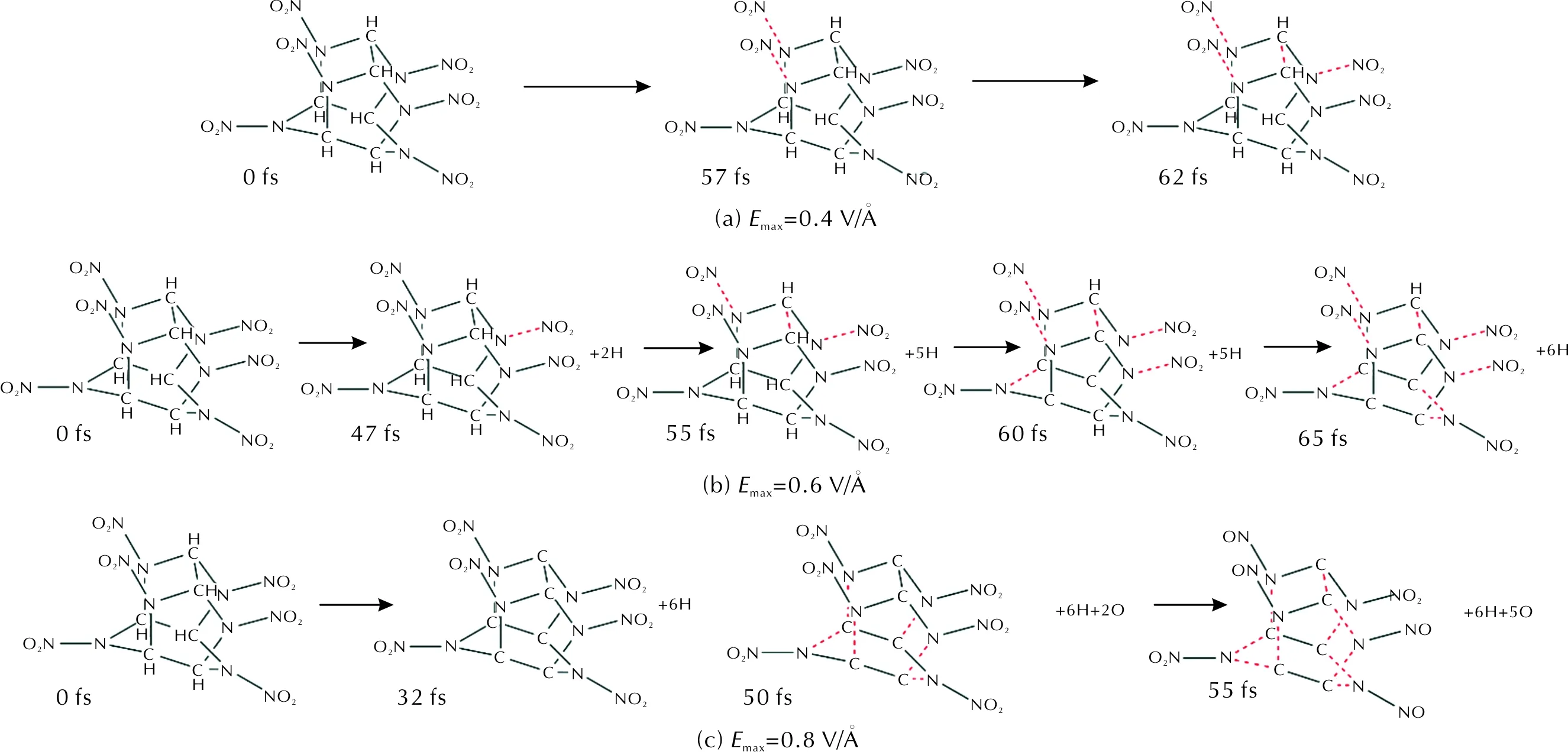
Fig.8 The decomposition process of CL-20 molecule under UV light
Next, we summarize the irradiation sensitivity and stability of the four explosive molecules in terms of decomposition reactions under UV. As could be seen from the decomposition process above, the initial bond breaking of the four explosive molecular are mainly R—H bonds, R—N bonds from R—NO2group or C—N bond from the ring structure. In previous thermal decomposition of NTO by DFTB-MD and DFT method, primary reaction channels are cleavage of nitro group (C—N bond fracture), rupture of C—N bond in the ring and H atom transfer to carbonyl[36], which are similar with the decomposition under UV light. But the dissociated H atom under UV light cannot bind carbonyl like in thermal decomposition. The UV light field causes the dissociated hydrogen atoms to break away from the matrix more completely than in thermal decomposition.
Some simulation results show that the detonation properties of explosives are related to the number and distribution of N—O bonds on nitro group[37-38], the reaction of nitro group and its product are also used as the descriptors of sensitivity under irradiation[3]. Therefore, we focus on the nitro group and compare the light intensity and reaction time of the nitro group detachment of the four molecules to estimate their irradiation sensitivities. The R—NO2bonds (R represent C or N) fracture of NTO, HMX, RDX, and CL-20 happen at 65fs under 0.6V/?,47fs under 0.6V/? and 58fs under 0.4V/?, and 57fs under 0.4V/?, respectively. Thus, the sensitivity rank will be CL-20—RDX>HMX>NTO. The experimental results of measuring the laser sensitivity of explosives by infrared laser are as follows, CL-20 (17.02J/cm), RDX (29.68J/cm), RDX (33.89J/cm), NTO (>70J/cm)[39], where lower value indicates more sensitive. Such irradiation sensitivity rank is consistent with our simulations, showing that the sensitivity under infrared laser is correlated with under UV laser. Compared with the infra-red laser, the ultraviolet laser can excite the electrons of the explosive molecules more directly, which will further deoxidize the nitro groups to generate NO molecule or N2gas.
The main reactions that occur in these four explosive molecules under UV light are dehydrogenation, ring opening, nitro group separation, and nitro group deoxygenation to form nitrogen gas. Studies based on reactive molecular dynamics indicate that production of NO2molecule coincides with rapid temperature increase and N2release in electric field induced decomposition of CL-20/HMX cocrystal[40]. Thus, we can take the N—N or C—N bond in N—NO2or C—NO2as the trigger bond of detonation under UV irradiation. Although dehydrogenation and ring opening reactions may serve as initial reactions and indicator of stability of the four explosive molecules, they contribute to detonation only indirectly, where free H atoms readily react with other radicals and produce lots of heat, and ring opening facilitates NO2production[40]. The dissociated hydrogen and oxygen atoms are not stable, but no obvious binding phenomenon is observed under the action of light field. H2O and N2are observed in the simulation of light field induced decomposition of CL-20/HMX cocrystal[40], so we infer that water molecules can be produced when the system is cooled down.
For polynitro explosive molecules in this study, we observe that the first broken bond in the ring structure is usually near the nitro group. The nitro group is a strongly electron-withdrawing group in the ground state. When the nitro group is irradiated by UV light, a large number of electrons near the group can be transferred, resulting in instability of the surrounding ring structure. N—NO2bonds with strong dissociation energy have high electron transfer ability and are more likely to activate surrounding rings. For the unique C—NO2bond of NTO, when the light is strong, the nitro group tends to deoxidize and form nitroso group. It is observed from the differential charge density diagram that when the light intensity increases, the center of the electron loss region changes from C—NO2to the five-member ring, indicating that light intensity affects the charge transfer and ring-opening. The oscillating nitro group decreases the stability of the structure under the effect of strong electric field[41], and the structure breaks through the induced electron transfer between the nitro group and the ring structure. For the four explosive molecules, we see detachment of the nitro group from the ring, as well as production of NO2within a certain range of moderate intensity. Differently, increasing the intensity of light field results in different production, i.e., the nitro group breaks away from the ring to form nitroso group or deoxidizes and combines with nitrogen atom to form N2.
3 Conclusion
(1) Using the number of excited electrons as a descriptor of the electronic stability of the four energetic molecules under UV irradiation, we obtain their electronic stability rank, which is NTO>CL-20>HMX>RDX. From nitro group detachment, we predict that their irradiation sensitivity rank is CL-20—RDX>HMX>NTO, NTO molecule shows the lowest irradiation sensitivity in our simulations. The sensitivity trend agrees with laser sensitivity of explosives by infrared laser in experiment.
(2) When the four molecules are exited under UV light, the main reactions are dehydrogenation, ring-opening, detachment of nitro group, and formation of nitrogen gas by nitro group deoxygenation. Since electron-withdrawing nitro group can transfer charge to nearby groups when excited, strong light intensity and large nitro separation energy facilitate the ring-opening process. For polynitro explosive molecules in this study (HMX, RDX, and CL-20), the N—NO2bonds with stronger dissociation energy are more likely to cause the ring-opening and form nitrogen molecules.
(3) For the four explosive molecules, the nitro group can be detached from the ring and form nitrogen dioxide within a certain range of moderate intensity. Increasing the intensity of light field, the nitro group undergoes deoxygenation to form nitroso group, and it may further deoxidize to N atom and bind each other to form nitrogen molecules.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (NSFC, Grant No. 51971037).
- 火炸藥學(xué)報(bào)的其它文章
- DNTF與PMMA熱穩(wěn)定性和力學(xué)性能的分子動(dòng)力學(xué)模擬
- 非均質(zhì)推進(jìn)劑燃燒演化模擬:算法與驗(yàn)證
- 準(zhǔn)靜態(tài)拉伸下PBX力學(xué)性能的三維細(xì)觀模擬
- Molecular Dynamics Simulation of CL-20/DNDAP Cocrystal Morphology at Different Temperatures
- 基于分子晶體序參數(shù)與K-means聚類的TNT晶型轉(zhuǎn)化有限溫度弦研究
- BTATz晶體及其共晶的熱分解及釋放氮?dú)鈾C(jī)理:從頭算分子動(dòng)力學(xué)模擬

