Preoperative and postoperative complications as risk factors for delayed gastric emptying following pancreaticoduodenectomy: A single-center retrospective study
Fang-Liang Xie, Li-Jun Ren, Wei-Dong Xu, Tong-Lei Xu, Xia-Qing Ge, Wei Li, Xu-Ming Ge, Wen-Kai Zhou, Kai Li, Yun-Hai Zhang, Zhong Wang
Abstract
Key Words: Delayed gastric emptying; Postoperation; Pancreaticoduodenectomy; Treatment
INTRODUCTION
Pancreaticoduodenectomy (PD) is a commonly performed surgical procedure for treating tumors in the head of the pancreas, the lower part of the common bile duct, and the ampullary region of the duodenum. It involves the resection of the pancreatic head, lower portion of the common bile duct, gallbladder, distal stomach, duodenum, and part of the jejunum[1]. With medico-technological advancements, PD has become a standardized surgical approach for treating pancreatic head cancer and periampullary benign and malignant tumors[2], decreasing serious postoperative complications such as severe pancreatic leakage, bile leakage, and massive hemorrhage[3]. However, the underlying mechanism of delayed gastric emptying (DGE), one of its complications, remains unknown, presenting a challenge for clinicians in its treatment[4]. Despite advancements in surgical techniques and perioperative management, which have reduced the occurrence of severe postoperative complications such as severe pancreatic leakage, bile leakage, and massive hemorrhage, some complications, particularly DGE, continue to be prevalent among those who underwent PD, with no known cause or effective treatment[5].
DGE is a syndrome characterized by a gastric motility disorder and gastric emptying disturbances, primarily caused by non-mechanical obstruction factors resulting from abdominal surgery[6]. The cause of DGE has remained unknown, resulting in poor treatment outcomes and prolonged hospital stays, posing challenges for clinicians. Evidence indicates that DGE is a functional gastric emptying disorder without organic lesions[7]. The incidence of DGE has been consistently high for many decades, with a study conducted by the International Study Group of Pancreatic Surgery (ISGPS) in 2007 reporting an incidence ranging from 19% to 57% following pancreatic surgery[5]. A recent foreign research review discovered that the incidence of DGE ranges from 3.2% to 59%, with 3234 (27.7%) out of 11669 patients experiencing post-PD DGE[8]. In recent years, numerous pancreas centers worldwide have conducted extensive studies and discussions on the factors inducing DGE after PD[9]; however, a definite and convincing conclusion is yet to be reached. Determining whether the cause lies in surgical techniques, perioperative management issues, or other factors is crucial in guiding treatment decisions.
Regarding treatment, the specific pathogenesis of DGE remains uncharacterized[5] without an established treatment plan for DGE, resulting in undesirable effects with various treatment approaches. Consequently, comprehensive measures have been generically adopted. For patients with postoperative DGE, the routine treatment plan of our team is to correct hypoproteinemia (HP) and electrolyte imbalances, maintain stable blood sugar levels, and utilize methods such as intramuscular metoclopramide, acupuncture, gastroscope-guided jejunal nutrition tube placement, and occasional intravenous erythromycin infusions[10-12]. However, past treatment attempts using these approaches have failed to improve gastric motility significantly or indicate the exact timing at which gastric function recovers in patients.
Therefore, this study aims to thoroughly investigate the risk factors associated with DGE and introduce a novel treatment approach, as detailed below.
MATERIALS AND METHODS
Baseline data
The clinical data of 114 patients who underwent PD between January 2015 and June 2018 at The First People's Hospital of Lianyungang were collected. Inclusion criteria: (1) Patients who underwent PD; (2) Patients who met the diagnostic criteria for DGE following PD; (3) Patients without cerebrovascular diseases who could tolerate surgery; (4) Patients with no history of abdominal surgery; and (5) Patients with complete clinical data. Exclusion criteria: (1) Patients with incomplete clinical data; (2) Patients with pre-existing gastrointestinal obstructive diseases; (3) Patients with Grade A DGE; (4) Patients with postoperative pulmonary infection; and (5) Patients undergoing re-operation due to postoperative complications.
Surgical methods
All the included patients underwent either PD or pylorus-preserving pancreatoduodenectomy (PPPD) under general anesthesia. The tumor was resected through a median abdominal incision, and lymph nodes were dissected from the pancreatic head, the lower part of the common bile duct, the gallbladder, the upper jejunum, the duodenum, and portions of the distal stomach. All digestive tract reconstructions were performed using the Child procedure. Anastomosis was performed as follows: Firstly, pancreaticojejunostomy was performed using a modified pancreatic duct-jejunal mucosato-mucosa one-layer anastomosis or pancreas-jejunal invagination anastomosis; secondly, the gastrointestinal anastomosis was performed by a full-thickness continuous absorbable suture with absorbable threads; finally, end-to-side gastrojejunostomy (for PD) or duodenal-jejunal end-to-side anastomosis (for PPPD) was adopted for gastrointestinal anastomosis. The gastrointestinal anastomosis stoma was located either in the anterior or posterior colon. The stomach and some jejunal feeding tubes were routinely placed during the operation, and two abdominal drainage tubes were inserted postoperatively.
Diagnostic criteria for DGE
The current diagnosis of DGE after pancreatic surgery follows the requirements recommended by the ISGPS in 2007. Gastroparesis (GP) can be diagnosed if the following conditions are met: (1) Continuous gastrointestinal decompression with a daily drainage volume of > 500 mL for > 3 d after pancreatic surgery; (2) Inability to consume solid food within 7 days after the operation; (3) Vomiting or bloating; and (4) Requirement for gastrointestinal excitomotors. When considering these symptoms, it is essential to rule out mechanical obstruction of the gastrointestinal outflow tract. ISGPS classifies DGE after pancreatic surgery into grades A, B, and C based on the severity. Grade A refers to the retention of the gastric tube for 4-7 d after the operation or the inability to consume solid food on the 7thpostoperative day; grade B refers to the retention of gastric tubes for 8-14 d after the operation, or the inability to consume a solid diet on the 14thpostoperative day; grade C corresponds to the postoperative gastric tube retention for > 14 d, significantly impacting patient recovery, or the inability to consume a solid diet even after 21 postoperative days. Patients with grades B and C, which have a significant impact on postoperative recovery, were included in the study[5].
Diagnostic criteria for complications
According to the Consensus on the Diagnosis, Treatment, and Prevention of Common Complications in Pancreatic Surgery (2017)[13], formulated by the Pancreatic Surgery Group of the Chinese Medical Association, postoperative complications such as pancreatic fistula, biliary fistula, postoperative bleeding, and chylous fistula were assessed. A pancreatic fistula is an abnormal channel between the pancreatic ductal epithelium and other epithelial surfaces, resulting in the flow of enzyme-rich fluid from the pancreas. The diagnostic criteria for pancreatic fistula are as follows: The amylase concentration in the drainage fluid, measured at least 3 d after the operation, exceeds three times the upper limit of the normal serum amylase concentration, alongside corresponding clinical manifestations. A biliary fistula is an abnormal passage through which bile flows out of the biliary system into the abdominal cavity or outside the body through a breach (or the cholangio-jejunal anastomotic stoma) of the biliary system. Postoperative bleeding is defined as bleeding that occurs after pancreatic surgery, typically indicated by bloody fluid in the abdominal drainage or gastrointestinal decompression tube or hematochezia. Simultaneous changes in vital signs, such as the heart rate and decreased hemoglobin (Hb) concentration, were observed. Chylous fistula is diagnosed when chylous fluid is drained from a drainage tube, orifice, or wound at ≥ 3 d after the operation, regardless of the amount of drainage, provided the concentration of triglycerides is > 1100 mg/L. Intra-abdominal infection is defined by symptoms such as chills, high fever, abdominal distension, and intestinal paralysis, occurring 3 d after the operation and persisting for > 24 h. Laboratory tests showing significantly elevated white blood cell count, with or without HP and anemia, and imaging evidence of intra-abdominal fluid accumulation contribute to the diagnosis of intra-abdominal infection. The diagnosis can be confirmed if the puncture extract is purulent or if bacteria are detected.
Observation indicators selected
Postoperative indicators were considered primary outcome measures, while general information and surgical factors were secondary.
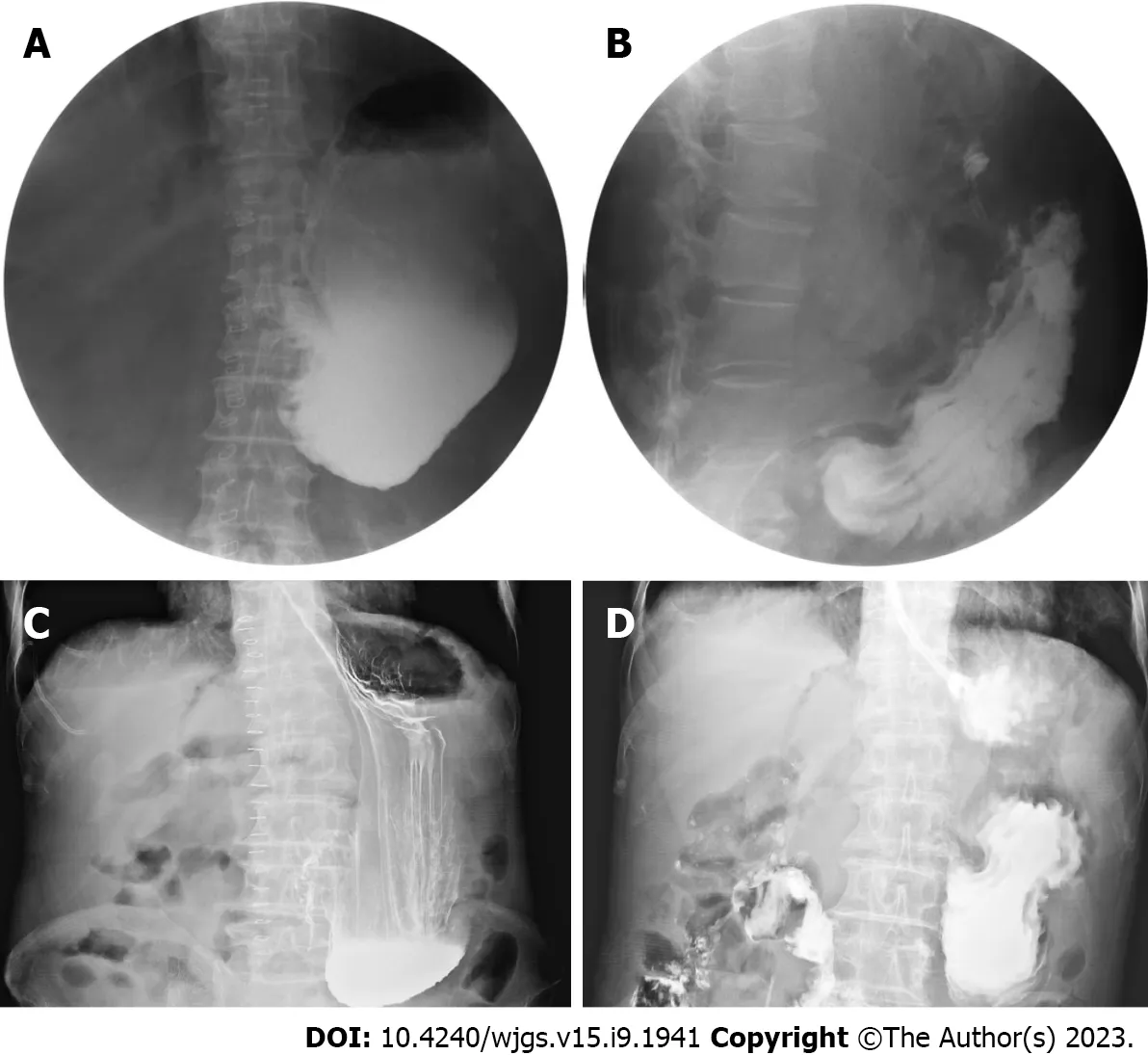
Figure 1 Gastrointestinal series showing the effect of different medications on gastrointestinal motility. Angiography was performed in patients A and B after oral administration of meglumine diatrizoate. A: On postoperative day 9, patient A showed no peristalsis and high tension in the stomach; B: On postoperative day 13, 24 h following the injection of neostigmine through the transdermal receptor pathway, peristaltic waves were observed in patient A; C: On postoperative day 11, patient B showed no peristalsis in the stomach; D: On postoperative day 13, 24 h after the injection of neostigmine through the transdermal receptor pathway, peristaltic waves were observed in patient B.
General information: Gender, age, preoperative systemic diseases, preoperative nutritional status.
Surgical factors: Pylorus preservation status, lymph node dissection, operation time (min), intraoperative blood loss (mL).
Postoperative indicators: Pancreatic fistula, abdominal complications, early enteral nutrition treatment (within 4 d), and Hb and albumin (ALB) levels on postoperative days 1, 4, and 7.
Statistics analysis
All data were statistically analyzed using SPSS 23.0. Enumeration data were expressed as proportions and compared using the Chi-square test or Fisher exact probability method. Measurement data were expressed as medians ± SD and analyzed using thet-test. Variables with a test value ofP< 0.10 in the univariate analysis were included in the multivariate analysis to identify independent risk factors associated with DGE using logistic regression. The estimated odds ratio with 95% confidence intervals was used to describe the relative risk. Statistical significance was indicated byP< 0.05.
RESULTS
Occurrence of DGE after PD
The study included 66 males (57.9%) and 48 females (42.1%) (male-to-female ratio is approximately 1.4:1), with a mean age of 62.5 years (range: 33-83 years). Sixty-three cases (55.3%) underwent PD, while 51 (44.7%) received PPPD. The primary diseases diagnosed were pancreatic cancer, cholangiocarcinoma, ampullary carcinoma, solid pseudopapillary tumor of the pancreas, intraductal papillary mucinous neoplasms, chronic pancreatitis, duodenal stromal tumor, and duodenal papillary adenomyoma, with 46, 32, 23, 3, 3, 3, 2, and 2 cases, respectively. DGE occurred in 33 patients, with an incidence of 28.9%. The angiography results are shown in Figure 1.
Analysis of risk factors for post-PD DGE
Univariate analysis of postoperative DGE showed that pylorus preservation, gastrointestinal anastomosis mode, postoperative abdominal complications, and ALB on a postoperative day 4 were significant risk factors for post-PD DGE (P< 0.05), as shown in Table 1.
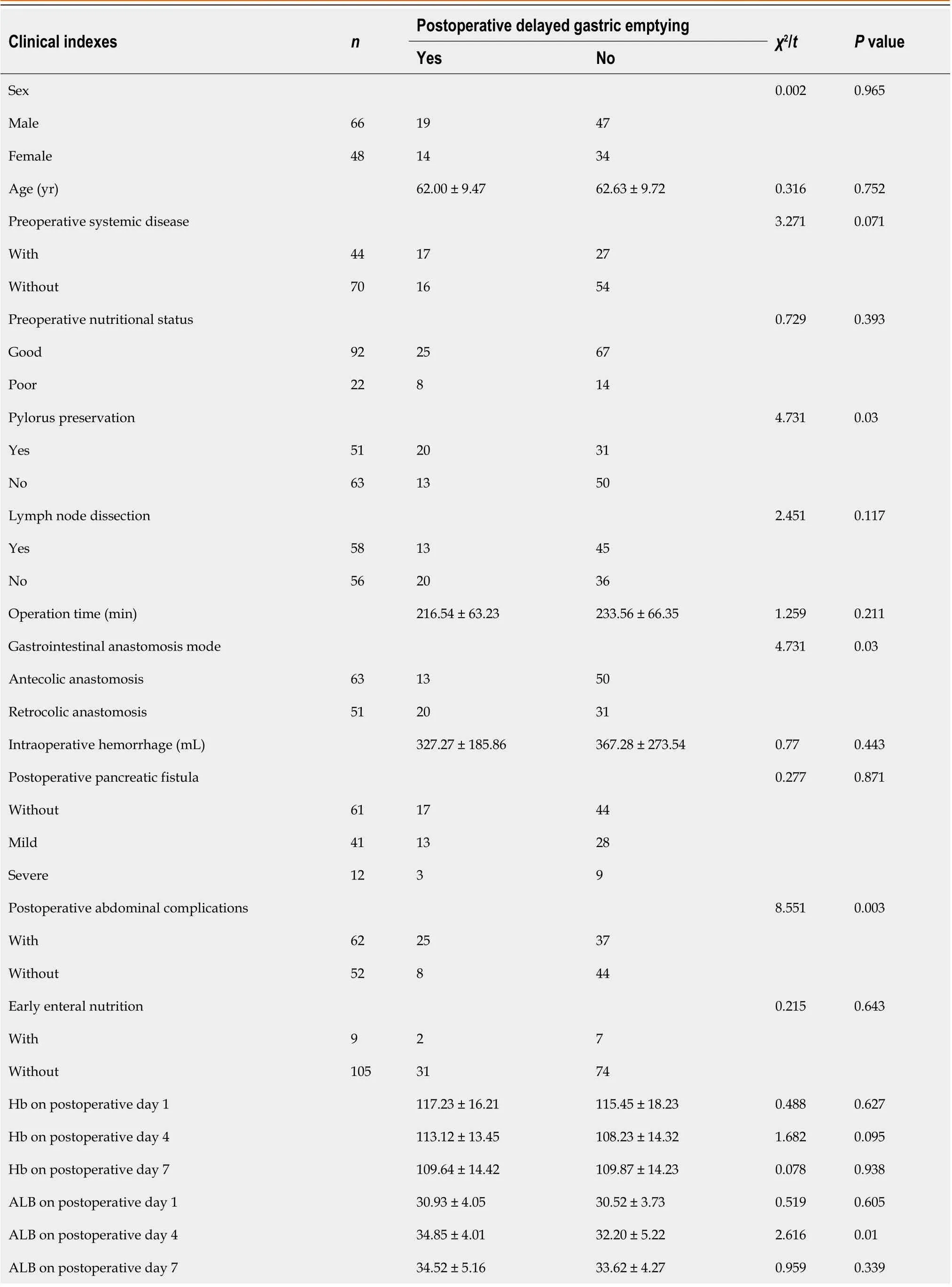
Table 1 Univariate analysis of delayed gastric emptying in 114 cases after pancreaticoduodenectomy
The six observation indexes (preoperative systemic disease, pylorus preservation, gastrointestinal anastomosis mode, postoperative abdominal complications, Hb and ALB on postoperative day 4), which were identified withP< 0.10 in the univariate analysis, were subjected to logistic regression analysis. The results are presented in Table 2.
Finally, the regression equation included postoperative abdominal complications, preoperative systemic diseases, and ALB on postoperative day 4 as factors influencing the occurrence of DGE after PD, as shown in Table 3.
DISCUSSION
This study enrolled 114 patients who underwent PD within a recent 5-year period at a single center, ensuring a brief timeframe and providing comprehensive and reliable data. Severe DGE grades of B and C were observed in 33 of the 114 patients after PD, resulting in an incidence rate of 28.9%, consistent with findings from other reports. We further investigated the potential impact of pyloric preservation on the risk of DGE, which has been controversial. A randomized controlled trial (RCT) study conducted by Japanese scholars[14] on 130 patients who underwent PD reported a significantly higher incidence of DGE in patients with pylorus preservation compared to those without pylorus preservation (17.2%vs4.5%,P= 0.02). However, another RCT study conducted by German scholars on 188 patients undergoing PD by German scholars[15] found no significant difference in the incidence of DGE in 95 patients with pylorus preservation and 93 patients without pylorus preservation (25.3%vs31.2%,P= 0.208). In the clinical setting, we have consistently observed patients undergoing PPPD to have a higher possibility of developing DGE. The univariate analysis of this study also showed a higher incidence of DGE in patients who underwent PPPD (P= 0.03), with 20 (39.2%) among the 51 patients developing DGE, compared to 13 (20.6%) among the 63 patients who underwent PD, which suggesting that PPPD patients have a high incidence of DGE in patients who have undergone PPPD. However, the multivariate analysis did not reveal a significant correlation between pylorus preservation and DGE. A further in-depth study found that patients with pylorus preservation experienced earlier and milder cases of DGE. If there were no other associated risk factors, the faster patient recovery would not compromise the safety of the procedure or the occurrence of postoperative abdominal complications.
Many studies have indicated postoperative abdominal complications as risk factors for DGE[14,16]. In this research, the univariate analysis revealed that postoperative abdominal complications are independent risk factors (P= 0.003) for DGE. Multivariate analysis identified postoperative abdominal complications as the most significant risk factors for DGE (OR = 4.768). Serious postoperative abdominal complications, such as abdominal infection, hemorrhage, chylous fistula, and anastomotic leakage, primarily occur due to pancreatic fistula and biliary fistula with infection. Although postoperative pancreatic leakage was considered an observation index in this study, both univariate and multivariate analyses showed no significant association with postoperative DGE. This finding could be attributed to the adequate abdominal drainage of a simple pancreatic fistula and the absence of local infection in the abdominal cavity, reducing the likelihood of developing postoperative DGE.
Some scholars have suggested[16] that local inflammation or infection foci caused by pancreatic fistula may contribute to DGE. Therefore, measures such as improving the operation quality and implementing early postoperative interventions to promote intestinal peristalsis could lower the incidence of postoperative pancreatic fistula; this ensures unobstructed drainage after the occurrence of pancreatic fistula. The prompt puncture and drainage of the fistula could prevent the accumulation of corrosive fluids, thereby lowering the risk of postoperative DGE. Furthermore, this study identified two other significant risk factors: early postoperative ALB and preoperative systemic diseases. Postoperative ALB, in the presence of systemic diseases, leads to anastomotic edema; suture cutting causes a significant increase in the incidence of anastomotic leakage, which can easily result in abdominal infection, bleeding, and other complications, resulting in postoperative DGE.
A study has investigated the use of neostigmine in treating GP following abdominal surgery[17]; it has shown encouraging results, particularly in the treatment of post-PD DGE. In this study, neostigmine was administered to a 73-year-old male patient with refractory GP after distal gastrectomy, demonstrating a certain degree of safety and clinical efficacy, suggesting that neostigmine is a safe and effective treatment in GP. Previous studies have reported[18-20] the presence of receptor pathways for spinal and sympathetic nerves in the skin and the clinical effectiveness of intradermal administration in treating conditions such as herpes zoster neuralgia and visceral pain[21,22]. Our study demonstrated the unique effects of neostigmine administeredviathe transdermal receptor pathway, which cannot be replicated through other routes of administration. Investigating the target of neostigmine in the receptor pathway is also a future research direction for our team.
CONCLUSION
Based on the results of this study, the following recommendations can be made for the prevention of DGE after PD: (1) Effective communication with patients and their families before the operation is crucial; this allows them to fully comprehend the potential postoperative complications, management strategies, and past experiences in handling complications, helping alleviate their anxiety; (2) Patients with systemic diseases such as hypertension and diabetes should receive careful perioperative treatment to maintain hemodynamic stability and a stable internal environment; (3) Standardizing and improving the procedure is essential to minimize postoperative pancreatic and bile leakage; and (4) Timely initiation of preoperative nutritional support therapy and postoperative ALB supplementation is essential to prevent tissue edema and maintain water-electrolyte balance. Continuous postoperative gastrointestinal decompression, and using patent gastric and abdominal cavity drainage tubes could help reduce the likelihood of abdominal complications.
However, this study has some limitations. As a single-center retrospective study, the potential biases in the data collection and analysis processes could have moderately influenced the final study results. Moreover, no specific analysis was performed during DGE treatment, and a larger sample size with more extensive data is needed to verify the therapeutic effect of neostigmine. Therefore, well-designed, multi-center studies with larger sample sizes are necessary for validation.
ARTICLE HIGHLIGHTS

Research objectives
This study aims to identify related risk factors for DGE after the PD procedure.
Research methods
In this retrospective, cross-sectional study, clinical data were collected from 114 patients who underwent PD from January 2015 to June 2018. They were analyzed regarding demographic factors, pre- and perioperative characteristics, and surgical complications. Univariate and multivariate analyses were performed to identify the risk factors for post-PD DGE.
Research results
The study included 66 males (57.9%) and 48 females (42.1%), aged 33-83 years (mean: 62.5), with a male-to-female ratio of approximately 1.4:1. There were 63 cases (55.3%) of PD and 51 cases (44.7%) of pylorus-preserving pancreatoduodenectomy (PPPD). Among the 114 patients who underwent PD, 33 (28.9%) developed postoperative DGE. Univariate analysis revealed significant differences in four of the 14 clinical indexes observed: Pylorus preservation, retrocolonic anastomosis, postoperative abdominal complications, and early postoperative albumin (ALB). Logistic regression analysis further identified postoperative abdominal complications [odds ratio (OR) = 4.768,P= 0.002], preoperative systemic diseases (OR = 2.516,P= 0.049), and early postoperative ALB (OR = 1.195,P= 0.003) as significant risk factors.
Research conclusions
Postoperative severe abdominal complications, preoperative systemic disease and early postoperative ALB are risk factors for post-PD DGE.
Research perspectives
The research perspective of this study is to thoroughly explore the risk factors for post-PD DGE.
FOOTNOTES
Author contributions:Xie FL and Ren LJ contributed equally to this work and are co-first authors; Xie FL and Ren LJ designed the research and wrote the first manuscript; Wang Z, Xu WD, Xu TL, Ge XQ, LW, Ge XM and Zhou WK contributed to conceiving the research and analyzing data; Wang Z, Li K and Zhang YH conducted the analysis and provided guidance for the research; all authors reviewed and approved the final manuscript.
Institutional review board statement:This study was approved by the Ethic Committee of Xuzhou Medical University Affiliated Hospital of Lianyungang (The First People's Hospital of Lianyungang) (Approval No. LW-20230411001-01).
Informed consent statement:Patients were not required to give informed consent to the study because the analysis used anonymous clinical data that were obtained after each patient agreed to treatment by written consent.
Conflict-of-interest statement:There is no conflict of interest.
Data sharing statement:All data and materials are available from the corresponding author.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:China
ORCID number:Zhong Wang 0009-0000-9467-8880.
S-Editor:Yan JP
L-Editor:A
P-Editor:Yan JP
 World Journal of Gastrointestinal Surgery2023年9期
World Journal of Gastrointestinal Surgery2023年9期
- World Journal of Gastrointestinal Surgery的其它文章
- Comparative detection of syndecan-2 methylation in preoperative and postoperative stool DNA in patients with colorectal cancer
- Preoperative prediction of microvascular invasion in hepatocellular carcinoma using ultrasound features including elasticity
- Surgical management of gallstone ileus after one anastomosis gastric bypass: A case report
- Hepatic ischemia-reperfusion syndrome and its effect on the cardiovascular system: The role of treprostinil, a synthetic prostacyclin analog
- Advances and challenges of gastrostomy insertion in children
- Surgical decompression for the management of abdominal compartment syndrome with severe acute pancreatitis: A narrative review
