Baseline radiologic features as predictors of efficacy in patients with pancreatic neuroendocrine tumors with liver metastases receiving surufatinib
Jianwei Zhang ,Haibin Zhu ,Lin Shen ,Jie Li ,Xiaoyan Zhang ,Chunmei Bai ,Zhiwei Zhou,Xianrui Yu,Zhiping Li,Enxiao Li,Xianglin Yuan,Wenhui Lou0,Yihebali Chi,Nong Xu,Yongmei Yin,Yuxian Bai,Tao Zhang,Dianrong Xiu,Jia Chen,Shukui Qin,Xiuwen Wang,Yujie Yang0,Haoyun Shi0,Xian Luo0,Songhua Fan0,Weiguo Su0,Ming Lu,Jianming Xu
1Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Department of Gastrointestinal Oncology,Peking University Cancer Hospital &Institute,Beijing 100142,China;2 Key Laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing),Department of Radiology,Peking University Cancer Hospital &Institute,Beijing 100142,China;3State Key Laboratory of Holistic Integrative Management of Gastrointestinal Cancers,Beijing Key Laboratory of Carcinogenesis and Translational Research,Department of Gastrointestinal Oncology,Peking University Cancer Hospital &Institute,Beijing 100142,China;4 Department of Medical Oncology,Peking Union Medical College Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College,Beijing 100730,China;5Department of Gastric Surgery,State Key Laboratory of Oncology in South China,Collaborative Innovation Centre for Cancer Medicine,Sun Yat-sen University Cancer Center,Guangzhou 510062,China;6Department of Pancreatic and Hepatobiliary Surgery,Fudan University Shanghai Cancer Center,Shanghai 200032,China;7 Department of Abdominal Oncology,West China Hospital,Sichuan University,Chengdu 332001,China;8Department of Medical Oncology,the First Affiliated Hospital of Xi’an Jiaotong University,Xi’an 710061,China;9 Department of Oncology,Tongji Hospital,Tongji Medical College,Huazhong University of Science and Technology,Wuhan 430030,China;10 Department of General Surgery,Zhongshan Hospital of Fudan University,Shanghai 200032,China;11Department of Medical Oncology,National Cancer Center/Cancer Hospital,Chinese Academy of Medical Sciences and Peking Union Medical College,Beijing 100021,China;12Department of Medical Oncology,the First Affiliated Hospital of Zhejiang University,Hangzhou 310009,China;13Department of Medical Oncology,the First Affiliated Hospital of Nanjing Medical University,Nanjing 210029,China;14 Department of Gastrointestinal Oncology,Harbin Medical University Cancer Hospital,Harbin 150081,China;15Department of Oncology,Union Hospital,Tongji Medical College,Huazhong University of Science and Technology,Wuhan 430022,China;16Department of General Surgery,Peking University Third Hospital,Beijing 100191,China;17Department of Medical Oncology,Jiangsu Cancer Hospital,Nanjing 214206,China;18Cancer Center of Jinling Hospital,Nanjing University of Chinese Medicine,Nanjing 210016,China;19Department of Medical Oncology,Qilu Hospital of Shandong University,Jinan 250012,China;20Department of Clinical and Regulatory Affairs,HUTCHMED Limited,Shanghai 200001,China;21 Department of Gastrointestinal Oncology,the Fifth Medical Center,Chinese PLA General Hospital,Beijing 100071,China
Abstract Objective: Currently,pre-treatment prediction of patients with pancreatic neuroendocrine tumors with liver metastases (PNELM) receiving surufatinib treatment was unsatisfying.Our objective was to examine the association between radiological characteristics and efficacy/prognosis.Methods: We enrolled patients with liver metastases in the phase III,SANET-p trial (NCT02589821) and obtained contrast-enhanced computed tomography (CECT) images.Qualitative and quantitative parameters including hepatic tumor margins,lesion volumes,enhancement pattern,localization types,and enhancement ratios were evaluated.The progression-free survival (PFS) and hazard ratio (HR) were calculated using Cox’s proportional hazard model.Efficacy was analyzed by logistic-regression models.Results: Among 152 patients who had baseline CECT assessments and were included in this analysis,the surufatinib group showed statistically superior efficacy in terms of median PFS compared to placebo across various qualitative and quantitative parameters.In the multivariable analysis of patients receiving surufatinib (N=100),those with higher arterial phase standardized enhancement ratio-peri-lesion (ASER-peri) exhibited longer PFS[HR=0.039;95% confidence interval (95% CI): 0.003-0.483;P=0.012].Furthermore,patients with a high enhancement pattern experienced an improvement in the objective response ratio [31.3% vs.14.7%,odds ratio(OR)=3.488;95% CI: 1.024-11.875;P=0.046],and well-defined tumor margins were associated with a higher disease control rate (DCR) (89.3% vs.68.2%,OR=4.535;95% CI: 1.285-16.011;P=0.019) compared to poorlydefined margins.Conclusions: These pre-treatment radiological features,namely high ASER-peri,high enhancement pattern,and well-defined tumor margins,have the potential to serve as predictive markers of efficacy in patients with PNELM receiving surufatinib.
Keywords: Neuroendocrine tumors;liver metastases;computed tomography;surufatinib
Introduction
The pancreas is one of the most common primary sites among gastroenteropancreatic neuroendocrine tumors(NET) (1).Among all metastatic sites in pancreatic NETs,pancreatic neuroendocrine tumors with liver metastases(PNELM) are most commonly seen,with a prevalence of 82% in registries and 64% in the SEER database in United States (1).Liver metastases (LM) are associated with poor prognosis and a 5-year survival of 13%-54% compared to 75%-99% without LM (2,3). The European Neuroendocrine Tumor Society consensus guidelines suggest that over 80% of PNELM can only be treated systemically (4-7).PNELM are usually accompanied by abundant blood supply,which is characteristically hypervascularized by a dense and specialized capillary network and high levels of vascular endothelial growth factor (8).These characteristics provide opportunities for the therapeutic use of anti-angiogenesis agents (9).
Surufatinib is a novel oral tyrosine kinase inhibitor that simultaneously targets angiogenesis [vascular endothelial growth factor receptor and tumor-immune evasion(colony-stimulating factor-1 receptor)].In the randomized placebo-controlled phase III trial,SANET-p,surufatinib demonstrated a prolonged median progression-free survival(PFS) than placebo [10.9vs.3.7 months;hazard ratio(HR)=0.49,95% confidence interval (95% CI): 0.32-0.76;P=0.001],indicating its clinical benefits for pancreatic NET (pNET) (10).Notably,PNELM accounted for 94.2% of the SANET-p population,hinting at the potential strong impact of surufatinib on a subgroup of patients with LM.
Contrast-enhanced computed tomography (CECT) is widely used for the diagnosis and evaluation of treatment responses in cancer patients (11-13).Pre-treatment radiological evaluation may have considerable clinical implications for identifying optimal subgroups.Radiologybased prognosis stratification has demonstrated the association between blood-supply-related imaging features and treatment efficacy (12).By using more quantitative and precise approaches,pre-treatment imaging has become a convenient method for predicting the prognosis and efficacy (14).High-vascularized lesions,for instance,the PNELM,theoretically had the future.They would most benefit treatment with anti-angiogenic therapy (15).Surufatinib,an efficacious angiogenic therapy,may also benefit from this approach.
This study aimed to investigate the association between qualitative and quantitative radiological parameters and the prognosis and efficacy of PNELM treated with surufatinib,hoping to optimize an effective therapy strategy for subgroups of patients receiving surufatinib treatment.
Materials and methods
Patient selection and eligibility
The analysis was conducted in all patients with PNELM from SANET-p,a multicenter,randomized controlled phase III trial.SANET-p was conducted in accordance with good clinical practice principles and relevant local regulations.Pathology was reviewed and diagnosis was confirmed by two independent pathologists according to the 2019 5th edition of the World Health Organization(WHO) Classification of Neuroendocrine Tumors.Clinical information was retrieved using the hospital information system.The basic eligibility criteria mainly complied with the trial.Key inclusion criteria in this analysis included: 1) above 18 years old;2) pathological diagnosis of unresectable or metastatic,well differentiated pancreatic NETs (pathological grade 1 or 2 according to the 2010 WHO classification);3) Eastern Cooperative Oncology Group performance status <2; 4) CECT completed within 4 weeks before the first dose of surufatinib;5) estimated survival of more than 3 months;and 6) at least one dose of surufatinib or placebo.Key exclusion criteria mainly included: 1) no confirmed liver lesions or no measurable lesions (diameter <1 cm) (n=12);2) no CECT assessment at baseline (n=3);or 3) images could not be analyzed because of artifacts.All subjects gave written informed consent and the study protocol was approved by the institutional review committee of Peking University Cancer Hospital according to the Helsinki Declaration.
CT technique and assessment criteria
All patients received pre-treatment CECT of the abdomen(liver dual phase) and pelvis,with the scanning coverage ranging from the dome of the right diaphragm to the symphysis pubis or lower according to the standard scanning protocol (the tube voltage was 120 kVp and current was 200 mA).The intravenous administration of 100-150 mL (150-300 mg/mL) of nonionic contrast material at a rate of 2-3 mL/s was required.Images were acquired first for the arterial phase and subsequently for the portal venous phase.The slice thickness was 5 mm with no slice gap,and followed by contiguous reconstruction increments of 5 mm.The image acquisition guideline(Supplementary Table S1) was followed by all centers involved in the SANET-p trial.All pre-treatment and subsequent CECT data were retrieved in a workstation(picture archiving and communication system,PACS) for further assessment.Evaluation of CECT images was performed by two qualified radiologists,and when inconsistencies occurred,the assessment was arbitrated by an independent third member.All the imaging reviewers were blinded to the clinical information.
Target liver metastatic lesions according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 were selected from baseline CECT images,avoiding blood vessels and normal liver parenchyma.The region of interest (ROI) was delineated manually in the arterial phase.The ROI were traced along the outer margin of the metastatic tumor of each slice to contain the whole lesion.Subsequently,the CT values of each region were measured in HU on the workstation (HUtumorART).ROI was then measured at the periphery of the lesion and at the maximum slice to record the parameter-peri (parameters are described below).The parameter-whole was measured as the averaged values of the peripheral area and central area (except for obvious necrotic areas in cores).HUliverARTand aorta (HUaortaART) were measured.Finally,HUtumor,HUliver,and HUaorta were measured in non-enhanced and HUtumorPORT,HUliverPORTwere obtained in portal venous phase slice target lesions.
Imaging parameters
The radiologic features involved qualitative and quantitative parameters. The qualitative parameters included the total hepatic lesion volume (<25%vs.≥25%)(16),hepatic tumor localization type classified by Frillinget al.[single metastasis (type I),isolated metastatic bulk accompanied by smaller deposits (type II),disseminated metastatic spread (type III)] (3),tumor margins (poorlydefined or well-defined),enhancement pattern [high enhancement and other (including low enhancement and heterogeneous enhancement) patterns],and necrosis proportions of lesions (≤25%vs.>25%) (17).
Quantitative parameters were mainly set as ratios between the tumor tissues and reference vessels,to avoid the inevitable variability between examinations and patients(18).Quantitative parameters included:
(a) Maximum diameter (MD) was defined as the longest diameter of the maximum lesion,measured by the longest transverse diameter axially in slices.
(b) Relative enhancement ratio (RER) was defined as the tumor density compared to the adjacent parenchyma in arterial (HUtumorART/HUliver,ARER) or portal venous phases (HUtumorPORT/HUliver,PRER).The RER-peri and RER-whole were calculated depending on the specific position of the lesions (19,20).
(c) Standardized enhancement ratio (SER) was defined as the tumor density compared with aortic enhancement(HUtumorART/HUaorta,ASER) of the arterial phase and portal phase,and portal enhancement (HUtumorPORT/HUaorta,PSER).The SER-peri and RER-whole were calculated depending on the specific position of the lesions(20,21).
Efficacy assessment
Patients were re-assessed by investigators using consistent imaging evaluating methods per RECIST version 1.1 every 8 weeks (±3 d) for the first 12 months and then every 12 weeks (±3 d) until progression disease (PD).SANET-p was performed in parallel by the investigators and blinded independent image review committee for efficacy analysis in SANET-p (10).Efficacy variables in the analysis included: the primary end point was defined as the time from randomization to documented PD or death,and the median PFS was the time taken for half of the patients to reach the primary end point.The objective response (OR)was defined as a complete response (CR) or partial response(PR).The disease control rate (DCR) was defined as the proportion of patients with CR,PR,or stable disease (SD).
Statistical analysis
Continuous variables were tested by thet-test (normally distributed variables),and categorical variables were compared using Chi-square test or Fisher’s exact test,where appropriate.We generated PFS curves using the Kaplan-Meier method and log-rank test.Forest plots depicting the PFS of subgroups were presented.The quantitative radiological parameters were stratified below and above the 50th percentile as low or high levels (e.g.ASER-peri low or high).To assess the association between PFS and the prognostic value of different radiological parameters,multivariate analysis following univariate analysis was conducted using Cox’s proportional hazards model.Logistical regressions with univariate/multivariate analysis were performed to evaluate the efficacy.Statistical significance was set at P<0.05.All analyses were performed with SAS Enterprise? (Version 8.2;Cary,NC,USA).
Results
Overview of whole cohort
Among 172 patients in the SANET-p trial,patients with no confirmed liver lesions or no measurable lesions (n=12);no CECT assessment at baseline (n=3);and inadequate images for analysis (n=5) were excluded according to the eligibility criteria.We included 152 patients [surufatinib (n=100) and placebo (n=52)] diagnosed with PNELM,who had baseline and at least one post baseline CECT evaluation from the SANET-p study.The flowchart illustrating the exclusion process is shown inSupplementary Figure S1.In the surufatinib group,the overall median (range) age was 50.0(25.0-75.0) years and 87% patients were G2.Baseline characteristics were balanced between the surufatinib and placebo groups (Table 1).The radiological deciphering of enhancement and margin-related features are shown inSupplementary Figure S2andFigure 1.
The summary of imaging parameters is listed inSupplementary Table S2.In the surufatinib group,56(56.0%) and 44 (44.0%) patients had well-or poorlydefined tumor margins,respectively.Thirty-two patients(32.0%) had high enhancement pattern,mean [standard deviation (SD)] of ASER-peri and ASER-whole was 0.46(0.15),and 0.38 (0.14);mean (SD) of PSER-peri and PSER-whole was 0.80 (0.14),and 0.69 (0.15);and mean(SD) of ARER-peri and ARER-whole was 1.51 (0.97) and 1.27 (0.90);mean (SD) of PRER-peri and ARER-whole was 1.11 (0.34) and 0.95 (0.31),respectively.No major differences in baseline imaging features were observed between the surufatinib and placebo groups.
Efficacy analysis of whole cohort
The PFS was 11.0 months in the surufatinib group compared to 3.7 months in the placebo group,with an HR of 0.44 (95% CI,0.29-0.68),indicating a 56% reduction in the risk of disease progression with surufatinib (Figure 2).Subgroup analyses of PFS consistently demonstrated benefit across most imaging parameters in the PNELM population,with no suggested influence of baseline covariates on PFS (Supplementary Table S3).
Notably,a significant reduction in PFS events was observed in subgroups defined by blood-supply-associated imaging parameters (HR<0.4),including high enhancement pattern,well-defined tumor margins,type III localization metastases,high MDs,necrosis proportions >25%,and hepatic tumor volume <25%.
In terms of response,the surufatinib group demonstrated a best overall response (BOR) of PR in 20 (20.0%) patients,SD in 60 (60.0%) patients,PD in 7 (7.0%) patients,and not evaluable (NE) in 13 (13.0%) patients.In comparison,the placebo group had a BOR of PR in 1 (1.9%) patient,SD in 30 (57.7%) patients,PD in 14 (26.9%) patients,and NE in 7 (13.5%) patients.The objective response ratio(ORR) was 20.0% in the surufatinib group and 1.9% in the placebo group.The ORR results were consistent with those observed in the SANET-p study (Supplementary Table S4).
Efficacy analysis of surufatinib group
Further exploration of qualitative and quantitative enhancement parameters and efficacy in the surufatinibgroup revealed potential predictive factors for longer mPFS using univariate Cox regression analysis: high ASER-peri(HR=0.094,95% CI: 0.009-0.931,P=0.043),low PRERwhole (HR=2.080,95% CI: 0.881-4.908,P=0.095),and low PRER-peri (HR=2.051,95% CI: 0.959-4.388,P=0.064).Proceeding with the multivariate analysis,ASERperi (HR=0.039,95% CI: 0.003-0.483,P=0.012) and PRER-whole (HR=2.872,95% CI: 1.299-6.348,P=0.009)were significantly associated with PFS (Table 2).PFS curves stratified by ASER-peri (low/high) and PRER-whole(low/high) are shown inSupplementary Figure S3andSupplementary Figure S4.These figures demonstrate that ASER-peri-high is associated with longer mPFS compared to the other groups.
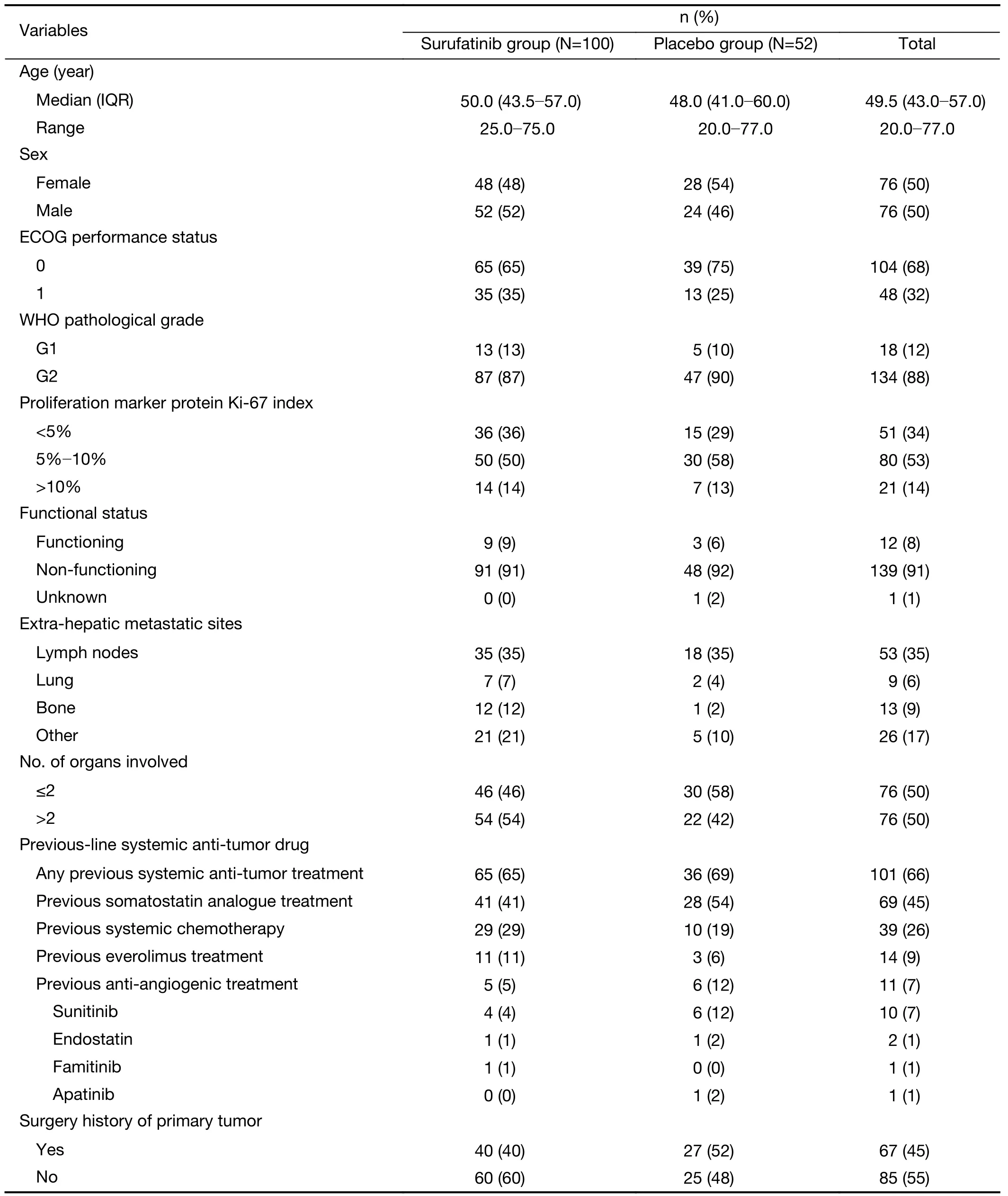
Table 1 Baseline clinical information of surufatinib vs. placebo in SANET-p of whole cohort
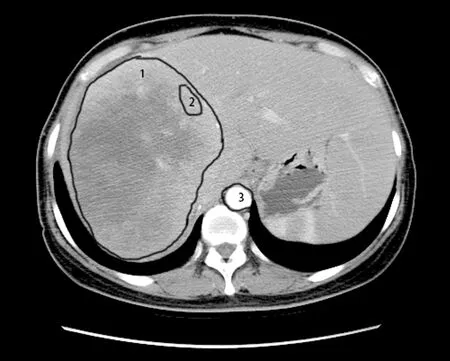
Figure 1 Delineation of target lesions and ROIs.ROI (number 1)was depicted along the contours of the tumor and the average of CT values was calculated as the whole-lesion values.In addition,the ROI (number 2) was generated on the periphery of the tumor;while the ROI (number 3) inside the aorta was depicted to calculate the peri-lesion values.ROI,region of interest;CT,computed tomography.
When examining the association between radiological factors and response in the surufatinib group using logistic analysis,we found that a high enhancement pattern was associated with a better ORR [high enhancement patternvs.other enhancement pattern,31.3%vs.14.7%,OR=3.488 (95% CI: 1.024-11.875),P=0.046].Welldefined tumor margins also showed a tendency towards better response in the surufatinib group [25.0%vs.13.6%,OR=3.142 (95% CI: 0.808-12.224),P=0.099] (Table 3).Furthermore,well-defined tumor margins were associated with a better DCR [89.3%vs.68.2%,OR=4.535 (95% CI:1.285-16.011),P=0.019] in the multivariate logistic regression analysis (Supplementary Table S5).No differences in ORR/DCR were observed in comparisons with different localization types,tumor volumes,necrosis proportions,and ASER-peri values.
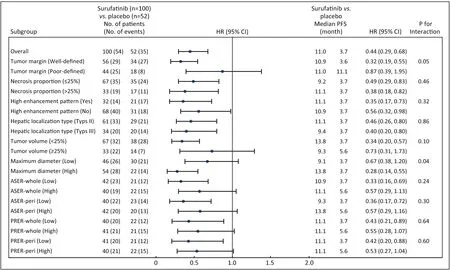
Figure 2 Forest plot of subgroup analysis in imaging characteristics and PFS assessed by the investigators.ASER,arterial standardized enhancement ratio;PRER,portal venous relative enhancement ratio;HR,hazard ratio;95% CI,95% confidence interval;PFS,progression-free survival.
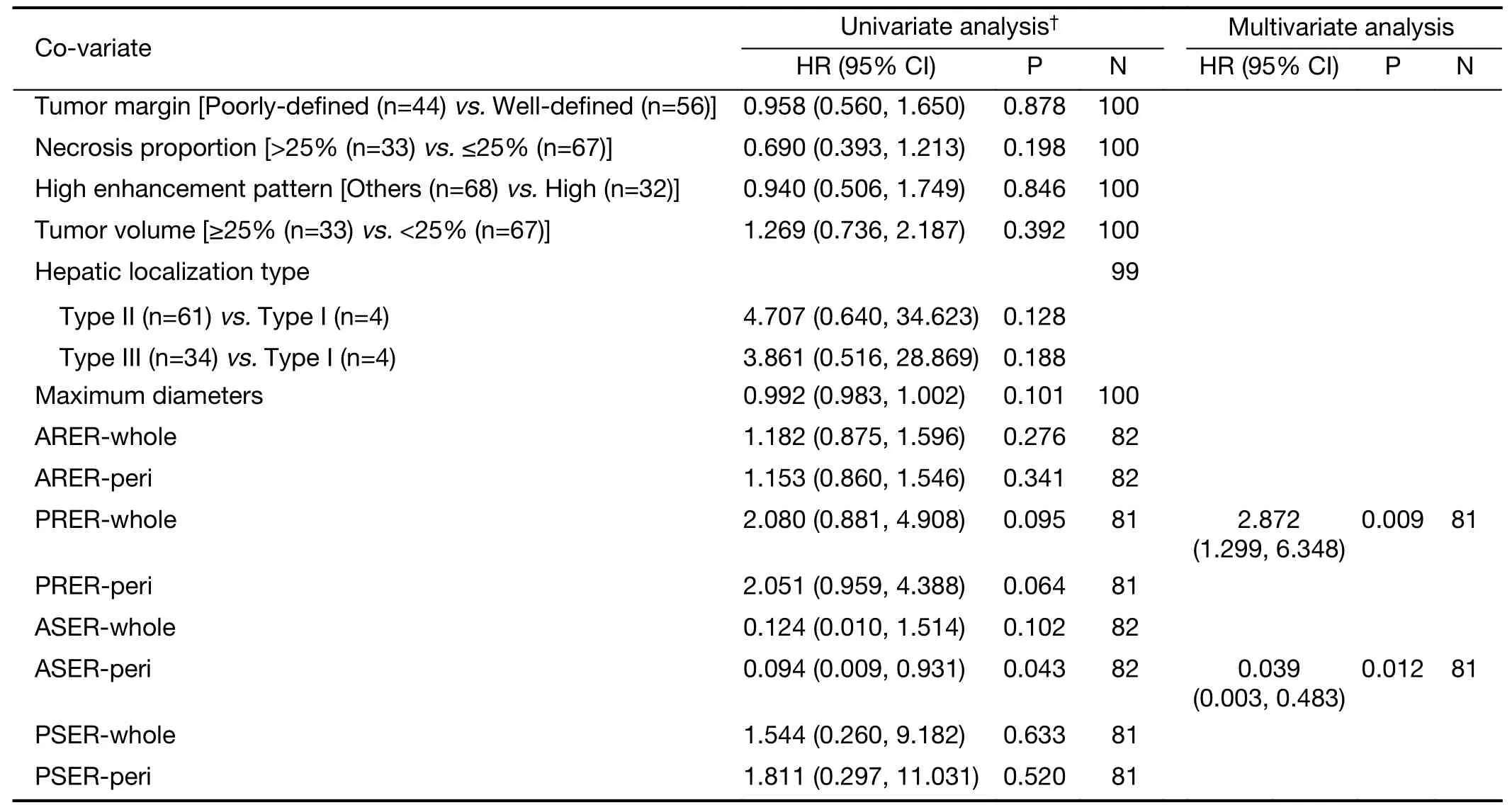
Table 2 Cox proportional hazards regression models of variables associated with PFS outcomes in surufatinib
Discussion
Although surufatinib provided significant clinical benefit regarding the PFS of PNELM patients,more precise methods are warranted to distinguish non-beneficiaries.We utilized blood-supply-associated imaging parameters as markers for surufatinib to evaluate its potential in PNELM.The results suggested that high ASER-peri,high enhancement patterns,and well-defined tumor margins could be predictors for better PFS and optimal ORR/DCR in surufatinib-treated patients.
We hypothesized that high blood-supply pre-treatment CECT parameters might correlate with the response to surufatinib,because vascularization positively correlated to efficacy,lesions with high blood supply may provide greater access to anti-angiogenesis agents (surufatinib) as well as markers of tumors with well-developed tumor vessels (9).Some identified radiological predictors,including the pre-treatment enhancement ratio (ER),have already been associated with treatment effectiveness(20,22).Such quantitative indexes have advantages in standardization for precise clinical practice and future research.Studies have mainly focused on enhancement patterns,which had positive relationships with promising objective responses in patients treated with antiangiogenesis agents (23).Volume of lesion enhancement and tumor micro-vessel density were also significant prognostic factors (21,22,24,25).The baseline CT arterial enhancement pattern/fraction also improved the PFS and response in patients with transcatheter arterial embolization,chemotherapy,radioembolization,or surgery(13,15,19,26-29).
We demonstrated that blood supply-associated radiological features (high ASER-peri and low PRERwhole values) revealed PFS benefit in the surufatinib group,which is applicable for future treatment judgement.Given the necessity to exclude the interference of differentscan conditions,we employed standard enhancement ratios.Among these parameters,the peri-lesion enhancement reflecting the blood supply may have similar practical value to whole-lesions.The measurement of central-lesion parameters,however,was inferior to that of peri/wholelesions (30,31),which may be due to the core of the lesions being filled with necrosis components and introducing some miscalculation.The ASER-peri,thereafter,is affirmed as the ideal precise measurement because it was positively correlated to the blood supply.
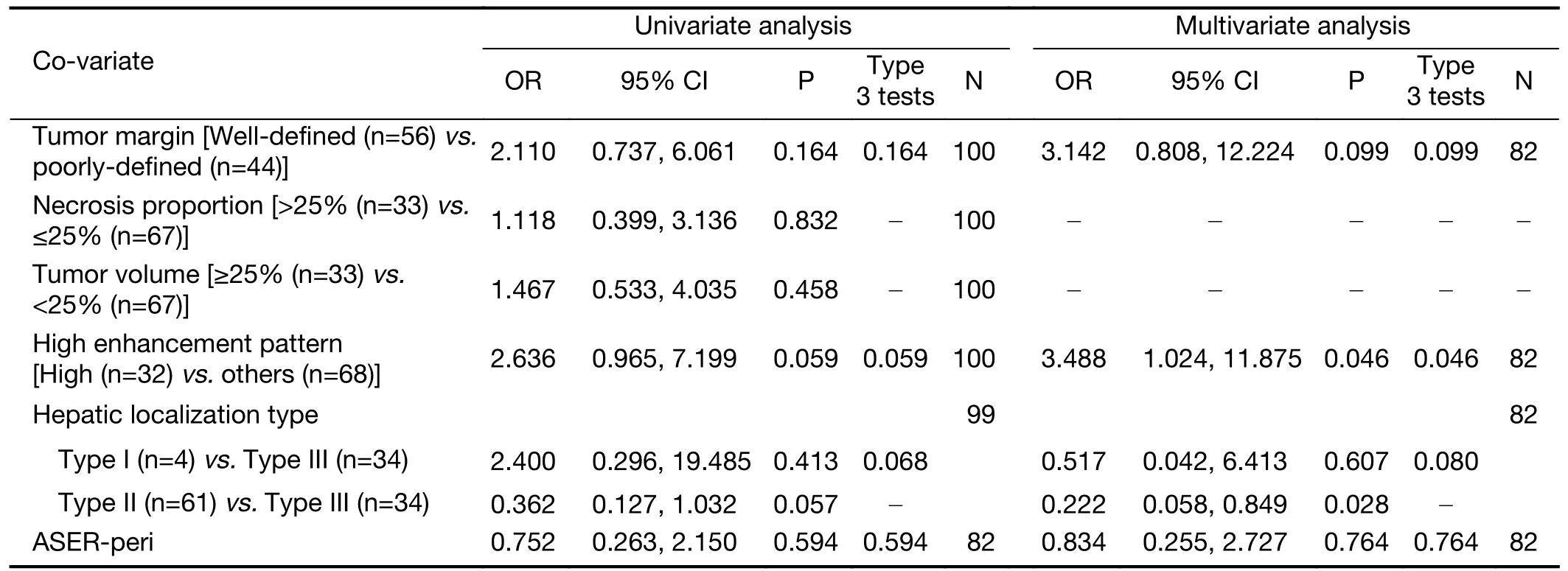
Table 3 Logistic regression of variables associated with ORR in surufatinib groups
Patients with well-defined margins and high enhancement patterns were more likely to have higher ORR (32).We observed the in-concordance of efficacy by imaging markers between ORR and PFS,which might be caused by the imperfect association between the PFS and ORR benefit generated from the slow progression nature of pNET (long duration SD was calculated as PFS benefit,but was not recorded as remission).
Our study had some noteworthy strengths.First,all data were collected from a multicenter double-blinding phase III clinical trial,excluding commonly-seen heterogeneity in retrospective cohorts.Second,we analyzed the data with large samples to increase the reliability.We revealed that ordinary pre-treatment CECT assessments can be extraordinary prognostic parameters for PNELM patients.Furthermore,the major populations were G2 patients,eliminating common pathological confounding factors(13,33,34).
Our study had some limitations.The analysis of CT scans was performed in different centers,which introduced variability in the measurement of parameters.Additionally,subjectivity was present in manual procedures such as defining the ROI on CT images.In the future,artificial intelligence might offer solutions to address these challenges and ensure more consistent and objective measurements (15).
Conclusions
Our study emphasized the association between pretreatment high blood-supply radiological parameters of tumors and the efficacy of surufatinib in patients with PNELM.Specifically,high ASER-peri emerged as a significant factor that benefited the PFS.Moreover,a high enhancement pattern and well-defined tumor margins were associated with a better ORR and DCR in these challenging cases of refractory PNELM.These pretreatment CECT characteristics have the potential to enhance patient selection and contribute to improving management strategies for PNELM.
Acknowledgements
None.
Footnote
Conflicts of Interest: Yujie Yang,Haoyun Shi,Xian Luo,Songhua Fan,and Weiguo Su are employees of HUTCHMED Limited and report personal fees from HUTCHMED Limited both during and outside the conduct of the study.The other authors have no conflicts of interest to declare.
 Chinese Journal of Cancer Research2023年5期
Chinese Journal of Cancer Research2023年5期
- Chinese Journal of Cancer Research的其它文章
- OpenNAU: An open-source platform for normalizing,analyzing,and visualizing cancer untargeted metabolomics data
- Genetic abnormalities assist in pathological diagnosis and EBVpositive cell density impact survival in Chinese angioimmunoblastic T-cell lymphoma patients
- A novel multimodal prediction model based on DNA methylation biomarkers and low-dose computed tomography images for identifying early-stage lung cancer
- Genetic susceptibility loci of lung cancer are associated with malignant risk of pulmonary nodules and improve malignancy diagnosis based on CEA levels
- Update of latest data for combined therapy for esophageal cancer using radiotherapy and immunotherapy: A focus on efficacy,safety,and biomarkers
- Inspired by novel radiopharmaceuticals: Rush hour of nuclear medicine
