Epidemic management in a measles outbreak in 2023,the return of vaccinepreventable diseases: A single center,retrospective observational study
Sevgi Aslan Tuncay,Gulsen Akkoc,Seyhan Yilmaz,Burcu Parlak,Pinar Canizci Erdemli,Aylin Dizi Isik,Didem Buyuktas Aytac,Meryem Cagla Abaci Capar,Eda Kepenekli Kadayifci
Marmara University Faculty of Medicine,Department of Pediatric Infectious Disease,Istanbul,Turkey
ABSTRACT Objective: To investigate the importance of immunization in preventing measles infection and to determine the most useful laboratory tests for confirmation of measles.Methods: This study included pediatric cases evaluated with a presumed diagnosis of measles between December 2022 and June 2023,at Marmara University Pendik Training and Research Hospital.The effects of vaccination status and underlying disease on the clinical course,treatments,and complications were evaluated.Results: In total,117 patients were enrolled in the study with a median age of 80 months (IQR: 32.5-125.0).Twelve patients with contact history were asymptomatic and had an underlying disorder,and intravenous immunoglobulin was given to them for postexposure prophylaxis.Fifty-one patients had confirmed measles diagnosis.Ribavirin treatment was given to three patients (a newborn,a girl with rhabdomyosarcoma,and a healthy boy) with respiratory distress.Seventy-eight percent of confirmed measles cases were unvaccinated,and all hospitalized cases were unvaccinated or undervaccinated.Four full-vaccinated children had confirmed measles infection.Measles PCR from nasopharyngeal swabs was negative in all of them,and their diagnosis was established with anti-measles IgM positivity.Conclusions: The measles vaccine is the most effective way to protect from measles and measles-related complications.Although measles can also occur in fully vaccinated patients,the disease is milder than in unvaccinated patients.Using ELISA and RT-PCR tests together may be beneficial in patients with high clinical suspicion for early diagnosis.
KEYWORDS: Measles;Outbreak;Vaccination;Vitamin A;Ribavirin
Significance
Our investigation was conducted to find out why we've seen an increase in measles cases since January 2023,despite high vaccination rates in Turkey.It was found that the majority of cases were in unvaccinated children.PCR confirmed the diagnosis in some symptomatic children,with anti-measles IgM levels detected just below the positive cut-off.The serologic cutt-off for measles diagnosis could be considered lower.This study clearly demonstrates the effectiveness of the measles vaccination in reducing the risk and severity of the disease.
1.Introduction
The measles virus is an enveloped RNA virus,classified as a member of the Paramyxoviridae family.Measles is transmitted by respiratory secretions of an infected individual and is one of the most contagious diseases.After an incubation period of 8-12 days,clinical signs appear with prodromal symptoms such as high fever,cough,cold,and conjunctivitis.The typical maculopapular rash occurs within 3-4 days following prodromal symptoms.The lack of an adequate antiviral treatment option,and the lack of an accepted treatment modality other than the supportive vitamin A treatment,make the 'prevention' of the disease more critical.The measles vaccine is the most effective way to prevent the disease.To ensure effective herd immunity and prevent epidemic diseases,the vaccination rate should be above 95%,as well as individual protection measures[1].As a result of its high contagiousness,measles outbreaks might arise if vaccination rates decline.
In the National Immunization Program of the Ministry of Health of Turkey,two vaccine doses are recommended in childhood: at the end of one year,and four years of age.In case of an epidemic,a third dose of measles vaccine can be administered in the ninth month of age,independent of the two doses.It has been conducted as a combined Measles,Mumps,and Rubella vaccination under the scope of the Measles Elimination Program since 2006 in Turkey[2].
Recently,there has been an increase in the number of children under-vaccinated against measles or who have not been vaccinated due to vaccine refusal/hesitancy and immigration.Therefore,measles cases are becoming more frequent in our country[3-6].In Turkey,the number of measles cases has increased since 2011.The first outbreak was seen in 2013 with 7 405 measles cases,mainly imported children.After the interventions,only 565 cases were detected by the end of 2014,and measles incidence continued to decline until 2019.The second outbreak was seen in 2019 with 2 892 measles cases.In the following years,the incidence of measles in Turkey gradually decreased and 103 measles cases were reported in 2022[7].
Even though no measles cases had been reported in our hospital following the 2013 measles outbreak,it was noticed on January 8,2023,that a current outbreak emerged after a child was diagnosed with measles,and 117 pediatric cases have been followed up with the suspicion of measles as an outpatient or inpatient.Turkey has reported 1 140 cases since the beginning of 2023 and has the highest number of cases in the World Health Organization (WHO) European Region.
The primary aim of this study is to show the effect of vaccination status on the measles infection and its clinical course.Secondary aim of the study is to investigate the most useful laboratory tests and serologic cut-off levels for confirmation of the measles.
2.Subjects and methods
2.1.Patient selection and data collection
The patients,aged 0 to 18,were admitted to Marmara University Pendik Training and Research Hospital between December 2022 and June 2023 with complaints of fever and rash and suspected measles cases screened using the hospital database.In these patients,the diagnosis of measles,the laboratory test used to diagnose measles,demographic characteristics,measles vaccination status,presence of underlying disease,presence and duration of clinical signs and symptoms of measles,course of measles,frequency of hospitalization,presence of measles-related complications(pneumonia,otitis,encephalitis,and myocarditis),treatment modalities,laboratory findings,and prognosis were evaluated.The impact of vaccination status and underlying disease on the clinical course were also investigated.
2.2.Case definitions and surveillance flow
The cases were classified according to definitions of the Ministry of Health as follows[8]: Suspected measles case: Any disease with a maculopapular rash.Clinical (possible) measles case: Fever higher than 38 ℃ and maculopapular rash and cough or runny nose or conjunctivitis.Definite measles case: Laboratory confirmation is required.Laboratory criteria required for a definitive diagnosis of measles: Detection of measles-specific IgM antibodies,or measles virus isolation,or detection of measles viral RNA by Reverse Transcriptase Polymerase Chain Reaction (RT-PCR),or significant(at least 4-fold) increase in measles-specific IgG antibody titer in serum samples taken at 2-4 weeks intervals.
When a suspected measles case admits to our center,the patient is directed to a separate area in the emergency room after triage according to epidemic measures.All subsequent procedures and follow-ups of the patient are performed in this separate area.Five milliliters of a serum sample from the measles-suspected case was taken into a sterile tube,centrifuged,and stored at 4 ℃-8 ℃ until sent to the reference laboratory.Qualitative anti-measles IgM,antimeasles IgG,anti-rubella IgM,and anti-rubella IgG tests were studied with the 'card-test' from this sample.All the tests' results were negative,positive,and intermediate.Additionally,urine and nasopharyngeal swab samples were taken for a polymerase chain reaction (PCR) test and sent to the reference laboratory.Furthermore,a test was also sent to study quantitative anti-measles IgM (positive:>15 U/mL) and IgG (positive >150 U/mL) tests for antibody levels in our laboratory.Patients with suspected measles are given oral vitamin A once daily for two days (Vitamin A dose: 50.000 IU under six months,100.000 IU between 6-11 months,200.000 IU for 12 months and above).
Tachypneic patients,who require oxygen support,have an ill appearance and poor oral intake,have been hospitalized,and airborne precautions are applied.They are followed up for complications of measles.
2.3.Statistical analysis
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS) 22.0 software (IBM Corp,Armonk,NY,USA).Numbers and percentages were used to express categorical variables.The mean ± standard deviation or the median with the Inter Quartile Range (IQR) was used to express continuous variables depending on whether they showed a parametric or nonparametric distribution.Categorical variables were compared using theChisquare test.The student’st-test and the Mann-WhitneyUtest were used to compare mean or median values between the two groups,depending on the sample distribution.Cut-off values were analyzed by ROC curve analysis.AP-value of less than 0.05 was regarded as the alpha (α) significance level.
2.4.Ethical approval
The study protocol was approved by the Institutional Ethics Committee of Marmara University (Protocol Code: 07.2023.877).
3.Results
3.1.Demographic,clinical and laboratory features of patients
In total,among 117 patients,61 males (61.6%) and 38 females(38.4%) were enrolled in the study.The median age of the patients was 80 months (IQR: 32.5-125.0).Eighteen asymptomatic patients that only had a contact history were excluded from the study.Among the included 99 patients,51 (51.5%) had confirmed measles diagnosis with anti-measles IgM (n=5),measles PCR (n=5)positivity,or both (n=41).The flow chart of the study is summarized in Figure 1.
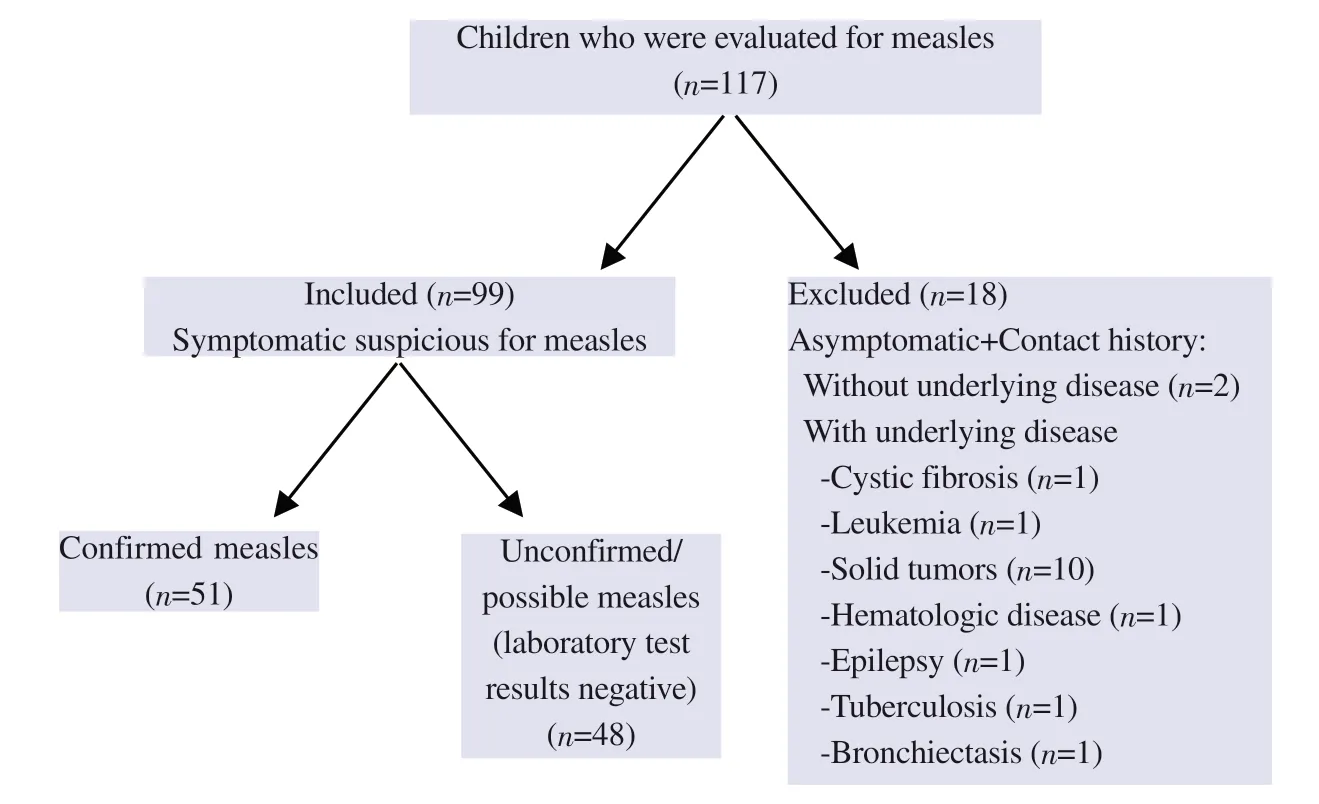
Figure 1. The flow diagram of the study shows the exclusion and inclusion criteria of the children.
Demographic features of the cases were compared in Table 1.Confirmed measles cases were older,and their vaccination rates were lower than unconfirmed/possible measles cases.
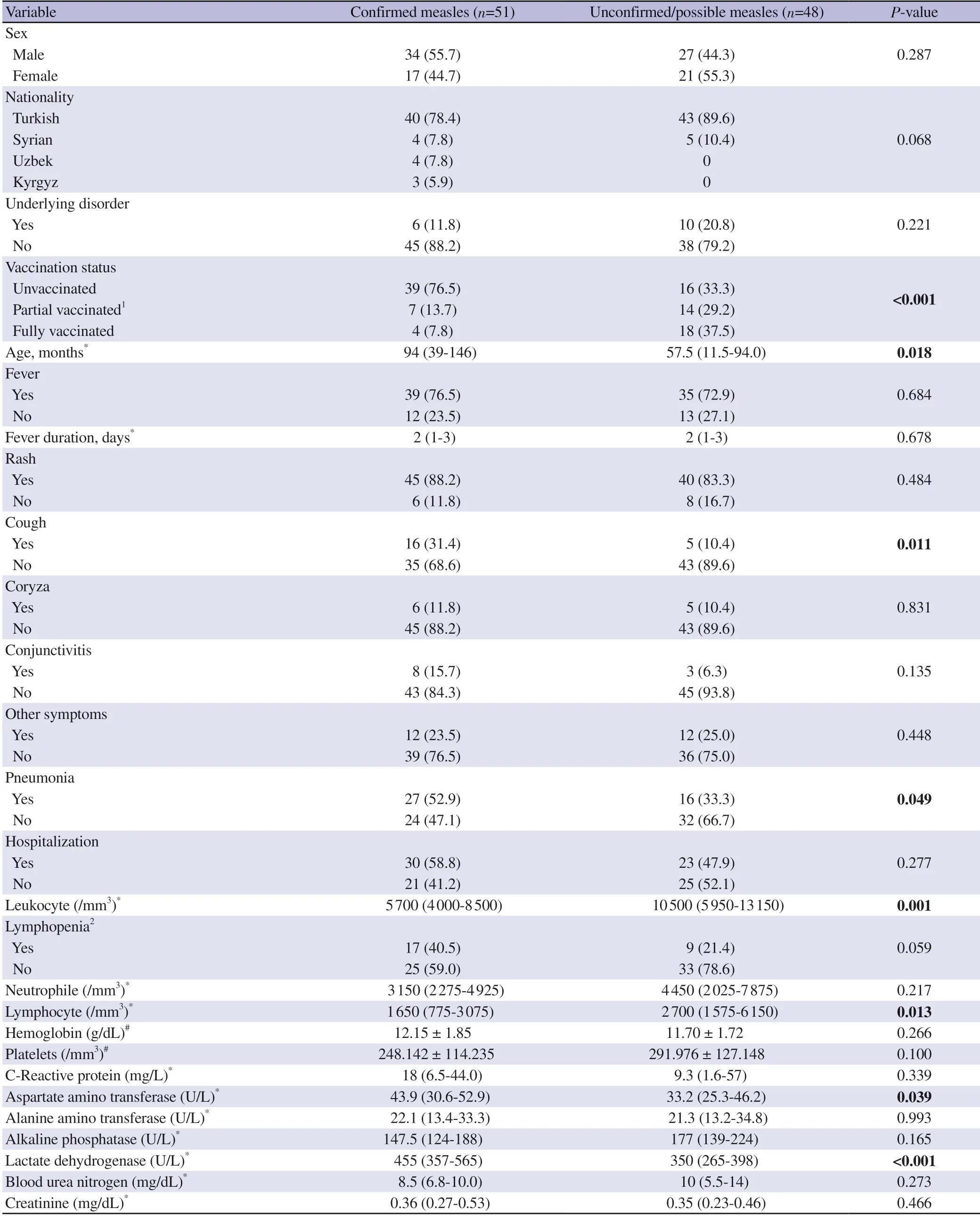
Table 1. Demographic,clinical features and laboratory findings of the cases [n (%)].
The clinical features of the cases are summarized in Table 1.There was no significant difference in the number of patients with fever,days of fever duration,and hospitalization rates between the two groups.The pneumonia complication rate was higher in the confirmed measles group.
Median lymphocyte counts of confirmed measles cases were lower than unconfirmed/possible cases (Table 1).Lymphopenia was an absolute lymphocyte count of less than 1 500/mm3.Lymphopenia was observed more in the confirmed measles group (n=17,40.5%vs.n=9,21.4%),but the difference was not significant (P=0.059).Median AST (P=0.039) and LDH (P<0.001) levels of confirmed measles cases were significantly higher (Table 1).
3.2.Clinical,laboratory features and management of the confirmed measles patients
A total of 51 patients had confirmed measles diagnosis,and the hospitalization rate was 58.8%.The median hospitalization duration was 4 days (IQR: 2-7 days).Of the hospitalized patients,36.7%needed any oxygen support with a median ventilation duration of 4 days (IQR: 2-7 days).Of all cases,vitamin A support was given to 36 patients (Table 2).
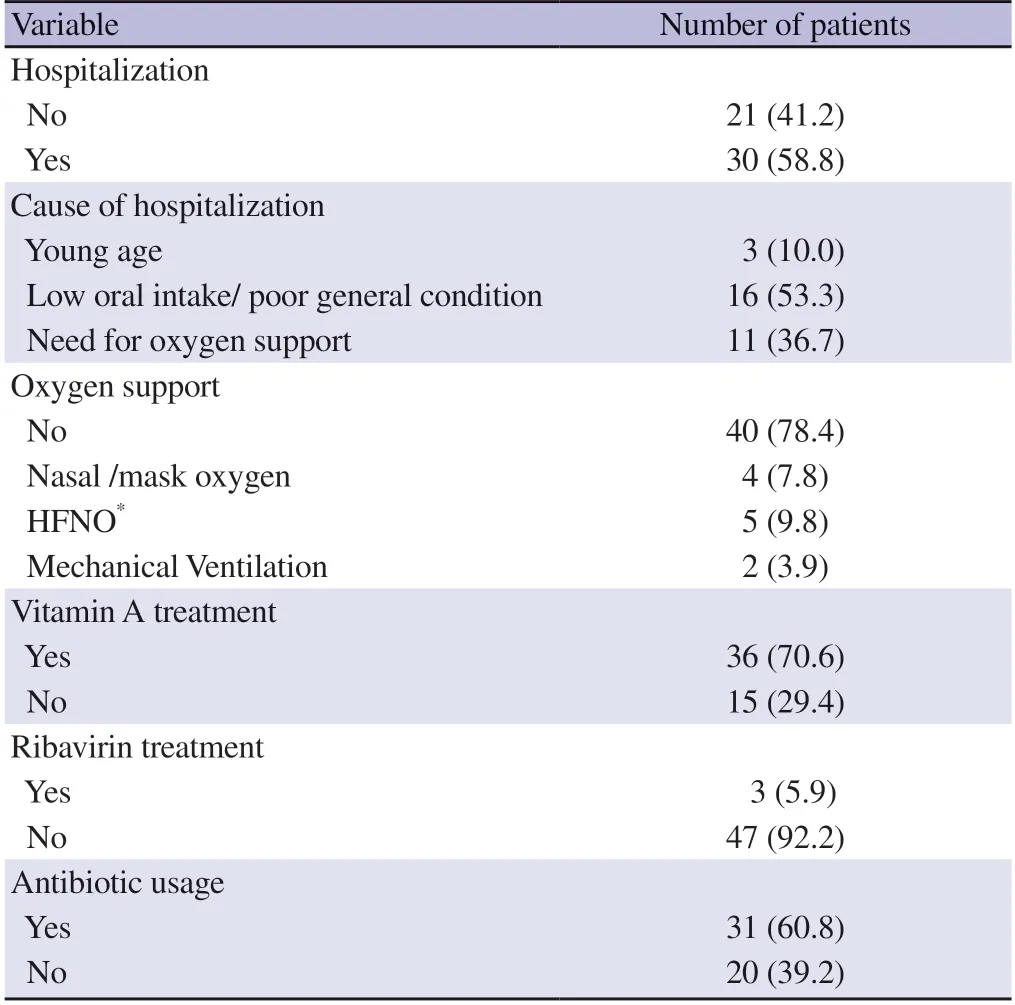
Table 2.Clinical,laboratory features and management of confirmed measles cases [n (%)].
Ribavirin treatment was commenced for three patients.The first patient was a neonate born from a mother with a measles infection at 34th weeks of gestation.After birth,the neonate was intubated.She had severe respiratory insufficiency and lung infiltrations on chest X-ray.Vitamin A supplementation and 400 mg/kg intravenous immunoglobulin (IVIG) were given twice.Ribavirin treatment was also given because the need for mechanical ventilation to be continued despite a supportive approach.The second patient was an 11-month-old girl with the diagnosis of rhabdomyosarcoma.She was admitted with a rash and fever and diagnosed with measles.Ribavirin was given due to severe respiratory distress,and she recovered on the fifth day of admission.The third patient was a twelve-year-old,partially vaccinated (1 dose) boy admitted with a rash and fever.He had respiratory distress and persistent fever and needed non-invasive mechanical ventilatory support.He was discharged in good condition after ribavirin treatment was given.Thirty-one of the patients received antibiotics for secondary bacterial pneumonia (15 patients received sulbactam-ampicillin/amoxicillinclavulanate,14 received third-generation cephalosporin,and two received cephalosporin+glycopeptide).
3.3.Complications of measles in confirmed measles
All of the confirmed measles cases (n=51) recovered,and 52.9%(n=27) of them had pneumonia.Complications occurred significantly higher in young infants [median age 58 (11-116) monthsvs.105(54-160) months].Four of 6 patients with an underlying disease had pneumonia,but there was no significant effect of the underlying disease on complications (Table 3).
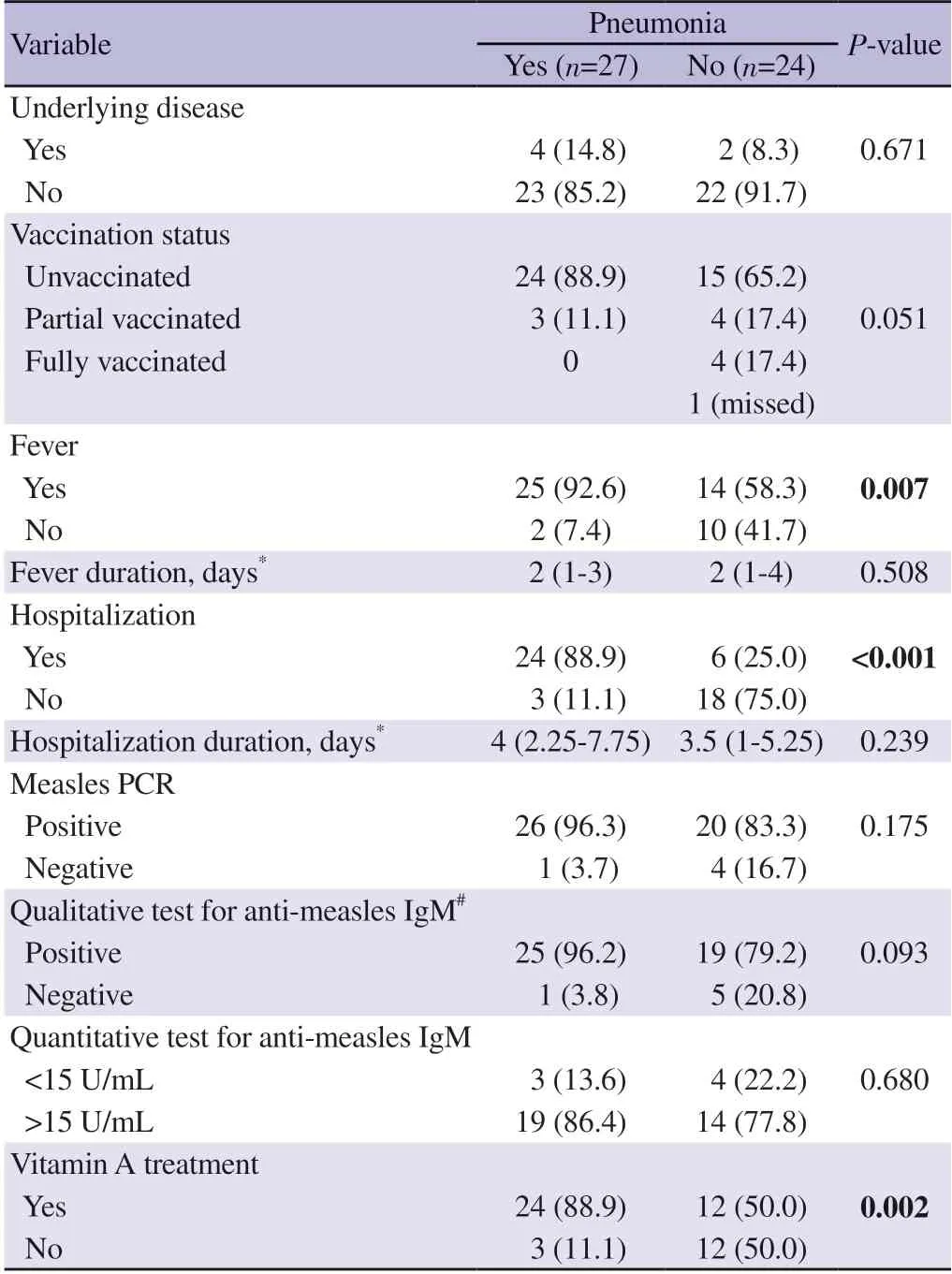
Table 3.Clinical characteristics of the confirmed measles cases that have pneumonia [n (%)].
Twenty-four patients with pneumonia were unvaccinated,although this was not statistically significant.High fever was found to be significant for complications,but the duration of the fever did not affect the development of complications.
Three patients with pneumonia were followed in outpatient settings.Six patients with pneumonia did not need oxygen support;their hospitalization was due to poor oral intake.The duration of hospitalization of patients with pneumonia was 0.5 days longer;it was not statistically significant.
Out of the confirmed measles group,4 out of 5 patients tested negative for PCR (P=0.175),5 out of 6 tested negative for antimeasles IgM did not develop pneumonia.However,this difference was not statistically significant (P=0.093,Table 3).
3.4.Diagnostic performance of anti-measles IgM value
ROC analysis showed that anti-measles IgM values greater than or equal to 9.1 U/mL had 90% sensitivity and 90% specificity for measles diagnosis (Figure 2).
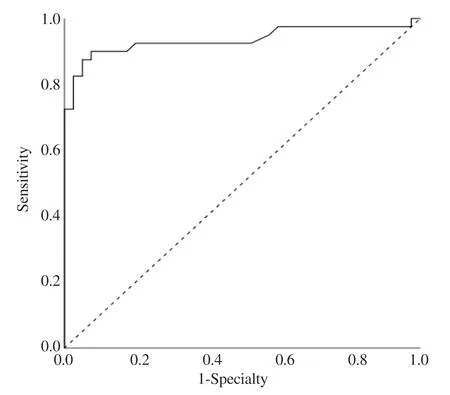
Figure 2.Diagnostic performance of anti-measles IgM value for diagnosis of measles at different cut off level on ROC curve.
4.Discussion
Measles is an airborne viral infection with a high contagiousness and attack rate.The measles vaccine is safe and effective and has been used for many years.Following successful immunization programs,the spread of the disease in the community has become limited,the number of measles cases has decreased,and death due to measles has been eliminated.The measles vaccine has been implemented in Turkey since 1970.It was administered as a single dose in the ninth month of age.The second dose was added in 1998 and was administered to first-year primary school children.After 2006,"the Measles and Rubella Elimination and Prevention of Congenital Rubella Syndrome Program" was initiated,and the triple combined Measles,Mumps,and Rubella vaccine started in two doses at 12-15 months and 4-6 years old[2].Since 2020,an additional dose has also been administered to 9-month-old infants in regions where measles cases were reported.
Measles vaccine coverage in Turkey is reported as 96% by the WHO[9].Despite high vaccine coverage,non-immune immigrants and vaccine refusal/hesitancy continue to be a risk for the measles epidemic.In Turkey,7 754 cases were reported between 2012-2013,and 534 cases in 2014.According to the WHO,in 2021,the estimated number of deaths due to measles is 128 globally,mostly among unvaccinated or under-vaccinated children under the age of 5 years[10].In a retrospective,multicenter study,the majority of children hospitalized for measles (53.1%) were not appropriately vaccinated according to age,and only 19% (23/120) of those aged>15 months had received at least one dose of vaccination[11].In our study,76.5% of the children with measles were unvaccinated,and 13.7% were partially vaccinated.Thirty (58.8%) of the 51 confirmed measles cases were hospitalized and all of them were unvaccinated or under-vaccinated.
Measles infection presents with rash,fever,cough,coryza,and conjunctivitis.Clinical diagnosis is difficult in areas where measles is uncommon because many other infectious diseases cause rash and fever[12].The approach algorithm to the patient presenting with rash and fever is described in Figure 3.Laboratory confirmation is essential for surveillance,especially using enzyme immunoassays to detect measles-specific IgM antibodies in acute serum samples.Over recent years,RT-PCR has been used increasingly to support the enzyme immunoassay test[13].Also,in some studies,rapid diagnostic tests (RDT) have been used that enable the rapid detection (<30 min) of measles-specific IgM antibodies in both serum and oral fluid specimens[14].In our study,among the 51 confirmed measles cases,five patients were measles PCR (+) and anti-measles IgM (-).This data highlights that the usage of RT-PCR and enzyme immunoassay tests simultaneously is helpful for the confirmation of suspicious cases.PCR may be a better modality for laboratory confirmation,provided tests are requested sufficiently early during the illness.

Figure 3.Approach algorithm to the patient presenting with rash and fever.
In confirmed measles cases,median days of ventilation support and hospitalization duration were equal.This data shows that patients who need hospitalization due to measles are discharged as soon as the need for ventilation support is over.With this approach,the hospital stay was tried to be kept as short as possible.
Measles mortality is higher in immunocompromised children,children with clinically important underlying diseases,and with severe malnutrition (including vitamin A deficiency)[1,15].Our study did not find any correlation between the underlying disease and measles-related complications.
Measles may cause immunosuppression and increases susceptibility to secondary infections.A study evaluated lymphocyte changes during measles,and they found lymphocyte counts were transiently decreased for measles patients and were more common in patients with fever and malnutrition[16].In our study,lymphopenia was more prominent in the confirmed measles cases,but it was not statistically significant and did not predict the risk of complications.
In our study,AST and LDH levels of confirmed measles cases were significantly higher.This finding may be compatible with viral infection.In a retrospective study,LDH and AST showed a higher course in the measles group than Kawasaki group.They suggested the combined use of LDH and AST in the differential diagnosis between viral infection and inflammatory diseases[17].
Measles may present with severe complications in unvaccinated patients.In a study from Indonesia,20% of the 75 children with measles developed bronchopneumonia.Of them,85%were unvaccinated and young children were more prone to complications[18].Similarly,in our study,27 (52.9%) of 51 confirmed measles children had pneumonia and 88.9% of them were unvaccinated.Also,pneumonia was more common in younger children.Most notably,among confirmed measles cases,none of the four fully vaccinated patients experienced any pneumonia-related problems.
Children with clinical or subclinical vitamin A deficiency have increased measles fatality rates,so the WHO recommends vitamin A supplementation.In the previous study[18],vitamin A supplementation was given in 85% of measles cases.In our study,vitamin A was given to 36 (70.5%) of confirmed measles cases.
Ribavirin is an antiviral agent that can treat severely affected and immunocompromised children with measles[1].The reported benefits of ribavirin include shorter hospitalization and reduced complications[19].In a report of a measles outbreak in a pediatric oncology unit from India,early ribavirin therapy prevented complications,and post-exposure prophylaxis with ribavirin prevented clinical disease in oncology patients[20].In our study,oral ribavirin was given to three confirmed measles cases.The first case was a neonate,the second was a patient with a solid tumor,and the third one was a boy without concomitant diseases.They all had pneumonia and respiratory distress and all of them recovered.Although randomized controlled trials cannot prove the benefit of ribavirin in measles infection,it may improve the course of the disease in complicated cases and immunosuppressed patients.
In confirmed measles cases,five of the six anti-measles IgMnegative children (Table 3),and four of the seven anti-measles IgM level <15 U/mL children (Table 3) did not develop pneumonia.We suggested that higher levels of anti-measles IgM may predict the risk of pneumonia,but it was not statistically significant (P=0.093).
A study showed that older age and poor nutritional status were independent risk factors for developing pneumonia in pediatric patients with measles[21].In our study,children who developed pneumonia were younger [median 58 (IQR: 11-116) monthsvs.105(IQR: 54-160) months].This may be associated with the immaturity of the immune system at a young age.
In our study,ROC analysis showed that anti-measles IgM values greater than or equal to 9.1 U/mL had good sensitivity and specificity for measles diagnosis.In our laboratory,anti-Meales IgM reference ranges are <10 U/mL negative,10-15 U/mL intermediate,and >15 U/mL positive.If clinical signs are suspicious for measles in patients with intermediate anti-measles IgM levels,we suggest that serology repetition or PCR testing can be used to confirm the diagnosis.
In our study,four fully vaccinated children aged 106-146 months had confirmed measles infection.Measles infection in fully vaccinated children (two doses >12 months) children can be explained by vaccine failure.Vaccine failure can either be primary,where a vaccine recipient does not develop protective immunity following vaccination,or secondary,where the protective immunity that develops postvaccination wanes.Measles,Mumps,and Rubella vaccine? (Serum Institute of India;Pune/ India),PRIORIX?(GlaxoSmithKline Biologicals;Rixensart/Belgium) are used for measles immunization in Turkey.They need to be stored between 2 ℃ and 8 ℃.For this purpose,a cold chain system is applied in which vaccines are kept and distributed in appropriate environments,temperatures,and conditions,from the production of vaccines to application to the patient.The ineffective content of the vaccine or disruption to the cold chain system may lead to a primary vaccine failure[22,23].
Two doses of measles vaccine are considered to provide longlasting immunity.Although antibody levels decline over time,it remains above the protective level[24].A recent study from California reported measles cases due to secondary vaccine failure.Patients with two or more vaccine doses demonstrated lower rates of hospitalization,cough,coryza,conjunctivitis,and fever[25].In our study,all of the four cases had only maculopapular rash,did not need hospitalization and their diagnosis was established with the anti-measles IgM and IgG positivity.These four cases may be nonimmune due to primary vaccine failure,and if more time had elapsed between rash onset and diagnosis,both IgG and IgM antibodies may have been elevated.Also,it may be explained by waning immunity(secondary failure).The distinction can be made with the IgG avidity test,but the avidity test is not being performed at our center.
In conclusion,although measles is a vaccine-preventable disease,increasing vaccine refusal/hesitancy and under-vaccinated immigrant people play a significant role in the initiation and eventually spread of outbreaks.Because measles is an airborne and rapidly transmitted infection with a high attack rate,epidemic management measures(vaccination,surveillance) should be taken immediately.By providing high vaccination rates in the community,future outbreaks can be prevented,and unvaccinated infants (<6 months) and immunocompromised children and younger children who are more vulnerable to disease can be protected from severe complications of measles.
Our study has several limitations.Firstly,it is a retrospective study,and the number of patients included is low.Secondly,there is no control group of healthy individuals.However,our study's strength lies in the fact that although vaccine coverage is high in our country,a large number of measles cases have been diagnosed.Moreover,patients with immunodeficiency were included in the study,and measles was also detected in fully vaccinated patients.More extensive clinical trials are necessary to establish rapid diagnostic methods,an appropriate cut-off point for measles diagnosis in children,and the most effective treatment for pediatric cases.
Conflict of interest statement
The authors declare no conflict of interest.
Funding
The authors received no extramural funding for the study.
Authors’ contributions
S.A.T,G.A,S.Y and E.K.designed the study;S.A.T researched the literature,wrote the manuscript,submitted the final version;S.A.T,S.Y,A.D.I,P.C.E,D.B.A,M.C.A.C collected the data;S.A.T and G.A made analysis and interpretation of data;G.A and E.K made critical review of manuscript.All authors read and approved the final manuscript.All authors meet the ICMJE authorship criteria.
Publisher’s note
The Publisher of theJournalremains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
 Asian Pacific Journal of Tropical Medicine2024年3期
Asian Pacific Journal of Tropical Medicine2024年3期
- Asian Pacific Journal of Tropical Medicine的其它文章
- Mitigating fire risks in hospitals: Are we primed and geared up?
- Navigating the future of malaria control: Insights from India's pioneering malaria slide bank
- Smoking of Carica papaya in Nigeria: The rationale,the public health effects and policies for intervention
- Dengue hemorrhagic fever with rectus sheath hematoma: A case report
- Characteristics of hospitalized patients with confirmed COVID-19 and their hospital management
- Prevalence and risk factors associated with long COVID symptoms in children and adolescents in a southern province of Vietnam
