Surgical decompression for the management of abdominal compartment syndrome with severe acute pancreatitis: A narrative review
Prashant Nasa, Gunjan Chanchalani, Deven Juneja, Manu LNG Malbrain
Abstract Intra-abdominal hypertension (IAH) and abdominal compartment syndrome (ACS) play a pivotal role in the pathophysiology of severe acute pancreatitis (SAP) and contribute to new-onset and persistent organ failure. The optimal management of ACS involves a multi-disciplinary approach, from its early recognition to measures aiming at an urgent reduction of intra-abdominal pressure (IAP). A targeted literature search from January 1, 2000, to November 30, 2022, revealed 20 studies and data was analyzed on the type and country of the study, patient demographics, IAP, type and timing of surgical procedure performed, post-operative wound management, and outcomes of patients with ACS. There was no randomized controlled trial published on the topic. Decompressive laparotomy is effective in rapidly reducing IAP (standardized mean difference = 2.68, 95% confidence interval: 1.19-1.47, P < 0.001; 4 studies). The morbidity and complications of an open abdomen after decompressive laparotomy should be weighed against the inadequately treated but, potentially lethal ACS. Disease-specific patient selection and the role of less-invasive decompressive measures, like subcutaneous linea alba fasciotomy or component separation techniques, is lacking in the 2013 consensus management guidelines by the Abdominal Compartment Society on IAH and ACS. This narrative review focuses on the current evidence regarding surgical decompression techniques for managing ACS in patients with SAP. However, there is a lack of high-quality evidence on patient selection, timing, and modality of surgical decompression. Large prospective trials are needed to identify triggers and effective and safe surgical decompression methods in SAP patients with ACS.
Key Words: Ⅰntra-abdominal hypertension; Ⅰntra-abdominal pressure; Decompression laparotomy; Midline laparotomy; Abdominal compartment syndrome; Acute pancreatitis
INTRODUCTION
Acute pancreatitis (AP) is a commonly-diagnosed gastrointestinal emergency that frequently requires hospitalization and admission to the intensive care unit (ICU). Despite a trend of decline in the mortality globally in the last 30 years for AP, the mortality rate of severe AP (SAP) is around 50% and is directly related to both duration as well as severity of persistent organ failure (POF)[1,2].
SAP is a recognized risk factor for intra–abdominal hypertension (IAH). The growing evidence emphasizes the importance of IAH in the pathophysiology of both new-onset as well as POF during the early phase of SAP[3-5]. The pathophysiological mechanisms that underlie IAH among SAP patients is yet to be explored in detail. A possible pathogenesis involves systemic inflammation because of the disease process, which in turn results in capillary leak and fluid sequestration, thus exhibiting retroperitoneal, visceral and bowel edema, ascites, and paralytic ileus. Gastric dilatation, abdominal pain with muscle contraction, and overzealous fluid administration for management of SAP tend to either sustain or exacerbates IAH[5-7]. However, once the IAH gets established, its clinical features overlap with that of SAP and are characterized either by rapid progression or new-onset of organ dysfunction. The incidence rate of IAH, among the patients with AP, varies in different studies and increases with severity,i.e.,it reaches up to 50% in patients with SAP[4]. Abdominal compartment syndrome (ACS) is defined as a sustained elevation of intra-abdominal pressure (IAP) of more than 20 mmHg and is associated with new onset organ dysfunction or failure, and also reflects an unabated progression of the IAH[8].
The prevalence rate of ACS among SAP patients is between 15% to 30%. The resultant multi-organ dysfunction observed in ACS, especially the respiratory and renal dysfunction, contributes to high morbidity and mortality rates in SAP[6,9]. On the other hand, the poly-compartment syndrome, characterized by simultaneous elevation of pressure in different compartments, is an extreme association of ACS that causes multi-organ dysfunction and requires immediate intervention[10]. ACS is a potentially-lethal complication with a staggering 50%-75% mortality rate among the patients diagnosed with SAP and ACS[11,12].
The optimal management of ACS involves a multi-disciplinary approach that starts from early recognition of the condition to initiating measures that are aimed at urgent reduction of IAP[13]. The 2013 consensus management guidelines of the Abdominal Compartment Society (www.wsacs.org) on IAH and ACS, recommended ‘decompressive laparotomy’ as an effective core strategic component in managing the overt ACS. In spite of the recommendation, the guidelines also acknowledged the morbidity risks involved in open abdomen and the associated complications such as the development of frozen abdomen and enterocutaneous fistula[8]. Moreover, the guidelines fail to specify recommendations for optimal timing, disease-specific patient selection and the role of less-invasive decompressive measures such as subcutaneous linea alba fasciotomy or component separation techniques. The morbidity of the open abdomen, after performing the decompressive laparotomy, should be weighed prior to the procedure against the potentially-lethal inadequately-treated ACS. In this background, the aim of the current paper is to systematically review the evidence on patient selection, optimal techniques and the uncertainties in evidence regarding surgical decompressive technique for the management of ACS and SAP.
LITERATURE REVIEW
For the current review paper, a targeted literature search was conducted through PubMed, Science Direct,Reference Citation Analysis(RCA), and Google Scholar using the MeSH keywords such as ‘Laparotomy’ OR ‘Intra–Abdominal Hypertension’ AND ‘Acute Pancreatitis’ and the study published between January 1, 2000 and November 30, 2022 was considered and the search revealed 16 results. When broader keywords such as ‘Intra-Abdominal Hypertension’ AND ‘Acute Pancreatitis’ were used for the same period, a total of 82 studies were found. Then, a total of 21 studies were analyzed through manual screening by the authors (Nasa P and Chanchalani G), excluding the reviews, non-human studies and non-English literature (Tables 1 and 2)[11,12,14-31]. One study was excluded due to unclear indications for surgical intervention[32]. The data was extracted from the selected studies with regards to type and country of the study, patient demographics, IAP value, type and timing of the surgical procedure performed, post-operative wound management, and the outcomes of patients with ACS.
Statistical analysis
The categorical variables were presented as frequency and percentage. Median [interquartile range (IQR)] or mean ± SD was used for continuous variables. A forest plot was drawn with standardized mean difference (SMD) and 95% confidence interval (CI) to exhibit the changes in IAP after surgical decompression with midline laparotomy. Unless otherwise indicated, all the statistical analyses were performed using SPSS (version 25.0, IBM SPSS Inc., Chicago, IL, United States).
RESULTS
No randomized controlled trial (RCT) was found in this topic. Out of the 20 studies considered for the analysis, 11 were observational and nine (81.8%) were retrospective while four (36.4%) studies were from China which included two largescale studies (with 94 and 273 patients, respectively). The median of the 20 (IQR = 14) patients with ACS was included in these studies, which ranged from 8 to 273 patients. A male predominance was observed in the results, with a mean age above 40 years; alcohol use and biliary pancreatitis were the most common etiology. Both lungs and kidneys were the two most common organ dysfunctions observed in all the studies (Table 1).
Out of the 225 patients who underwent surgical decompression for ACS in the observational studies, 200 (88.9%) patients underwent midline laparotomy. The rest of the patients also underwent other surgical procedures such as subcutaneous linea alba fasciotomy (17 patients, 7.5%) and subcostal laparostomy (8 patients, 3.6%). There was a considerable decline in IAP rate after the decompression surgery was performed using midline laparotomy (SMD = 2.68, 95%CI: 1.19-1.47,P< 0.001; 4 studies) among patients with ACS (Figure 1)[11,12,17,19]. Most of the patients underwent a secondary abdominal closure. The mortality rate, reported in different studies, varied widely from 12.5% to 75%. Further, the studies that included more than 25 patients reported a mortality rate between 25%-75% (Table 3)[11,12,14-22].
For the current review, a total of 17 patients, with a mean age of 45.7 ± 13.8 years from six case reports and three case series with individual patient data, was analyzed separately. Out of the total 17 patients, six (35.2%) were females. Alcohol use (8, 47.1%) and biliary (4, 23.5%) were found to be the common etiologies of AP. The mean cumulative fluid balance of eight patients after 24 h was 5698.7 ± 2638 mL. All the patients required invasive mechanical ventilation whereas eight patients (47.1%) required vasopressors (Table 2). Most patients (14, 82.4%) underwent midline laparotomy and delayed vacuum-assisted closure (VAC) (13, 76.5%). The median number of days of open abdomen was 18 (IQR = 42) while the total time was in the range of 2 to 210 d. The median ICU and hospital length of stay were 30 (IQR = 15) d and 54 (IQR = 41.5) d, respectively, with only one fatal outcome (5.9%). The abdomen was primarily closed in only one patient whereas the rest (16/17, 94.1%) of the patients managed with an open abdomen and delayed primary closure, assisted with VAC, for 10 d to 7 mo. Only one out of the 18 patients died (Table 4).
DISCUSSION
General findings
SAP is a common risk factor of ACS with considerable morbidity and mortality rates, despite the existence of established treatment methods[33,34]. The demography of the patients with ACS was found to be similar like AP patientsi.e.,a mean age of 40 years and a male predominance. Biliary and alcohol-related factors were found to be the most common etiologies for AP[1]. Elevated IAP, especially ACS are detrimental, not only for the intra-abdominal organs like kidneys, intestines and liver, but it may also impact other organs such as heart, lungs, and brain[10,35,36]. The guidelines recommend an early recognition of ACS using IAP measurement and urgent management in case of positive IAH[8,37].
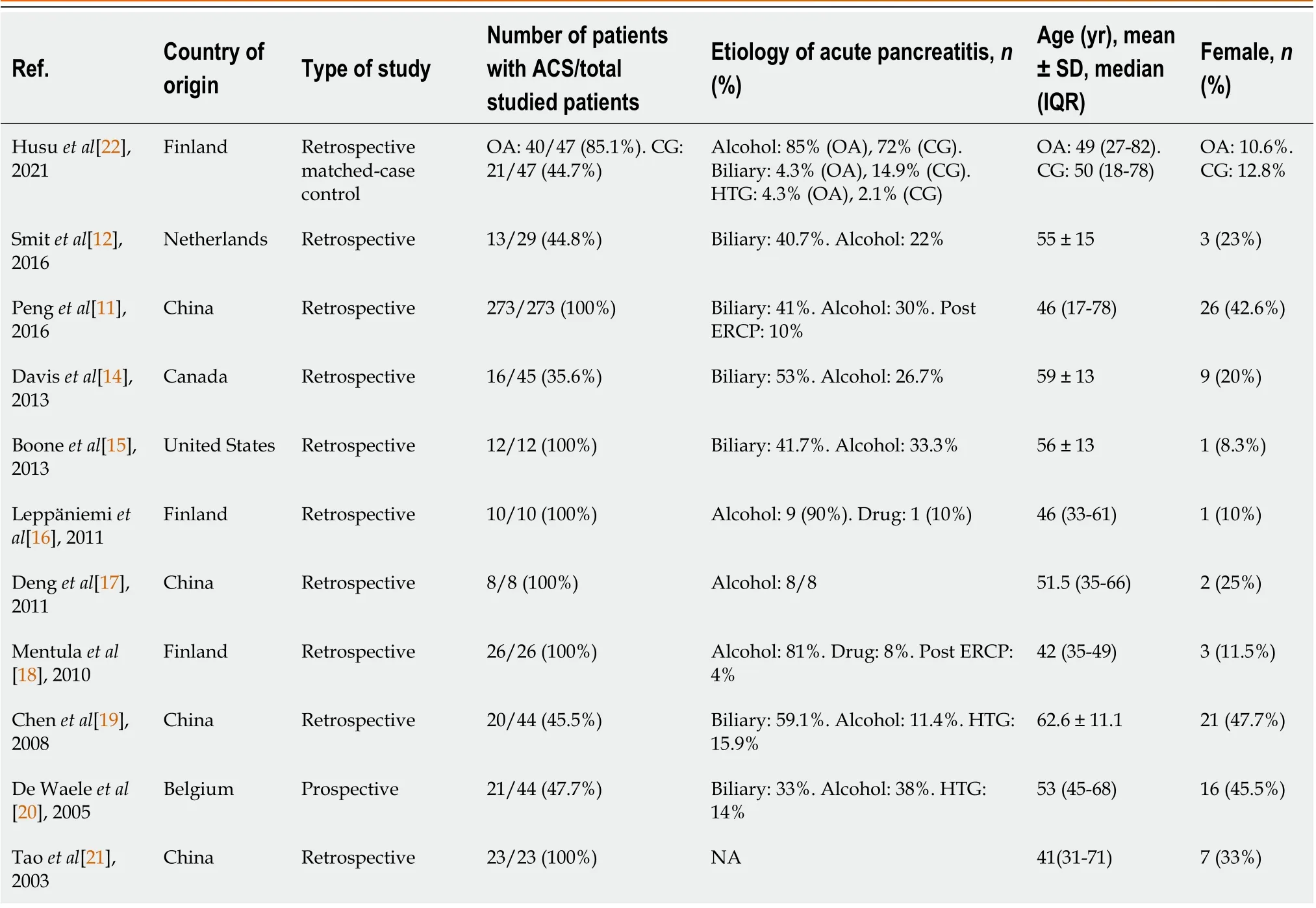
Table 1 Demographic data of observational studies on abdominal compartment syndrome with acute pancreatitis

Figure 1 Forest plot of observation studies showing the mean change in intra-abdominal pressure before and after surgical decompression after midline laparotomy. SMD: Standardized mean difference; CI: Confidence interval.
Medical management
SAP treatment is primarily a supportive one, except for acute gallstone pancreatitis. However, the guidelines are controversial in terms of the role played by urgent endoscopic retrograde cholangiopancreatography (ERCP) with sphincterotomy in managing the acute gallstone pancreatitis[37]. In a recently-conducted multi-center RCT, an urgent ERCP with sphincterotomy was compared with a conservative treatment to treat the acute gallstone pancreatitis without cholangitis. The study found no significant difference in the primary endpointi.e.,a composite outcome of mortality and major complications such as new-onset POF, cholangitis, bacteremia, pneumonia, pancreatic necrosis, or pancreatic insufficiency at six months from the randomization[38]. Hence, ERCP should be considered only for acute severe gallstone pancreatitis associated with cholangitis or persistent cholestasis.
The management of ACS among patients with SAP depends on its severity and the course of the primary disease. The treatment ranges from conservative medical management to surgical decompression laparotomy. The medical management includes hemodynamic support and the optimization of regional and systemic perfusion, improvement of abdominal compliance (e.g.,with adequate sedation and analgesia with or without neuromuscular blockade) and reduction of intra-luminal volume (e.g.,with nasogastric or colonic decompression) or reduction of intra-abdominal volume (e.g.,paracentesis)[7,34,39].
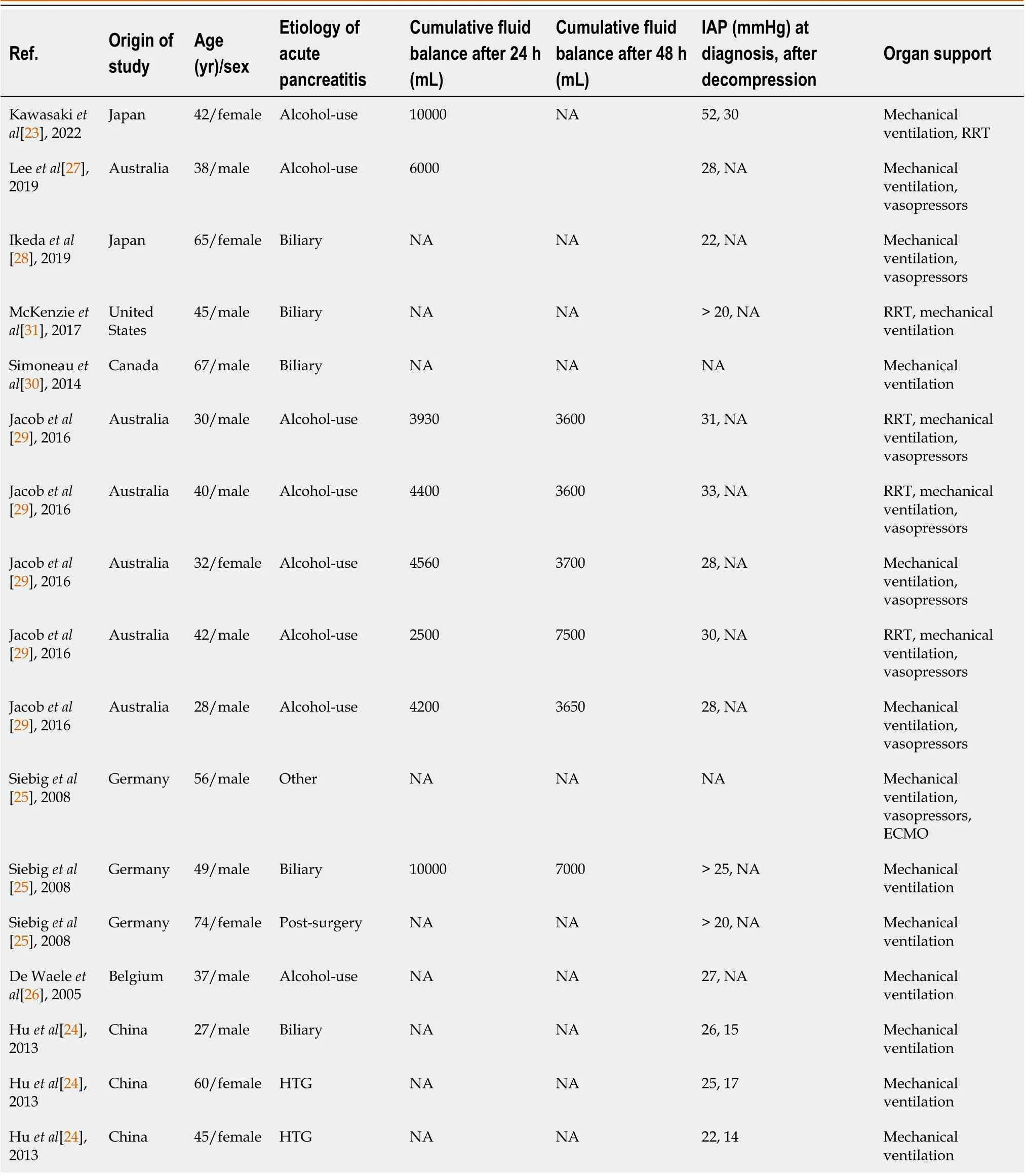
Table 2 Demographic data of case reports or case series on abdominal compartment syndrome with acute pancreatitis
Fluid management
The local and systemic inflammation of the AP results in extravascular fluid accumulation and the depletion of intravascular fluid[7]. Hence, the AP management guidelines recommend early rapid hydration to restore the intravascular volume, improve circulatory dysfunction and ameliorate both tissue and organ dysfunction[37,39]. However, injudicious and aggressive fluid resuscitation may propagate fluid accumulation, increase the risk of fluid overload and promote or exacerbate the secondary IAH or ACS. Moreover, the fluid accumulation also impairs the wound healing process which in turn promotes infection[7].
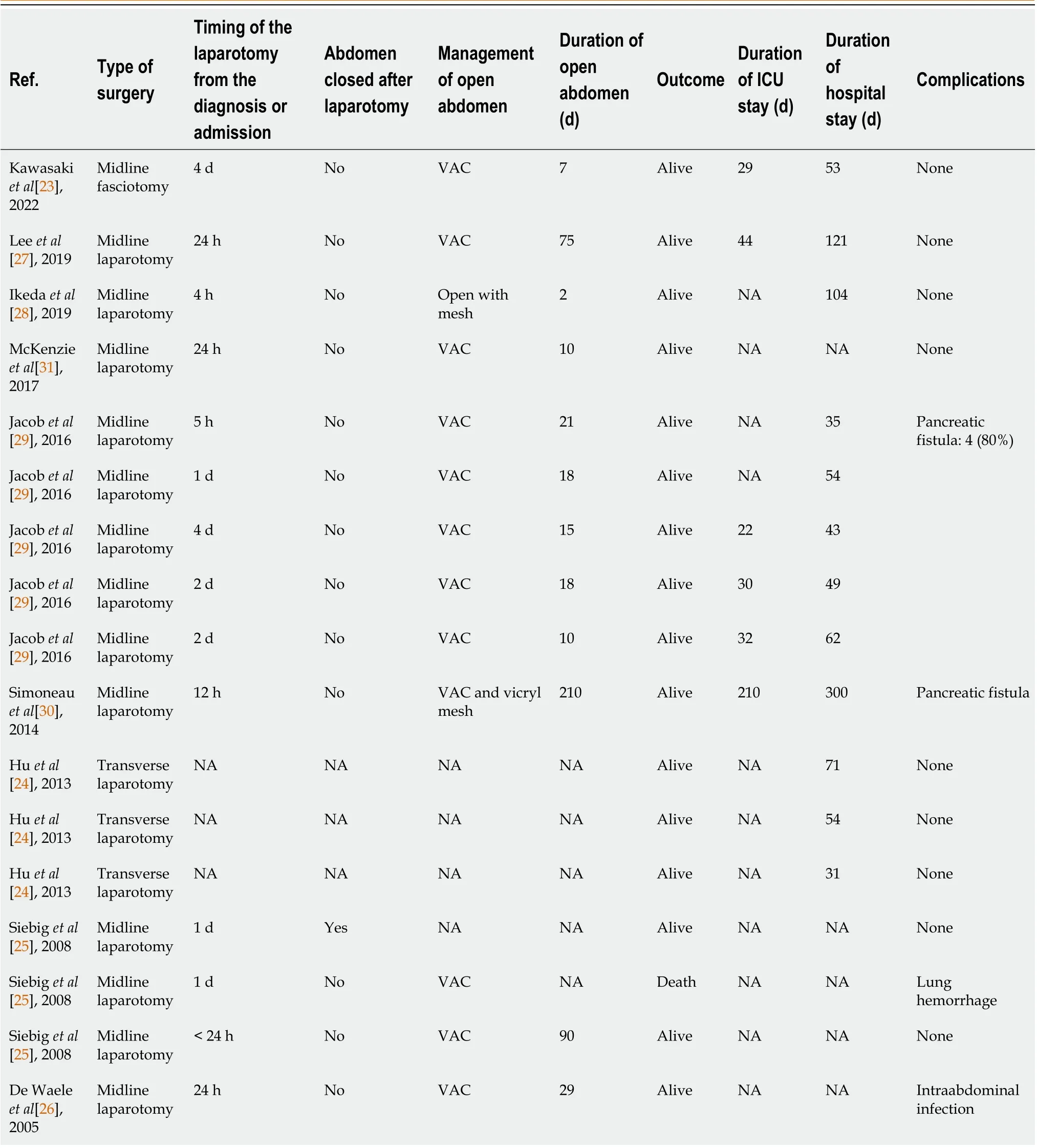
Table 4 Demographic data of case reports or case series on abdominal compartment syndrome with acute pancreatitis
In a recently-published RCT, the incidence of ACS was found to be lower (32.5%vs72.2%,P< 0.05) with controlled intravenous fluid administration than the rapid fluid expansion[40]. In another recent RCT, the goal-directed fluid administration was found to be associated with less fluid overload among AP patients than the early aggressive fluid resuscitation method. However, no significant or meaningful difference was found in terms of clinical outcomes[41]. Fluid management in IAH patients is a challenging task that needs to be individualized and titrated to the clinical endpoints[7,42]. Besides, the intravenous fluid administration in patients with IAH may not ameliorate organ dysfunction despite increasing the cardiac output. Functional hemodynamic monitoring such as pulse pressure or stroke volume variation should be considered prior to fluid administration in these patients[43]. On the other hand, fluid removal may be considered for selected IAH patients using diuretics or continuous veno-venous hemofiltration so as to achieve negative fluid balance[44].
Surgical management
Patients with SAP who develop ACS have extremely high chances of mortality and so, early recognition of this condition and timely intervention may help in improving the organs’ functions, morbidity and mortality[39,45]. A high incidence of visceral ischemia was found among the patients with SAP and ACS, thus contributing to a high mortality rate of this group of patients[14].
Various surgical interventions have been tried in patients with ACS, who failed the medical management process. Ultrasound or computed tomography scan-guided percutaneous drainage of intra-abdominal collections is a minimallyinvasive procedure available to reduce the IAP in selected patients[12,21]. However, urgent surgical decompression is highly effective and potentially, a life–saving treatment for ACS, especially in those patients with refractory ACS. Decompressive laparotomy helps in improving the abdominal compliance by rapidly lowering the IAP[10,39,45]. In this review, the authors found a significant reduction of IAP after surgical decompression in four observational studies that measured pre- and post-decompression IAP levels. However, the impact of surgical decompression on multi-organ dysfunction was found to vary. In porcine model that involved 32 animals with SAP and ACS, the early surgical decompression (within 6 h) was associated with a significant improvement in systemic hemodynamics, alleviation of organ dysfunction and reduced mortality rate compared to the decompression performed at 9 or 12 h[46]. In a retrospective study conducted by Mentulaet al[18], the surgical decompression procedure improved both respiratory as well as renal functions in nearly half of the patients. A prominent improvement was observed in respiratory function only among those patients with severe hypoxemia[18,20]. Further, early surgical decompression was also found to be associated with reduced mortality[18]. However, high morbidity and the complications associated with open abdomen like ventral hernia, frozen abdomen, pancreatic and enterocutaneous fistula and sepsis result in the recommendation of surgical decompression by the guidelines, only after the failure of medical management to reduce IAP[9,13,41]. Nonetheless, a patient–centric approach and the role of clinical evaluation beyond IAP are lacking in these guidelines[8,9]. In addition to this, there is a lack of evidence and agreement regarding IAP values, timing, and the techniques that may trigger surgical decompression.
Surgical decompression technique
No trials have been conducted so far, comparing the surgical technique for decompression. Often, the choice is individualized as per the available expertise and common sense. The current review found that midline laparotomy is the most common surgical procedure performed for decompression. Midline laparotomy involves a full–thickness (skin, fascia, peritoneum) vertical midline skin incision from xiphoid to pubis.
Midline decompressive laparotomy can rapidly reduce IAP and improve organ dysfunction like hemodynamic, respiratory and renal dysfunctions[18,36]. Other surgical approaches, reported in the literature, include full-thickness bilateral subcostal transverse laparotomy and subcutaneous linea alba fasciotomy (Figure 2). Transverse laparotomy is a quick procedure with a high success rate for fascial closure. However, the incision is done upon the abdominal muscles (rectus and external oblique), which may require a complete reconstruction with temporary mesh in case of loss or retraction of fascia[12,18,24]. On the other hand, subcutaneous linea alba fasciotomy is a less invasive approach that involves 2-3 skin incisions at the linea alba, without opening the peritoneal cavity[47]. It avoids both morbidity and the complications associated with open abdomen. Further, the procedure can also be used as a bridge before committing to perform decompression laparotomy[48]. However, the success rate is only 50%-70%, with a higher risk of incisional hernia[16,49].
Timing of surgery
The timing of surgical decompression is a matter of ongoing debate. The dichotomy of earlyvslate decompression should consider a variety of factors. The meta-analysis of 15 studies (that included both adults and children) by Van Dammeet al[45], demonstrated the effectiveness of surgical decompression in reducing the IAP and halting the progression of systemic organ failure. However, the overall mortality was 49.7% in adults. In the current review, the authors found the mortality of patients with ACS varied between 25%-75% in spite of undergoing surgical decompression. Higher mortality, observed in these patients, may reveal the higher severity of the disease at the baseline. Another hypothesis is that the delay in the surgical intervention, in the background of progressive multi–organ failure and irreversible visceral ischemia, contributed to higher mortality of these patients[22]. The patients, in most of the studies included, underwent surgical decompression after the failure of medical management whereas the granular data on patients who may benefit from early surgical decompression was missing.

Figure 2 Techniques of surgical decompression for abdominal compartment syndrome. 1: Bilateral subcostal transverse laparotomy; 2: Midline laparotomy; 3: Subcutaneous linea alba fasciotomy.
As mentioned earlier, Mentulaet al[18] found that early surgical decompression (within the first four days of diagnosis) in patients with IAP > 25 mmHg can be associated with low mortality (18%vs46%). Taoet al[21] observed the mortality rate to be merely 16.7% in 18 patients with surgical decompression for ACS and SAP. For these patients, definitive closure was performed within 3-5 d of surgical decompression. An early intervention (5-22 h after the diagnosis of ACS) and using a lower IAP trigger (> 20 mmHg) could explain about the better outcomes[21]. This outcome aligns with the guidelines that suggest an early closure within the first week, or whenever feasible, to reduce the complications. However, various factors should be considered prior to decision of early closure is made, such as the resolution of cardiorespiratory compromise, no further surgical exploration being considered and no concerns for the recurrence of ACS[50].
In the absence of high–quality evidence, the timing of the surgery should be individualized based on the factors such as the evolution of IAP over time, the severity of organ dysfunction and the response to medical management.
Triggers for surgical decompression
Most of the studies did not identify any cut–off for IAP to guide the surgical decompression whereas intervention was primarily implemented based on the rapid progression of organ dysfunction and medical management failure. The largest retrospective study, conducted on ACS in SAP, found the percutaneous catheter drainage to be superior to open laparotomy with temporary closure, in terms of reducing the need for ICU stay, complications, and mortality. However, open laparotomy was found to be highly effective than the percutaneous drainage procedure in immediate restoration of the physiological variables like hemodynamics or oxygenation (PaO2/FiO2 ratio). The higher mortality in open laparotomy group was linked to increased rate of infections (100%vs55%,P< 0.001) and complications (80%vs41%,P< 0.001)[11]. However, the patients were recruited in this study only after the failure of medical management whereas those patients with a need for immediate surgery were excluded. There are no studies available so far on prophylactic surgical management to reduce the risk of ACS. The results of the only multi-centric, randomized controlled study (the DECOMPRESS study) comparing decompressive laparotomy and percutaneous drainage are yet to be published[51]. The potential triggers for surgical decompression include compromised oxygenation and/or ventilation, hemodynamic instability and worsening organ dysfunction, despite medical management.
Post-surgical decompression complications
Midline laparotomy with temporary abdominal closure (TAC) is associated with its own complications such as infection, bleeding, fistula, failed fascia closure and incisional hernia. The incidence rate of these complications varied in different studies (Tables 3 and 4). Penget al[11] found a high complication rate in patients with open laparotomy compared to percutaneous drainage (80%vs41%;P< 0.001). Fistula (24.6%), especially pancreatic (7.5%), and bleeding (11.4%) were the common complications. Further hepatic, portal, or mesenteric vein thrombosis were also reported in 2 (3.2%) patients.
Open abdomen management
In general, the presence of open abdomen is the consequence after surgical decompression for ACS, because of the need for frequent re-operations and the risk of recurrence. However, it is challenging to manage the open abdomen after surgical decompression as it needs a careful and a dynamic plan. Open abdomen can be managed with TAC techniques like skin-only closure, mesh, bags (e.g.,Bogota bag) or the use of a non-adhesive plastic layer (e.g.,polyethylene film, opposite dressings), non-absorbable zipper or VAC therapy with close monitoring of IAP for recurrence of IAH[48,51,52]. A common misconception is that open abdomen protects against the recurrence of IAH and ACS, though it is not the case. TAC reduces the complications of an open abdomen like evisceration, contamination, fluid and temperature loss, enterocutaneous fistula, and fascial retraction[53,54].
The current review found that the primary closure got delayed in most of the studies. The least early closure rate in these patients can be explained by the risks involved in recurring IAH after early closure, reported higher rate of intraabdominal infections, and fistula[11]. Further, a higher proportion of these patients developed infected necrotizing pancreatitis that requires multiple episodes of necrosectomy[18].
In the meta-analysis of randomized and case-controlled studies, the negative pressure wound therapy or VAC for the open abdomen was found to be associated with better outcomes[55]. Negative pressure wound therapy or VAC is also recommended by an international expert panel as the preferred technique for the management of open abdomen[50,56]. VAC has been used in most of the patients in published case reports and case series. When leaving the abdomen open, the most crucial issue is to plan for its closure again. If one fails to plan the closure within the first week after opening, then the possibilities are high for failure with a ventral hernia repair at a later stage.
CONCLUSION
Patients with SAP are prone to develop IAH and ACS and are at risk for worse outcomes. Anticipation and regular monitoring of IAP and organ function are necessary for a timely diagnosis of ACS in patients with SAP. It is challenging to manage ACS in patients with SAP since it needs a multi-modal approach. Surgical decompression is an effective intervention, which can rapidly reduce the IAP and may be considered only in those patients with progressive cardiorespiratory compromise or medical management failure. There is a lack of quality evidence on a few parameters such as the patient selection, timing, and the modality of surgical decompression. Further research is required in this domain in the form of large, prospective controlled trials to identify the triggers and effective and safe modality of surgical decompression in patients with ACS and SAP.
FOOTNOTES
Author contributions:Nasa P conceptualized and designed the article; Nasa P and Chanchalani G performed acquisition of data, analysis and interpretation of data, and drafted the article; Juneja D and Malbrain ML revised the article; and all authors have read and approved the final manuscript.
Conflict-of-interest statement:Nasa P declared to be on the advisory board of Edwards life sciences. Malbrain ML is Professor of Critical Care Research at the 1stDepartment of Anesthesiology and Intensive Therapy, Medical University of Lublin, Poland. He is co-founder, past-President and current Treasurer of WSACS (The Abdominal Compartment Society, http://www.wsacs.org). He is member of the medical advisory Board of Pulsion Medical Systems (now fully part of Getinge group), Serenno Medical, Potrero Medical, Sentinel Medical and Baxter. He consults for B. Braun, Becton Dickinson, ConvaTec, Spiegelberg, Medtronic, MedCaptain, and Holtech Medical, and received speaker’s fees from PeerVoice. He holds stock options for Serenno and Potrero. He is co-founder and President of the International Fluid Academy (IFA). The IFA (http://www.fluidacademy.org) is integrated within the not-for-profit charitable organization iMERiT, International Medical Education and Research Initiative, under Belgian law. Other authors do not declare any conflict of interest in relation to the content of the present paper.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United Arab Emirates
ORCID number:Prashant Nasa 0000-0003-1948-4060; Gunjan Chanchalani 0000-0001-8429-8526; Deven Juneja 0000-0002-8841-5678; Manu LNG Malbrain 0000-0002-1816-5255.
S-Editor:Wang JJ
L-Editor:A
P-Editor:Zhao S
11Peng T, Dong LM, Zhao X, Xiong JX, Zhou F, Tao J, Cui J, Yang ZY. Minimally invasive percutaneous catheter drainage versus open laparotomy with temporary closure for treatment of abdominal compartment syndrome in patients with early-stage severe acute pancreatitis.J Huazhong Univ Sci Technolog Med Sci2016; 36: 99-105 [PMⅠD: 26838748 DOⅠ: 10.1007/s11596-016-1549-z]
 World Journal of Gastrointestinal Surgery2023年9期
World Journal of Gastrointestinal Surgery2023年9期
- World Journal of Gastrointestinal Surgery的其它文章
- Preoperative and postoperative complications as risk factors for delayed gastric emptying following pancreaticoduodenectomy: A single-center retrospective study
- Comparative detection of syndecan-2 methylation in preoperative and postoperative stool DNA in patients with colorectal cancer
- Preoperative prediction of microvascular invasion in hepatocellular carcinoma using ultrasound features including elasticity
- Surgical management of gallstone ileus after one anastomosis gastric bypass: A case report
- Hepatic ischemia-reperfusion syndrome and its effect on the cardiovascular system: The role of treprostinil, a synthetic prostacyclin analog
- Advances and challenges of gastrostomy insertion in children
