Atomically Dispersed Ruthenium Catalysts with Open Hollow Structure for Lithium–Oxygen Batteries
Xin Chen, Yu Zhang, Chang Chen, Huinan Li, Yuran Lin, Ke Yu, Caiyun Nan,Chen Chen
ABSTRACT Lithium–oxygen battery with ultrahigh theoretical energy density is considered a highly competitive next-generation energy storage device, but its practical application is severely hindered by issues such as difficult decomposition of discharge products at present.Here, we have developed N-doped carbon anchored atomically dispersed Ru sites cathode catalyst with open hollow structure (h-RuNC) for Lithium–oxygen battery.On one hand, the abundance of atomically dispersed Ru sites can effectively catalyze the formation and decomposition of discharge products,thereby greatly enhancing the redox kinetics.On the other hand, the open hollow structure not only enhances the mass activity of atomically dispersed Ru sites but also improves the diffusion efficiency of catalytic molecules.Therefore, the excellent activity from atomically dispersed Ru sites and the enhanced diffusion from open hollow structure respectively improve the redox kinetics and cycling stability, ultimately achieving a high-performance lithium–oxygen battery.
KEYWORDS Atomically dispersed; Open hollow structure; Discharge product; Lithium; Oxygen battery
1 Introduction
Lithium–oxygen (Li–O2) batteries, due to their ultra-high theoretical energy density, have shown enormous application potential in facilitating energy transformation in the future and achieving large-scale energy storage [1–5].However,due to the insolubility and insulation of the discharge product lithium peroxide (Li2O2), the redox kinetics in the battery is slow, exhibiting a large overpotential in the charge and discharge process [6–10].Meanwhile, the insolubility of the Li2O2and the low diffusion efficiency of catalytic molecules, such as Li+and O2, consistently result in inadequate stability [11–16].The aforementioned two challenges pose significant obstacles to the commercialization progress of Li–O2batteries [17–19].Based on this, there is a pressing need to design effective air cathode catalysts that not only possess an adequate number of highly active sites but also demonstrate accessible active sites and enable the rapid transfer of reactant molecules [20–26].
The advantages of single-atom catalysts such as ultra-high atomic utilization and unsaturated coordination of active sites endow them with high catalytic activity, demonstrating significant catalytic advantages in many fields such as fuel cells, water splitting and organic small molecule catalysis[27–35].In Li–O2battery, some single-atom catalysts have been studied in recent years, and good results have been achieved in the improving of battery performance [36–40].Among them, the atomically dispersed metal–nitrogen–carbon (M–N–C) catalyst prepared with metal organic frameworks (MOFs), especially zeolite imidazolate frameworks(ZIFs) as the precursor, have been widely used due to their advantages of rich micropores and high specific surface area,but the solid structure reduces the mass activity of catalysts and the accessibility of the internal active site [41, 42].And the microporous structure also limits the diffusion of catalytic molecules.To address these issues, fortunately, the open hollow structure has the capability to effectively expose a greater number of active sites [43–45].For example,Zhuang et al.developed a hollow RuIrOxnano-netcages with three-dimensional structure to achieve efficient pH-universal for overall water splitting [46].Han et al.[47] obtained a hollow, mesoporous, atomically dispersed M–N–C catalysts with enhanced catalytic diffusion, achieving efficient conversion of organic macromolecules.In addition, the hollow structure can also show greater advantages in Li–O2battery, providing sufficient storage space for the generation of Li2O2and improving the mass transfer efficiency of Li+and O2[48].Therefore, based on these considerations, it is reasonable to expect that the design of atomically dispersed catalysts with open hollow structure for Li–O2battery will improve the reaction kinetics effectively and achieve the improvement of battery cycling stability.
Here, we reported a N-doped carbon anchored atomically dispersed Ru sites catalyst with open hollow structure.Using ZIF-8 as the precursor, the internal dead volume was holed out by chemical etching to obtain a cavity of about 200 nm, and the surface was rich in mesopores,which improved the diffusion efficiency of catalytic molecules and realized the rapid mass transfer of electrolyte and oxygen.X-ray absorption spectroscopy (XAS) and aberration-corrected high-angle annular dark-field scanning transmission electron microscopy (AC HAADFSTEM) confirmed the existence of abundant atomically dispersed Ru sites in the catalyst, providing a large number of oxygen adsorption and Li2O2nucleation sites, while the open hollow structure further improved the dispersity and accessibility of the Ru active sites.With the excellent activity from atomically dispersed Ru sites and the enhanced diffusion from open hollow structure, the generation and decomposition efficiency of Li2O2in Li–O2battery was improved, a large discharge specific capacity and cycling stability of nearly 150 cycles were obtained.This provides more ideas for improving catalyst activity.
2 Experimental Section
2.1 Preparation of Materials
2.1.1 Preparations of ZIF-8
In a typical synthesis [46], 5.6752 g 2-methylimidazole was dispersed in 87.2 mL deionized water containing 17.5 mg hexadecyl trimethyl ammonium bromide (CTAB)labeled as solution A.0.3626 g Zn(NO3)2·6H2O was dispersed in 12.5 mL deionized water labeled as solution B.Under stirring, solution B was injected into solution A,and the mixture was stirred for 5 min and then left at rest at 28 °C for 3 h.The products were obtained by centerfugation, washed several times with deionized water, and dried.
2.1.2 Preparations of h-ZIF-8
The ZIF-8 obtained above was dispersed in 20 mL ethanol.350 mg tannic acid (TA) was dissolved in 300 mL of deionized water and ethanol (1:1 volume of the two mixed solutions).Then, stirring at room temperature for 10 min.
2.1.3 Preparations of s-NC and h-NC
s-NC was obtained by annealing the ZIF-8 at 900 °C for 2 h in the atmosphere of nitrogen.h-NC was obtained by annealing the H-ZIF-8 at 900 °C for 2 h in the atmosphere of nitrogen.
2.1.4 Preparations of h-RuNC, s-RuNC and h-RuNPNC
h-NC powder was dispersed in 20 mL of isopropanol, and then Ru solution with 2% mass fraction was dissolved in 5 mL of isopropanol.The Ru solution was added drop by drop to the dispersion of h-NC under untrasound and continued for 30 min, follwed by stirring for 12 h.Centrifuge the precipitate, wash with isopropanol for 3 times, and dry.Then, the powder annealed at 500 °C for 2 h in the atmosphere of nitrogen, and obtained h-RuNC.The synthesis of s-RuNC was similar to that of h-RuNC, except that h-NC was replaced by s-NC.The synthesis of h-RuNPNC was similar to that of h-RuNC, only 5% Ru solution was added.
2.2 Characterizations
The crystal phases were identified using powder X-ray diffractometer (XRD) on a Phillips X’pertProMPD with Cu Kα radiation.Catalyst morphology was obtained using scanning electron microscope (SEM) on a S-4800 Hitachi.Transmission electron microscopy (TEM) images were observed on a Hitachi H-800 TEM working at 100 kV.High angle annular dark field scanning TEM (HAADF–STEM) and corresponding energy dispersive X-ray spectroscopy (EDS)images were performed on a Titan 80–300 STEM operated at 300 kV.N2adsorption–desorption measurements were carried out at 77 K, the Brunauer–Emmett–Teller (BET)surface area was calculated using experimental points at a relative pressure ofP/Po= 0.05–1.00.Elemental analysis of Ru in the samples was performed on a Thermo IRIS Intrepid II inductively coupled plasma-optical emission spectrometry(ICP-OES).X-ray photoelectron spectroscopy (XPS) analyses were conducted on ESCALAB 250 Xi X-ray photoelectron spectrometer with Al Kα radiation.Raman spectra were obtained on a microscopic confocal Raman spectrometer(LabRAM Aramis, Horiba Jobin Yvon) with a 532 nm laser.The X-ray absorption fine structure (XAFS) spectra were collected at 1W1B station in BSRF (Beijing Synchrotron Radiation Facility, P.R.China) operated at 2.5 GeV with a maximum current of 250 mA.
2.3 Battery Assembly and Performance
The air electrode was composed of 90% as-synthesized catalyst and 10% polyvinylidene fuoride (PVDF).The total mass of the electrodes is 0.6–0.7 mg.The assembled button battery model was CR2032, and the battery consisted of a lithium foil, glass fiber filter, air electrode and electrolyte(1 M lithium bis(trifluoromethylsulfonyl)imide, LiTFSI dissolved in a tetra(ethylene) glycol dimethyl ether, TEGDME),assembled in a glove box filled with argon gas.The galvanostatic discharge–charge curve was tested on a Neware battery test system at the voltage range of 2.0–4.5 V.
3 Results and Discussion
3.1 Morphological Characterization of h-RuNC
Figure 1a shows a schematic diagram of the design synthesis process of catalysts.We first synthesized ZIF-8 in uniform size (Fig.S1), and then used ZIF-8 as a precursor to hollow out the cube through the action of tannic acid(TA) as surface functionalized and etching agent to obtain a hollow structure, the internal cavity diameter was about 150–200 nm (Figs.1b and S2), denoted as h-ZIF-8.Then,after heat treatment at 900 °C in nitrogen atmosphere for 2 h, hollow nitrogen-doped carbon nanocages were obtained, denoted as h-NC (Fig.S3).Ru sites were loaded on the surface of the hollow nanocages through electrostatic adsorption, denoted as h-RuNC (Figs.1c and S4).It can be observed by EDS images that Ru, C and N are uniformly distributed on the three-dimensional framework in h-RuNC(Fig.1d).The dispersion state of Ru in h-RuNC was further observed by AC HAADF-STEM, and it is found that Ru existed in an atomically dispersed state on h-RuNC, which is the highlight position in the figure (Figs.1e and S5).The above results indicate that we have successfully synthesized nitrogen-doped carbon anchored atomically dispersed Ru cathode catalyst with open hollow structure.XRD patterns also further indicates that h-RuNC is amorphous carbon and there is no crystalline phase of Ru (Fig.1f).The specific surface area of h-NC is 940 m2g–1(Fig.S6), and the surface has a large number of porous structures, and a bimodal pore size distribution of 3.5 and 28 nm, respectively (Fig.1g).In addition, we compared the solid cube that had not been hollowed out by etching agent and denoted as s-NC.The specific surface area of s-NC is 668 m2g–1, indicating that after etching with an etchant, h-NC has a higher specific surface area and generates more abundant pore structures on its surface.
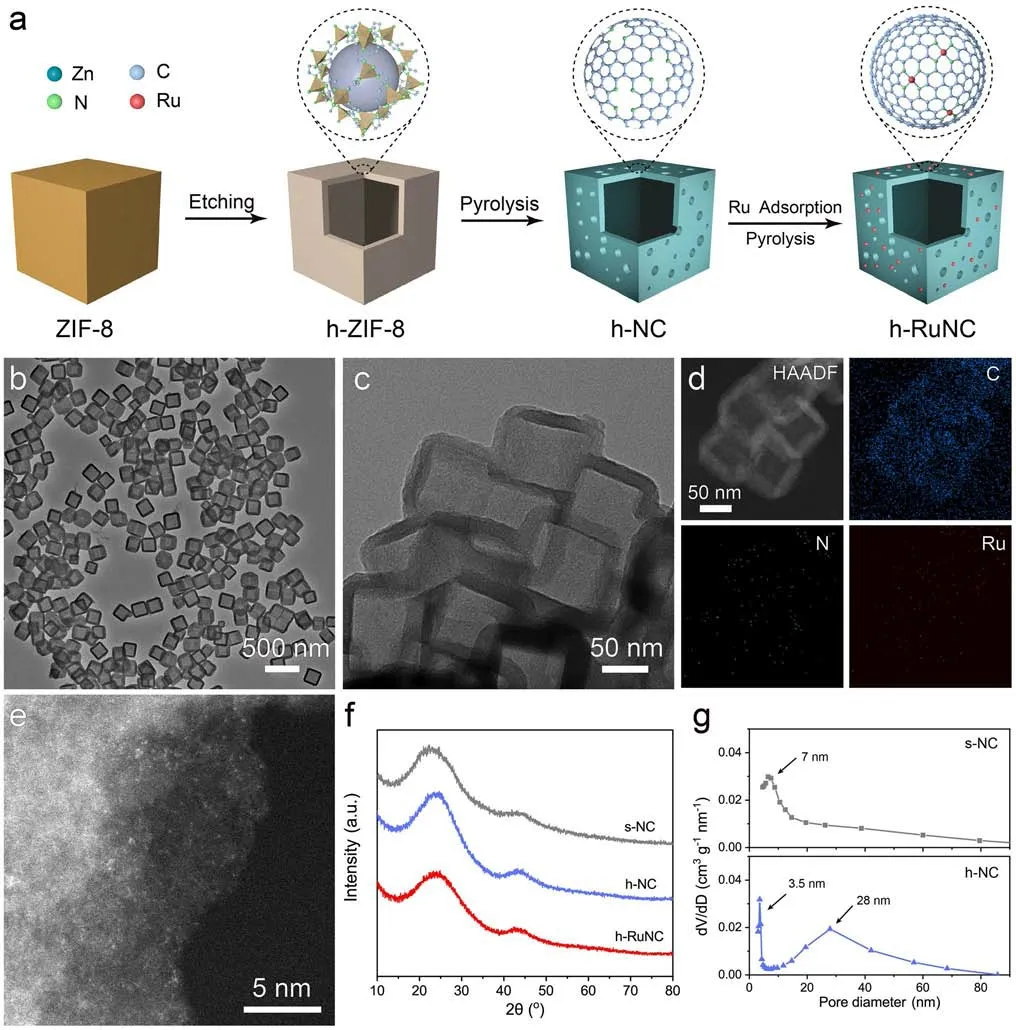
Fig.1 a Schematic illustration of the fabrication of h-RuNC.b TEM image of h-ZIF-8.c TEM image of h-RuNC.d HAADF-STEM and corresponding elemental mapping images of h-RuNC.e AC HAADF-STEM image of h-RuNC.f XRD patterns of catalysts.g Pore size distribution of s-NC and h-NC
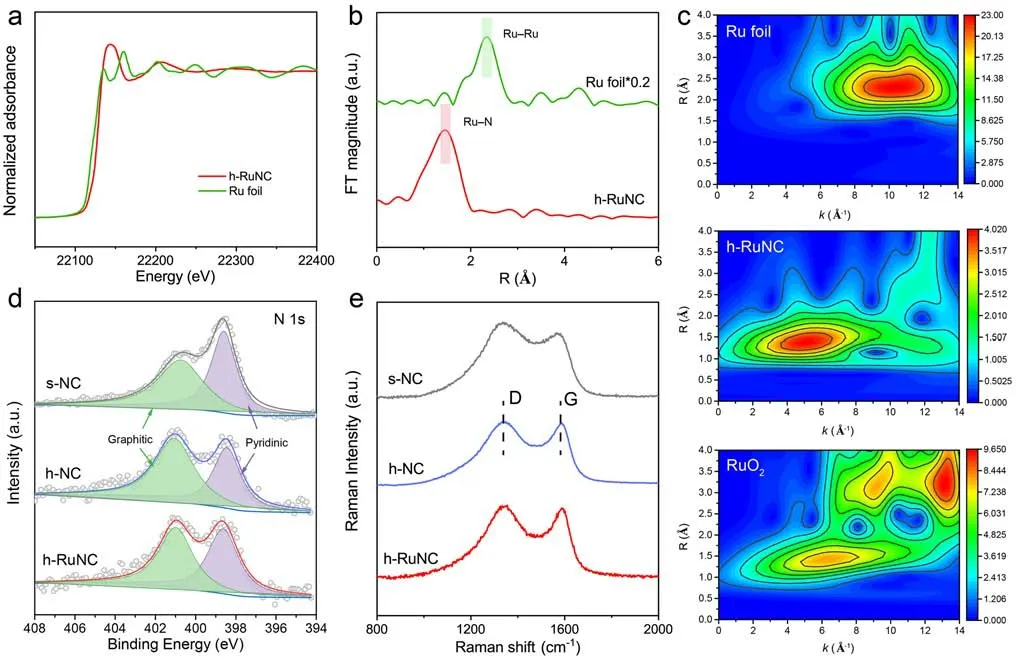
Fig.2 a Normalized XANES spectra of Ru K-edge.b Fourier-transform EXAFS spectra of R space.c Wavelet transform of Ru foil, h-RuNC and RuO2.d XPS N 1s spectra of s-NC, h-NC and h-RuNC.e Raman spectra of s-NC, h-NC and h-RuNC
We further studied the electronic structure and coordination environment of Ru atoms in h-RuNC by XAFS.The X-ray absorption near edge structure (XANES) spectra of Ru k-edge show that Ru atoms are positively charged in h-RuNC (Fig.2a).The extended edge X-ray absorption fine structure (EXAFS) spectrum of h-RuNC has a main peak at 1.4 ?, corresponding to the first coordination shell of Ru–N [29], and no obvious Ru–Ru peak, indicating that Ru is atomically disperse anchored on the carrier (Fig.2b).Then, the wavelet transform (WT) analysis of EXAFS signal of Ru K-edge (Fig.2c), it can be observed that the maximum intensity at 2.3 ?–1belongs to Ru–Ru in the spectrum of Ru foil [49], the maximum intensity at 1.4 ?–1belongs to Ru–O in the spectrum of RuO2, and the maximum WT contour of h-RuNC assigned to Ru–N contribution, further confirming the atomic dispersion state of Ru.EXAFS fitting reveals the coordination environment of Ru in h-RuNC, it could be deduced that each Ru atom is coordinated with four N atoms, forming the RuN4site (Fig.S7 and Table S1).Next, we characterized the surface electronic structure of these catalysts by XPS, and Ru, N, C and O elements have obvious signals (Fig.S8).In the N 1sspectra (Fig.2d), two well-separated peaks are shown, corresponding to graphite N (401.1 eV) and pyridine N (398.5 eV), respectively [50].Surprisingly, we find that the ratio of graphiteN/pyridineNin s-NC is 1.04.However, after modification with etchants, the ratio of graphiteN/pyridineNin h-NC is 1.43, the proportion of graphiteNin h-NC is significantly increased,becoming the dominant type of N species.Therefore, after modification with etchants, not only does it remove the dead volume inside the cube, but it also regulates the type ofNspecies.After the further introduction of Ru, a high proportion of graphiteNwas still maintained.As shown in Fig.S9 of Ru 3pspectrum, the oxidation state of Ru in h-RuNC is positively charged.The C 1sspectra show that the carbon structure does not change significantly during etching agent treatment and Ru loading (Fig.S10).In addition, Raman spectroscopy shows that the proportion of D and G peaks in h-NC and h-RuNC decreased after etching agent treatment,indicating a better graphitic structure and higher conductivity, which is conducive to the electron transfer for charging and discharging processes (Fig.2e).
In order to further understand the catalytic active center,we loaded a higher content of Ru on the surface of h-NC,denoted as h-RuNPNC (Fig.S11).The introduction of a high load of Ru did not significantly affect the structure of h-NC,and the crystalline phase of Ru was not shown in XRD (Fig.S12).Therefore, we further observed h-RuNPNC through HAADF-STEM (Fig.S13), and found that the content of Ru on its surface increased.Ru, C and N were uniformly distributed on the three-dimensional framework, and Ru particles were observed in h-RuNPNC.The content of Ru in h-RuNC and h-RuNPNC was 1.24% and 2.70%, respectively,by ICP-OES analysis (Table S2).
3.2 Electrocatalytic Performance
This series of catalysts were applied to Li–O2battery to investigate the effect of catalyst structure and active site on the performance of batteries.The full discharge in Fig.3a shows that after modification by etching agent,the discharge capacity of h-NC is significantly improved,which is the result that we can foresee, because the cavity in h-NC can provide more storage space for discharge products Li2O2, and the discharge specific capacity of Li–O2battery is achieved by the reaction of lithium ion and oxygen to generate Li2O2.The more Li2O2can be generated, the higher the discharge capacity.In addition,h-NC greatly increases the surface quantity and unit mass activity of the catalyst due to the removal of internal dead volume.h-RuNC obtained more active sites due to the introduction of atomically dispersed Ru, and the discharge specific capacity was further increased.However, the discharge specific capacity of h-RuNPNC decreased after the formation of Ru particles.Then the current density was increased to 500 mA g-1, h-RuNC still shows a high discharge capacity (Fig.3b).When the current density is 200 mA g–1and the limited capacity is 500 mAh g–1, the first charge and discharge curve show that h-RuNC has the lowest charge and discharge overpotential, showing good reversibitliy (Fig.3c).Subsequently, we investigated the long-term stable cycling of the battery.By observing the turn-by-turn charge–discharge curves (Fig.3d-f), we found that the overpotential of s-NC increased rapidly,h-NC could delay the growth of overpotential to a certain extent, and h-RuNC could effectively control the groth of overpotential.This indicates that the formation of open hollow structure and mesopores can accelerate the diffusion efficienty of the catalytic molecules and improve the reaction rate of the discharge process, while h-RuNC further introduces atomically dispersed Ru sites with high catalytic activity, effectively improving the catalytic decomposition ability of Li2O2.The final cycling results showed that h-RuNC could stably cycle for nearly 150 times, while s-NC cycles for 60 times, h-NC cycles for 90 times, and h-RuNPNC cycles for less than 100 times(Fig.3g, h).
In addition, we tested the cyclic voltammetry curves of the catalyst before and after the introduction of atomically dispersed Ru sites (Fig.S14), and found that the peak current intensity of h-RuNC invreased significantly in oxygen reduction reaction (ORR) and oxygen evolution reaction (OER), indicating that the introduction of atomically dispersed Ru sites had significantly improved the catalytic activity of the catalyst.We further validated the advantages of open hollow structure by loading Ru with the same content as h-RuNC on solid s-NC, denoted as s-RuNC (Fig.S15).Through XAFS, it could be seen that Ru also exists in atomically dispersed state in s-RuNC(Fig.S16), which is consistent with the XRD result in Fig.S15b.By observing the cycling curves of battery based on s-RuNC, we find that the overpotential of s-RuNC is larger than that of h-RuNC, and the number of cycles also decreased, which can cycle for 100 times (Fig.S17).This shows that the open hollow structure has an impact on the diffusion of catalytic molecular, which in turn affects the cycling of the battery.The electrochemical activity of s-RuNC has also been significantly improved compared to s-NC, indicating the importance of atomically dispersed Ru for the improvement of activity.We also observed the morphology of h-RuNC after 150 cycles.The three-dimensional strucutre of the catalyst remained intact, with the majority Ru still existing in an atomically dispersed state,while a small amount of Ru was also agglomerated into clusters (Fig.S18).

Fig.3 a Full discharge/charge curves of as-synthesized catalysts at the current density of 200 mA g–1.b Comparison of discharge capacities of different catalysts at different current densities.c First discharge/charge curves of as-synthesized catalysts.Discharge/charge curves of d s-NC, e h-NC and f h-RuNC.g Plots of medium voltage against cycling number.h Cycling performances of the as-synthesized catalysts
3.3 Morphology of Discharge Product
Furthermore, we observed the morphology and phase of the discharge products.Firstly, the discharge products catalyzed by these catalysts were characterized by XRD (Fig.S19),and it was found that the discharge products were Li2O2.Subsequently, we observed the morphology of Li2O2and conducted ex-situ SEM analysis on the surface of the electrodes with different Li2O2(Figs.4 and S20).Firstly, the catalyst, KB and binder can be observed on the surface of pristine electrode (Fig.4a, d, g).After the discharge process,the discharge products can be clearly observed on the surface of these catalyst electrodes.With the extension of the discharge depth, the discharge products further grow.The discharge products catalyzed by s-NC and h-NC show a typical toroidal shape (Fig.4b, e), while the discharge products catalyzed by h-RuNC show a film shape with higher dispersion(Fig.4h).Therefore, after subsequent recharging, the discharge products generated by h-RuNC are more easily and efficiently decomposed (Fig.4c, f, i) [51, 52].It is precisely due to the efficient decomposition of discharge products that the cycling stability of battery has been significantly improved.We further analyzed the electronic structures of the discahrge products on the electrode surfaces after the discharge process of these catalsyts using XPS.As shown in Fig.S21, in addition to the main discharge product Li2O2,a small amount of Li2CO3was also produced.However, it is obvious that among several catalysts, the proportion of byproduct Li2CO3in the discharge product of h-RuNC is the least.Thus, the h-RuNC catalyst can decompose efficiently the discharg product and maintain a long-term stable cycle of the battery.

Fig.4 SEM images of the cathodes after discharge and recharge a-c s-NC.d-e h-NC.g-h h-RuNC at different states.a, d, g pristine, b, e, h after full discharge, c, f, i after recharge.j Schematic diagram of catalytic activity enhancement from s-NC to h-NC to h-RuNC
On the whole, there are a large number of atomically dispersed Ru sites on the surface of h-RuNC, which can adsorb a large amount of oxygen during the discharge process, providing more nucleation sites for Li2O2, improving the discharge specific capacity of the battery, and guiding the discharge products to grow into a film shape with higher dispersion through surface growth mode; Subsequently, during the charging process, the discharge products are more easily decomposed, resulting in a low charging overpotential for the battery, improving the performance of the battery.Conversely, the discharge products of other catalysts continuously migrate on the catalyst surface and grow into large toroidal shape, resulting in poor decomposition efficiency during charging.
4 Conclusions
In summary, we obtained N-doped carbon anchored atomically dispersed Ru with open hollow structure as cathode catalyst by etching strategy.By creating open hollow structures and regulating the dispersion state of Ru on N-doped carbon, h-RuNC exhibited superior electrochemical performance compared to Ru particle (h-RuNPNC) and solid structure (s-RuNC) in Li–O2battery, and could cycling stably for nearly 150 cycles.The open hollow structure can improve the diffusion efficiency of catalytic molecules, which is very suitable for the mass transfer process of Li+and O2in Li–O2battery, and improve the accessibility of active sites of the catalyst.And through the analysis results of XPS, we found that after the etching agent treatment, the type of N in the carrier has a regulatory effect, which can increase the proportion of graphite N.The large number of atomically dispersed Ru sites also improved the formation and decomposition efficiency of discharge products effectively, and the battery also achieved a discharge specific capacity of up to 1000 mAh g–1.Thanks to the dual optimization of structure and active site (Fig.4j), h-RuNC has excellent performance in Li–O2battery, which provides an important idea for optimizing the application of high-performance atomically dispersed M–N–C catalysts in Li–O2battery.
AcknowledgementsThis work was supported by National Key R&D Program of China (2021YFF0500503), National Natural Science Foundation of China (21925202, U22B2071), International Joint Mission on Climate Change and Carbon Neutrality.
FundingOpen access funding provided by Shanghai Jiao Tong University.
Declarations
Conflict of interestThe authors declare no conflict of interest.
Open AccessThis article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing,adaptation, distribution and reproduction in any medium or format,as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material.If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.To view a copy of this license, visit http:// creat iveco mmons.org/ licen ses/ by/4.0/.
Supplementary InformationThe online version contains supplementary material available at https:// doi.org/ 10.1007/s40820- 023- 01240-0.
- Nano-Micro Letters的其它文章
- Coaxial Wet Spinning of Boron Nitride Nanosheet-Based Composite Fibers with Enhanced Thermal Conductivity and Mechanical Strength
- Deep Insight of Design, Mechanism, and Cancer Theranostic Strategy of Nanozymes
- Internal Polarization Field Induced Hydroxyl Spillover Effect for Industrial Water Splitting Electrolyzers
- Flexible and Robust Functionalized Boron Nitride/Poly(p-Phenylene Benzobisoxazole) Nanocomposite Paper with High Thermal Conductivity and Outstanding Electrical Insulation
- Exploring the Roles of Single Atom in Hydrogen Peroxide Photosynthesis
- Advances of Electrochemical and Electrochemiluminescent Sensors Based on Covalent Organic Frameworks

