Advances in Zika virus vaccines and therapeutics: A systematic review
Shiza Malik ,Khalid Muhammad ,Omar Ahsan ,Muhammad Tahir Khan ,Ranjit Sah ,Yasir Waheed0?
1Bridging Health Foundation,Rawalpindi 46000,Pakistan
2Department of Biology,College of Sciences,UAE University,15551,Al Ain,United Arab Emirates
3Department of Medicine,Foundation University Medical College,Foundation University Islamabad,Islamabad 44000,Pakistan
4INTI International University,Persiaran Perdana BBN Putra Nilai,71800 Nilai,Negeri Sembilan,Malaysia.
5Institute of Molecular Biology and Biotechnology,the University of Lahore,KM Defence Road,Lahore 58810,Pakistan
6Zhongjing Research and Industrialization Institute of Chinese Medicine,Zhongguancun Scientific Park,Nanyang 473006,China
7Department of Microbiology,Tribhuvan University Teaching Hospital,Institute of Medicine,Kathmandu 44600,Nepal
8Department of Microbiology,Dr.D.Y.Patil Medical College,Hospital and Research Centre,Dr.D.Y.Patil Vidyapeeth,Pune 411018,Maharashtra,India
9Department of Public Health Dentistry,Dr.D.Y.Patil Dental College and Hospital,Dr.D.Y.Patil Vidyapeeth,Pune 411018,Maharashtra,India
10Gilbert and Rose-Marie Chagoury School of Medicine,Lebanese American University,Byblos 1401,Lebanon
ABSTRACT Zika virus (ZIKV) is the causative agent of a viral infection that causes neurological complications in newborns and adults worldwide.Its wide transmission route and alarming spread rates are of great concern to the scientific community.Numerous trials have been conducted to develop treatment options for ZIKV infection.This review highlights the latest developments in the fields of vaccinology and pharmaceuticals developments for ZIKV infection.A systematic and comprehensive approach was used to gather relevant and up-to-date data so that inferences could be made about the gaps in therapeutic development.The results indicate that several therapeutic interventions are being tested against ZIKV infection,such as DNA vaccines,subunit vaccines,live-attenuated vaccines,virus-vector-based vaccines,inactivated vaccines,virus-like particles,and mRNA-based vaccines.In addition,approved anti-ZIKV drugs that can reduce the global burden are discussed.Although many vaccine candidates for ZIKV are at different stages of development,none of them have received Food and Drug Authority approval for use up to now.The issue of side effects associated with these drugs in vulnerable newborns and pregnant women is a major obstacle in the therapeutic pathway.
KEYWORDS: Zika virus;Infection;Therapeutics;Antiviral agents;Vaccines;Therapies;Treatment;Novel therapeutic;Clinical management
1.Introduction
Despite extensive research and development in the fields of vaccinology,pharmaceuticals,and therapeutics,infectious disease agents continue to pose a significant threat.The COVID-19 pandemic has specifically highlighted the need for the scientific authorities all over the world to take a mitigative and proactive approach against widespread infectious viruses before they escalate into global pandemics[1,2].ZIKV infection is one such concern,in terms of the serious health problems it takes,especially in the susceptible population such as pregnant women,newborns,and the elderly[3].It is believed to be the major causative agent of microcephaly and autoimmune disorders in infants[3,4].It mainly exhibits clinical symptoms including rash,fever,headaches,dizziness,malaise,anorexia,and stomach ache like many other viral infections[5].However,the links with neurological complications in newborns and adults are specific characteristics associated with Zika virus infection[5,6].
ZIKV is a zoonotic single-strandedFlavivirusthat belongs to the family Flaviviridae.It is transmitted to people primarily through the bite of an infectedAedesspecies mosquito (Ae.aegyptiandAe.albopictus).It was first isolated from the blood of theRhesusmonkey and theAe.africanusmosquito in the mid-20th century[7].This isolation not only indicated its zoonotic origins but also provided insights into its transmission routes and infectious vector,in the form of theAedesmosquito,similar to the dengue virus[1,8].Scientists also discovered ZIKV-specific antibodies in the blood of people living in forest regions where the ZIKV vectors are common.Apart from mosquito bites,ZIKV can also spread through sexual transmission,blood transfusion,and the exchange of body fluids like breast milk[1,2,7].
The global presence of ZIKV has been confirmed through isolates from various origins on different continents.Recent outbreaks of Zika infection in the past decade in different regions of the globe are of major concern to the scientific community[8,9].ZIKV has been associated with numerous medical conditions,including Guillain-Barré-syndrome,congenital malformations,and microcephaly.The World Health Organization has identified ZIKV as a global public health concern,with cases reported from more than 60 countries[8].
Despite the low mutation rates and strain diversity of the virus,currently there are no specific treatment options for ZIKV[10-12].However,efforts are underway to develop vaccines and therapeutics by following similar pathways of therapeutic development that have been used for other infectious viral agents,such as dengue (DENV),Ebola,and HIV,etc.[13,14].It is predicted that insights gained from these studies into the molecular targets and vaccination designs will contribute to the development of a successful vaccine and therapeutic candidates.Hence,trials are ongoing to create efficient therapeutic and vaccination options for the mitigation of ZIKV infection[15,16].This article discusses effective vaccination and therapeutic interventions against ZIKV infection.
2.Methodology
A methodological search strategy was adopted to gather data from online sources,including Google Scholar,PubMed,National Library of Medicine,Web of Science,European Database,Springer,and Embase databases.Statistical results were obtained from the official websites of the World Health Organization,National Library of Medicine,Centers for Disease Control and Prevention,and Food and Drug Administration.We also thoroughly consulted and searched through the official website https://clinicaltrials.gov/ in order to incorporate information regarding the latest ongoing and completed clinical trials in our study.The study included original research articles,sections from books,letters to the editors,short and lengthy reviews,and some published recently case studies.
The search terms mainly included Zika virus;Zika virus infection;ZIKV transmission,ZIKV epidemiology,ZIKV therapeutics;antiviral agents against ZIKV;vaccines;therapies;treatment;novel therapeutics;clinical management and future prospects related medication for ZIKV.After thorough analysis of the data,abstracts,titles,and journals of the research publications were included in this review.The information gathering covers a wide range of sources including original research articles,reviews,short commentaries,case reports,and letters to the editors.The search strategy focus on incorporating data from recent years especially past five years(2019-2023) to add only the most recent advances related to ZIKV vaccination efforts.Moreover,the studies of mostly English origin have been included in this review for analysis (Figure 1).

Figure 1.Flowchart for the literature slection process.
3.Zika virus
As there are currently no approved and specific drugs for ZIKV,preventive measures such as increased liquid intake and rest are initially recommended[14].However,in the case of severe illness states and when infection prevalent beyond control,some therapeutic options could be considered with proper consultation from doctors[17].This section will discuss the data gathered from different studies to elaborate on the development efforts behind therapeutics and vaccines for ZIKV.It will also outline ongoing vaccine trials and therapeutic options for ZIKV[15,18-26].
3.1.Outlining vaccine development for ZIKV
Several vaccination trials are being conducted worldwide againstFlavivirusinfection,including vaccines against yellow fever virus,tick-borne encephalitis virus,Japanese encephalitis virus,and DENV[23,24,27].Following a similar pathway,efforts are underway to develop vaccines against ZIKV.Several studies predicted that inactivated and attenuated vaccines could also be used against ZIKV infection[28,29].However,the limited information regarding ZIKV biology,immune response,host interaction,and disease development mechanism poses challenges to vaccination efforts[9,11].Otherattractive approaches may include subunit vaccines,DNA vaccines,and viral vector-based vaccines that utilize the structural proteins derived from ZIKV[14,28,29].Given the seriousness of Zika infection,it is crucial for the scientific community to offer an effective vaccine against ZIKV or better disease management.Approximately 50 candidate vaccine trials for ZIKV have been conducted in recent years;some of them have been completed while others are currently underway[23,29,30].This section will briefly outline different vaccination approaches,their mechanism of action,and ongoing vaccines trials for ZIKV.Important examples and ongoing trials are presented in Table 1 and Figure 2.

Table 1.Summary of vaccine candidates currently in clinical trials against Zika virus (ZIKV).
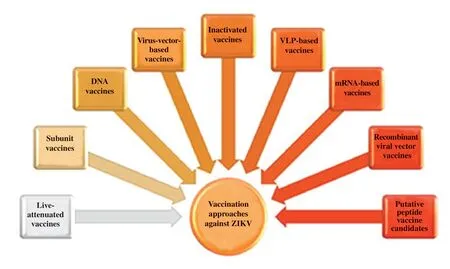
Figure 2.Vaccination approaches under trial against Zika virus.
3.1.1.Attenuated candidate vaccines
Virus particles are rendered ineffective through chemical or traditional treatments,reducing the infectivity yet retains the immunogenicity of the viral pathogens[31].Inactivated virus particles are directly administered to stimulate multiple antigenic targets,activating various immune system cells and leading to the production of neutralizing antibodies against ZIKV[32].
3.1.2.DNA vaccines
Genes of interest,such asprM/Egenes and viral segments,are incorporated into the plasmid structure.The plasmids are then transferred to the host cell,which takes up the DNA through antigenpresenting cells[29,34].The expression of viral genes leads to the production of targeted antigens and immunoglobulins,cytokines,and neutralizing antibodies,among other immune system responses against specific viruses[35].DNA vaccine trials against ZIKV have been conducted to evaluate the efficacy and safety of such vaccines in preventing ZIKV infection.Several research groups and pharmaceutical companies have initiated clinical trials to test DNA vaccines against Zika.These trials involve administering the DNA vaccine to participants and monitoring their immune response and subsequent protection against ZIKV virus infection development[40-45].These trials and others have shown promising results in terms of inducing an immune response against ZIKV virus.However,further research is required to evaluate the long-term efficacy and safety of these vaccines in larger populations.
3.1.3.mRNA vaccine candidates
These vaccines use the open reading frame of RNA to encode the genes of interest.Upon entry into the host cells,these vaccine candidates help to activate the host immune system to translate specific antibodies upon detection of viral antigens in the immune system.mRNA vaccines are considered superior to DNA vaccines as they do not integrate into the host’s genetic material and do not cause mutations[33].mRNA vaccines work by delivering a small piece of mRNA into cells,containing instructions to produce a specific viral protein that stimulates an immune response in the body[48].The mRNA vaccine is injected into the body usually through an intramuscular shot.Once inside the body,the mRNA molecules are taken up by cells,particularly antigen-presenting cells,such as dendritic cells.The mRNA is then transported to the cell’s cytoplasm where it is translated into viral proteins by the cell’s own ribosomes[46,48].For example,in the case of ZIKV,structural proteins such as proteins C,prM,and E are primarily utilized to design potential vaccines candidates that enhance the antigenic expression and regulate the immunological response.The synthesized viral protein is then processed by the cell and presented on its surface using major histocompatibility complex (MHC)molecules[41].
The presented viral protein acts as an antigen,stimulating the immune system to recognize it as foreign and triggers an immune response.Adaptive immune cells (B-cells) produce large amounts of virus-specific antibodies[25].Additionally,the viral protein presentation activates other immune cells,such as T cells,which play a role in cell-mediated immunity,including the killing of infected cells.After the immune response,some activated B and T cells transform into memory cells that remember the virus’s antigen,providing long-term immunity.Viral structural genes and proteins are used to create mRNA-based therapeutics.The mRNA technology has shown potential in the development of vaccines against various diseases,including COVID-19,due to its ability to rapidly produce targeted immune responses.mRNA vaccines,like the Pfizer-BioNTech and Moderna COVID-19 vaccines,utilize a lipid nanoparticle (LNP) delivery system to protect and deliver the fragile mRNA molecules into cells.LNPs are composed of lipid molecules that form a protective shell around the mRNA,allowing it to enter cells more efficiently and trigger an immune response.Scientists are working to develop similar mRNA-based vaccine technology against the Zika virus or other similar pathogens,improvising the use of LNPs or other delivery systems for efficient delivery into cells.One such example is the vaccine approach involving the use of lipid nanoparticles to encapsulate genetically modified mRNA(mRNA-LNP)[41,48].This modified mRNA carries the genetic information for ZIKV structural genes.Through a prime-boost immunization strategy,mice were administered modified mRNA encoding ZIKVprM-Egenes,leading to the production of virus-like particles.This approach included significant levels of neutralizing antibodies (nAbs),providing protection in both immunocompetent mice (C57BL/6) and immunocompromised mice (AG129,lacking interferon alpha/beta/gamma receptors).Thus,the synthetic mRNA machinery serves as a rapid and flexible platform for designing vaccines against ZIKV[41].
3.1.4.Zika virus-like particle (VLP) based vaccine
VLPs are molecular entities that exhibit similar characteristics to virus particles and they do not require chemical inactivation to prevent pathogenic responses in the host.VLPs maintain epitope structures within the immune system[46].They mostly utilize Zika structural proteins such as CprME and non-structural proteins like NS2B/NS3 proteins,among other viral epitopes[47].The remaining viral segments can sometimes automatically assemble into VLPs,which do not express the viral pathogenicity as the original viruses[46-49].
3.1.5.Subunit vectors,viral vectors and inactivated vaccine candidates against the ZIKV
Subunit vector platforms use certain antigenic proteins of the ZIKV for testing in different clinical trials.The envelope gene (E) and domain Ⅲ (EDⅢ) proteins of the ZIKV are attractive candidates in subunit vaccine design[51-53].The ZIKV E protein is composed mainly of three ectodomains (DⅠ,DⅡ,and DⅢ),which refers to a specific region of the viral envelope protein in the virus’s structure and thus often act as the primary target of neutralizing antibodies(nAbs) in host immune system.Specifically,EDⅢ domain plays a crucial role in the virus’s interaction with host cells and the immune response.EDⅢ contains important antigenic sites and is involved in receptor binding and virus entry into host cells[31-35].It is also a major target for neutralizing antibodies and potential vaccine development.In the case of virus-vector-based vaccines,the genetic material of the ZIKV is introduced into another virus that acts as a carrier but is nonpathogenic to the host.Commonly used vector platforms include lentiviruses,retroviruses,and the most commonly knownAdenovirusvectors[38].Purified inactivated Zika vaccines involves killing the original ZIKV particles through heat,UV treatment,chemical or radiational treatment and introducing the virus shells or remaining viral segments into the testing subjects[38,54].
3.2.Some novel vaccination approaches for Zika vaccination
In addition to the traditional vaccination approaches described in the previous section,new scientific approaches are being experimented in different trials to combat Zika infection.These vaccination approaches include development of vaccines by using recombinant viral vectors,and peptides-based vaccine candidates,among others.Details of these vaccines’ approaches are described below.
3.2.1.Recombinant viral vector vaccines
These vaccines use the viral genome of another viral species to carry ZIKV genes,hence the name “recombinant viral vector vaccine”.Adenovirusvector platforms are commonly used for this purpose[36].These vectors function similarly to RNA and DNA vaccination,with the only difference being the genes carried by the vehicle[18].Important examples of ongoing trials include: VRCZKADNA085-00-VP and VRC-ZKADNA090-00-VP,ZIKV wt prM/E,VRC-ZKADNA090-00-VP,ZIKV wt prM/E,Adenovirusserotype 52 vector vaccine (RhAd52–prM-Env vaccine),measles virus-based vaccine (MV-Zika-sE),vesicular stomatitis virus (VSV)-capsid vaccine,live measles virus-Zika (MV-Zika),recombinant(VSV-Zika E260-425) based trials,and stomatitis virus (rVSV)based trials among others[36-45,55].
3.2.2.Putative peptide vaccine candidates
Zika epitopes that specifically correlate with B-cell and T-cell epitopes,as well as MHC epitopes,have been identified to generate specific immune responses in different vaccine protocols[56].Various peptide sequences have been identified in different studies and mapping platforms,which are often checked for developing effective vaccine candidates.Peptide-based vaccination is a novel and more focused approach to design and precisely locating the epitope region of the antigen,eliciting specific immunological responses[57].Multiple epitopes can be combined to create a widespectrum infectious response.Important peptide candidates under investigation include 641 B-cell epitopes and peptide sequences,1458 T-cell epitopes and peptide sequences,6725 MHC class I epitope and peptide sequences and 1631 MHC class Ⅱ epitope and peptide sequences,among others[38,57].
3.3.Therapeutics against ZIKV
Since no vaccine has been approved yet for ZIKV infection,scientists are carefully driving efforts to design effective therapeutics,antiviral drugs,and immunotherapeutic strategies to deal with the threatening ZIKV infection.One strategy adopted by scientists is to test already approved therapeutics with established efficacy against other infectious viruses[14].Hundreds of small interfering RNAs (siRNA),single guide RNAs,and micro RNAs play a role in the infectious cycle of ZIKV,originating from either the virus or the host,and are used to design specific targeted therapies[52,53].By understanding the potential function of these small candidate molecules,disrupted functioning occurs,creating hurdles within the infectious cycle.This new class of therapeutics is rapidly progressing fromin-silcotoin vitrotrials[51,58].The similar characteristics and genetic backgrounds of these viruses make it possible that the therapeutics for one viral species may be effective for others.This strategy has been carried out for HIV,DENV,Ebola,and many other infectious diseases[58,59].Similar approaches have also been carried out for ZIKV in different studies.
Another strategy is the development of complete new drugs against ZIKV infection by properly understanding its genome,viral infectious cycle,host interaction,and infection output in the form of immune responses in hosts[28,58].This category is gradually advancing as more research is conducted on the molecular biology of ZIKV infection.Certain inhibitor compounds are proposed in different studies based on the molecular targets in viral infection and the replication cycle[60].In this regard,the experience gathered during DENV and Ebola drug development can be applied to design effective therapeutics for ZIKV[19,61].However,this is easier said than done in the case of ZIKV infection because it mostly affects pregnant women and newborns,which could create a hurdle for testing the efficacy of proposed therapeutics[3,22,65].
The major purpose of the designed therapeutics is to reduce viremia,symptomatology,and the risk of neurological complications in newborns[28,62].All candidate drugs are in clinical trials and require further work and combination trials before being subjected to a susceptible human population[28].A trial of drug testing is essential since there are several ethical issues regarding the usage of antiviral agents for ZIKV infection in pregnant women and newborns[3,22].Thus,there is a long way before the vaccines can be approved for direct application on women and children.
3.4.Repurposed drugs against ZIKV
Drugs used for other infectious viral diseases,such as DENV and Ebola,are being tested for their efficacy against ZIKV infection.This is a common strategy and thousands of drugs are being tested to determine their potential against ZIKV infection[19,61].A few examples are polymerase inhibitor 7-deaza-2′-C-methyladenosine(7DMA),pyrimidine synthesis inhibitors (e.g.,brequinar),mycophenolate mofetil,daptomycin,and sertraline,emricasan,a pan-caspase inhibitor,sofosbuvir,Alpha-L-Fucose,Ribavirin Monophosphate,S-Adenosyl-L-Homocysteine,25-Hydrocholesterol(25HC),which have previously been tested against HIV,Ebola,and VSV.Galidesivir (BCX4430),an adenosine nucleoside analogue,is also being tested.Most of these drug candidates have already been checked for their established efficacy against other viral infections such as yellow fever,DENV,Ebola,influenza and marburg,among others[28,60-63].
3.5.Homeopathic prescriptions and Ayurveda medicines for the treatment of ZIKV
These durgs are most commonly used against ZIKV infection due to their minimal side effects.The major reason is their established therapeutic efficacy,general wide-scale acceptability and limited or no side effects reported against other infections such as cholera,yellow fever,malaria,DENV,conjunctivitis,and others[28,60,62].This is the best therapeutic option in the case of widespread ZIKV infections.Important examples may include: homeopathic products derived fromEupatorium perfoliatum;Atropa belladonna;andRhus tox,among others[12,53,58,64,65].Similarly,the use of herbal medicines for preventing and healing the infectious outcomes of viral diseases is traditionally broad-spectrum.Their natural origin limits their side effects and makes them safe in human-based trials.Some of the reported compounds against ZIKV infection may includeTinospora cordifoli,andGymnema sylvestre-containing saponins,flavonoids,alkaloids,and sterols with their antiviral effects[40,66,67].
3.6.Immunotherapeutic drug candidates
Immunoregulatory molecules are activated through vaccination or therapeutic treatment to regulate immune responses against ZIKV.These compounds can limit the side effects associated with ZIKV infection.Various studies are being conducted on monoclonal and neutralizing antibody-based therapies against ZIKV infection for their associated prophylactic and therapeutic efficacies[21].A number of monoclonal antibodies (MAbs) against E proteins of ZIKV have been identified to date,exhibiting a neutralizing effect against virus epitopes[18].These MAbs-based therapeutic interventions are a good approach to tackling the ZIKV;however,the issue of viral genomic mutation and escape from immune responses is a great hurdle to establishing effective MAbs-based treatment options[19,21].Thus,the scientific community is working on a kind of therapeutic MAbbased cocktail,similar to the combination therapy to tackle the mutative nature of viruses from multiple streams.Important antibody candidates may include E protein-mediated and NS1 monoclonal antibodies,ZKA190,(2A10G6),ND,(ZKA64),(ZV54/ZV-67),(Z3L1/Z23/Z20),(ZIKV-117) and (Z004),among others[18,19,21].
3.7.Zika vaccination and pregnancy
ZIKV is known to cause congenital syndromes such as microcephaly,spasticity,Guillain-Barré syndrome,ocular abnormalities,craniofacial disproportion,and sometimes even miscarriage in implanted fetuses.Thus,the primary goal for most vaccination trials independent of the vaccination type is the prevention of these congenital syndromes and other adult health syndromes[68].This is to prevent ZIKV infection during pregnancy being transmitted to children and to prevent the sexual transmission of diseases to mutually protect the risk of individuals.Despite pregnant women being the most urgently needed candidates for vaccines,trials on them are certainly limited owing to the risk associated with two lives.For this cause,vaccination trials are being conducted in mouse models,including the targeted small extracellular vesicles (sEVs) encapsulating antiviral siRNA compounds,which have been tested to inhibit ZIKV[69].This vaccine formulation was designed for neuro-specific targetingviaengineering the EVs membrane with lamp2b protein along with fused neuronspecific rabies virus glycoprotein-derived peptide (RVG)[34,69].The administration of this vaccine formulation protected the pregnant mouse models against ZIKV transmission to the fetus owing to the transversing abilities of sEVsRVG-siRNA against the blood-brain barrier and placenta of the mother.Moreover,it also reduced the neurological damage and inflammation linked with ZIKV in model mice.This approach thus provided a sEVs-based targeted system to be further checked in humans for fetus protection against ZIKVrelated secondary infections[68,69].
Similarly,some vaccine trials on inactivated vaccines are also undergoing trial phases on animal models since inactivated vaccines have a long record of safety in pregnant women.One such trial is linked with the purified formalin-inactivated vaccine formulation (ZPIV) derived basically from the 2015 Puerto Rican ZIKV strain (PRVABC59;ZIKV-PR).This vaccine formulation showed effectiveness in animal models and also demonstrated benefits for non-pregnant women by preventing viremia loads along with elucidation of neutralizing antibody and cross-protective B-cell mediated immune responses against Zika and dengue infections[34,71].
A similar inactive ZPIV vaccine trial by name (Takeda’s TAK-426) demonstrated tolerance and a safety potential for creating immunogenicity in bothFlavivirus-naive andFlavivirus-primed adults.Furthermore,a purified inactive virus vaccine candidate along with the Vero-cell-adopted ZIKV strain (GMZ-002) exhibited significant productivity in Vero cell lines[72].In case of administration of a similar formulation in IFNAR1-blocked C57BL/6 mice models,they were shown to be fully protected against the lethal dose challenge with protective effects via robust and persistent immunity development[73].Such clinical trials have paved the way for further assessment of these ZPIV vaccines in pregnant women to protect the fetuses against transmissible ZIKV infections[69].
4.Discussion
To the best of our knowledge,no particular drugs or vaccines have been licensed against ZIKV infection to date.The present therapeutics are only helpful for mitigating infectious symptoms up to a certain level[74,75].The main causes of delay in vaccination may be the susceptible groups of patients,such as pregnant women,newborns,and elderly individuals,all of whom experience immunocompromised conditions[76,77].Another cause is the significant amount of effort required for these infectious diseases,and the limited epidemic nature of the ZIKV may have resulted in a delay in vaccination development.
However,it should be kept in mind that major pandemics of the past were endemic and epidemic for some years,but later several outbreaks were reported for them,which created havoc in the healthcare industry[65,78].Thus,before the ZIKV infection emerges as a pandemic,it is crucial for the scientific community to consult,coordinate and carry out integrated efforts for the development of effective therapies against ZIKV[79,80].Moreover,different modern therapeutic approaches such asin-silicomodeling,viral peptide sequencing trials,proteome-scale screening,genomic studies,immunoinformatic and past trials of vaccination development should be followed to establish a strong vaccine candidate[61,81].Additionally,modern therapeutic interventions such as nanotechnology-based drug additives,drug conjugation protocols,and plant-driven antiviral agents in newly arriving organic products should be experimented against ZIKV infections[58,82].
Further,the scientific community should follow the of previous vaccination trials and promptly assess the efficiency of drugs and vaccines that have already shown promising outcomes in phase 2 clinical trials[83,84].Concerns about better understanding of ZIKV infection host immune responses and viral-drug-host relations should also be given due consideration by carrying out rapid molecular diagnostics against the ZIKV[86,87].For this purpose,properin-silicoandin-vitroexperimental settings should be devised so that the next phase of clinical trials can progress at a rapid pace[7].
Additionally,the use of controlled human infection models(CHIMs) is imperative for vaccination development.CHIMs involve deliberately infecting human subjects with a specific pathogen under controlled conditions in order to study the disease and evaluate potential treatments.While CHIMs have been used successfully in the past for diseases such as malaria and influenza,their use for a ZIKV vaccine development raises ethical considerations due to the potential risks involved.A CHIM for ZIKV would involve administering the virus to healthy volunteers and monitoring their immune response and symptoms[89,90].Researchers could then test the efficacy of experimental vaccines by vaccinating participants and exposing them to the virus.This approach allows for faster identification of potential vaccines and a better understanding of their effectiveness.However,using a CHIM for ZIKV vaccine development requires careful consideration of the risks involved.ZIKV can cause severe birth defects,and its long-term effects on adults are still not fully known[91,92].Exposing healthy volunteers to the virus involves potential harm and researchers must ensure strict safety protocols are in place to protect participants.Ethical guidelines,such as informed consent,strict participant selection criteria,and constant monitoring and care during the study,must be followed rigorously[92].The potential benefits to public health must outweigh the risks to individual participants.Before using a CHIM,extensive preclinical research should be undertaken to identify potential vaccine candidates and evaluate their safety and efficacy in animal studies.It is essential to minimize harm to participants and ensure any potential benefits gained from a CHIM study are significant and necessary[92-94].
However,it is evident that the perspective regarding the consideration of ZIKV CHIM has undergone significant changes since the ethics consultation in 2016[88].There is now a greater recognition of societal value and a more comprehensive understanding of how to reduce risks.Those attending the meeting,which included representatives from regulatory bodies such as the US Food and Drug Administration and EMA,reached a consensus that CHIM could serve as a valuable tool for assessing ZIKV vaccine candidates.Before CHIMs can be employed to gauge vaccine effectiveness,various scientific aspects concerning CHIMs must be resolved.These include factors such as the challenge strain,method of administration,dosage,and the timing of challenge postvaccination[88,93,94].Moreover,there are numerous ethical concerns regarding testing subjects such as pregnant women and newborns;proper consideration should be given to the fact that the lack of experimentation and lack of prevention of treatment may also lead to fatalities,which is certainly not the desired outcome[94].Therefore,ethical concerns,clinical trials,standard operating procedure,and proper updated scientific efforts should be combined together to ensure the availability of treatment against ZIKV infection and to prevent its entry into the healthcare system in the coming 5-10 years[93].Given the seriousness of this problem,we need to proceed with a sense of urgency as we do not want to face another pandemic in this century[94].
5.Limitations and perspectives
The review,while providing valuable insights into Zika virus therapeutics and vaccination development,has notable limitations.Firstly,the absence of licensed drugs or vaccines against ZIKV infection to date underscores the challenging landscape of therapeutic advancements.The review acknowledges the current therapeutics' limited efficacy in mitigating infectious symptoms,but it may not extensively explore alternative approaches or the underlying reasons for the delay in vaccination development.Moreover,the discussion on the delay in vaccination development points to the challenges posed by susceptible groups,such as pregnant women and newborns,yet the review may not delve deeply into the complexities of addressing immunocompromised conditions in these populations.Additionally,the emphasis on the limited epidemic nature of ZIKV raises questions about the generalizability of findings to potential future pandemics,requiring a more nuanced exploration of the evolving epidemiological landscape.
Furthermore,the review proposes modern therapeutic approaches,includingin-silicomodeling and nanotechnology-based interventions.However,it may lack a comprehensive evaluation of the feasibility,scalability,and potential challenges associated with implementing these approaches in real-world scenarios.The discussion of CHIMs introduces ethical considerations and potential risks.While the review mentions the evolving perspective on CHIMs,it could benefit from a more in-depth exploration of the unresolved scientific aspects,ethical concerns,and the necessity of strict safety protocols in employing CHIMs for ZIKV vaccine development.Additionally,the urgency expressed in the conclusion underscores the need for a more detailed examination of the timeline and feasibility of preventive measures to avoid potential pandemics in the coming years.Thus,addressing these limitations would enhance the review's comprehensiveness and contribute to a more robust understanding of the challenges and prospects in combating ZIKV infection.
6.Conclusions
ZIKV is a potential threat to the global healthcare system.Its complex clinical picture,lack of molecular insight,and gaps in its therapeutic options make it an emerging threat to the scientific community.Although work is underway to establish its epidemiology,pathogenesis,and therapeutic options,more focused and stringent efforts are needed to overcome the gap left in therapeutic trials.A sustained and coordinated effort is required from the scientific,medical,and community sectors to understand the threats associated with the ZIKV and to take mitigation measures for effectively dealing with it in case of future outbreaks.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Funding
This work is supported by the United Arab Emirates University UPAR (Grant No.G3458).
Authors’ contributions
Conceptualization: S.M.and Y.W.;methodology: S.M.,K.M.,O.A.,M.T.K.,R.S.,Y.W;formal analysis: S.M.,K.M.,O.A.,M.T.K.,R.S.,Y.W;investigation: S.M.,K.M.,O.A.,M.T.K.,R.S.,Y.W;data curation: S.M.,K.M.,O.A.,M.T.K.,R.S.,Y.W;original draft preparation: S,M.;review and editing: K.M.,Y.W.;visualization:S.M.,K.M.,O.A.,M.T.K.,R.S.,Y.W;supervision: Y.W.;project administration: Y.W;funding acquisition: K.M.,Y.W.
Publisher’s note
The Publisher of theJournalremains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
 Asian Pacific Journal of Tropical Medicine2024年3期
Asian Pacific Journal of Tropical Medicine2024年3期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Mitigating fire risks in hospitals: Are we primed and geared up?
- Navigating the future of malaria control: Insights from India's pioneering malaria slide bank
- Smoking of Carica papaya in Nigeria: The rationale,the public health effects and policies for intervention
- Dengue hemorrhagic fever with rectus sheath hematoma: A case report
- Characteristics of hospitalized patients with confirmed COVID-19 and their hospital management
- Prevalence and risk factors associated with long COVID symptoms in children and adolescents in a southern province of Vietnam
