Expression of Cyclooxygenase-2 and Its Relationship with Mismatch Repair and Microsatellite Instability in Hereditary Nonpolyposis Colorectal Cancer△
Peng Jin,Jian-qiu Sheng*,Ying-hui Zhang,Ai-qin Li,Zi-tao Wu,and Shi-rong Li
Department of Gastroenterology,The Military General Hospital of Beijing,Beijing 100700,China
HEREDITARY nonpolyposis colorectal cancer(HNPCC) is an autosomal dominant inherited disease caused by germline mutation in mismatch repair (MMR) gene.An important phenotype of MMR gene inactivation is microsatellite instability(MSI).1,2Studies have shown high cyclooxygenase-2(COX-2) expression in familial adenomatous polyposis (FAP)and sporadic colorectal carcinoma,and long-term use of COX-2 inhibitors was found to reduce the risk of developing digestive tract tumors such as colorectal cancer.3,4However,the role of COX-2 in HNPCC is still uncertain.Therefore,with sporadic colorectal adenoma and carcinoma as control,we investigated the expression of COX-2 in HNPCC adenoma and carcinoma samples and analyzed its relationship with MMR expression and MSI status.
MATERIALS AND METHODS
Patient selection and sample collection
A total of 28 patients of colorectal adenoma and 14 of colorectal carcinoma were collected from 33 HNPCC families (distributed in Beijing,Hebei,He’nan,Inner Mongolia,Shandong,and Hubei provinces),all of whom were identified in accordance with Amsterdam criteria II from July 2003 to July 2007.5,6As controls,32 patients with sporadic colorectal adenoma and 24 patients with sporadic colorectal carcinoma were randomly selected during the same period from patients visiting the colonoscopy clinic of the Military General Hospital of Beijing.Patients who had taken nonsteroidal anti-inflammatory drugs within 1 year or had family history of hereditary colorectal carcinoma or inflammatory bowel diseases were excluded in the present study.
Three to six samples of adenoma or carcinoma (all confirmed by histology) were collected in each enrolled patient for DNA extraction,routine histological test (HE staining),and immunohistochemical assay.Two samples of normal colonic mucosa 10 cm from the adenoma or carcinoma,either proximal or distal,were also collected for DNA extraction and MSI detection.All the samples were obtained with colonoscopy by HNPCC Collaboration Group of the Hospital.For histological and immunohistochemical tests the tissues were fixed with formalin and embedded in paraffin,while for DNA extraction the samples were stored in liquid nitrogen till use.
The study protocol was approved by the Ethics Committee of the Military General Hospital of Beijing PLA.All the enrolled patients signed written informed consents for participation.
Immunohistochemical assay
The monoclonal antibodies against two MMR proteins hMLH1 and hMSH6 were purchased from BD Pharmingen(San Diego,CA,USA),and the monoclonal antibody against hMSH2 was from Calbiochem (EMD Chemicals,Cincinnati,OH,USA).Anti-COX-2 monoclonal antibody and DAB color reaction kit were purchased from Beijing Zhongshan Goldenbridge Biotechnology Co.,Ltd.(China).Immunohistochemical staining and results determination for hMLH1,hMSH2,and hMSH6 were carried out according to the method described by Sheng et al,7while staining for COX-2 detection was performed according to the method described by Lim et al.8
In hMLH1,hMSH2,and hMSH6 immunostaining,the results were graded as negative when there was a complete absence of staining in all the nuclei of tumor cells,and positive when there was positive staining in the nuclei.For each COX-2 immunostaining,an integrated score was calculated by adding the intensity of staining (3 for strongly positive,2 for moderately positive,1 for weakly positive,and 0 for negative) to the percentage of stained cells (0 when no cell was stained,1 when 1%-25% of the cells were stained,2 when 26%-50% were stained,3 when 51%-75% were stained,and 4 when 76%-100% were stained).8The final score provided the basis for the judgment of low expression (0-2) or high expression (3-7) of COX-2.
DNA extraction and MSI Analysis
DNA of normal colonic mucosal tissue,adenoma,and carcinoma were extracted with ZR Genomic DNA II kit (ZYMO Research Corporation,Irvine,CA,USA) following the manufacturer’s instructions.Five standard Bethesda microsatellite loci were chosen for polymerase chain reaction(PCR) amplification,includingBAT25,BAT26,D2S123,D5S346,andD17S250.Primers used in this study are as follows∶BAT25,FAM-5’-TCGCCTCCAAGAATGTAAGT-3’(sense),5’-TCTGGATTTTAACTATGGCTC-3’ (anti-sense);BAT26,FAM-5’-TGACTACTTTTGACTTCAGCC-3’ (sense),5’-AACCATTCAACATTTTTAACC-3’ (anti-sense);D2S123,FAM-5’-AAACAGGATGCCTGCCTTTA-3’ (sense),5’-GGACTTTCCACCTATGGGAC-3’ (anti-sense);D5S346,FAM-5’-ACTCACTCTAGTGATAAATCGGG-3’ (sense),5’-AGCAGATAAGACAAGTATTACTAG-3’ (anti-sense);D17S250,FAM-5’-GGAAGAATCAAATAGACAAT-3’ (sense),5’-GCTGGCCATATATATATTTAAACC-3’ (anti-sense).The primers were synthesized by Shanghai Sango Co.,Ltd.(China).The 5’-ends of all the forward primers were labeled with carboxyfluoresceinaminohexyl amidite (FAM).PCR was performed in a 20 μL reaction system containing 0.5 μL primer (10 pmol),10 μL Master Mix (containing dNTPs,Taq enzyme,and magnesium ion buffer),0.1 μL DNA template (50-100 ng),and 8.5-9.5 μL deionized water.The PCR was conducted in three steps∶pre-denaturation at 95°C for 5 minutes,then 32 cycles at 95°C for 45 seconds,at 56°C for 45 seconds,and at 72°C for 45 seconds,followed by the final extension at 72°C for 7 minutes.PCR products were detected with 6% polyacrylamide gel electrophoresis on ABI Prism 3700 DNA Analyzer (Life Technologies Corporation,Carlsbad,CA,USA).The results of electrophoresis were analyzed using GeneMapper 3.0 software (Life Technologies Corporation).Microsatellite instability was assumed when the segment length of a studied locus was changed or a new segment was present when compared with the results of corresponding normal mucosa.The presence of two or more instable loci were determined as high-frequency MSI (MSIH),1 instable locus as low-frequency MSI (MSI-L),while the decision of microsatellite stability (MSS) was reached when no instable locus was detected.
Statistical analysis
SPSS 10.0 software was used for statistical analysis.Numeration data were compared using chi-square test and Fisher’s exact test.APvalue under 0.05 was considered statistically significant.
RESULTS
General information of the enrolled patients
Among the 28 HNPCC adenoma patients,there were 19 males and 9 females,with a mean age of 48±12 years(range,21-73 years).Among the 14 HNPCC carcinoma patients,11 were males and 3 were females,aged 54±15 years on average (range,33-74 years).
The 32 sporadic adenoma patients included 20 males and 12 females,with a mean age of 59±15 years (range,28-84 years).The 24 sporadic carcinoma patients included 16 males and 8 females,aged 62±13 years (range,40-86 years).
COX-2 expression,MMR protein (hMLH1,hMSH2,and hMSH6) expression,and MSI in colorectal adenoma and carcinoma
As revealed in immunohistochemical assay,the rate of COX-2 high-expression was 53.6% (15/28) and 42.9%(6/14) in HNPCC adenoma and carcinoma tissues,but 62.5% (20/32) and 91.7% (22/24) in sporadic adenoma and carcinoma,respectively (Table 1).Among these four groups,the COX-2 high-expression rate in HNPCC carcinoma tissue was significantly lower than that in sporadic colorectal carcinoma (χ2=9.49,P<0.05).
The absence of MMR protein was observed in 67.9%(19/28) HNPCC adenoma and 71.4% (10/14) HNPCC carcinoma,both significantly higher than their counterparts in the sporadic cases [3.1% (1/32),χ2=25.32;12.5% (3/24),χ2=13.64;bothP<0.01](Table 1).
The percentage of MSI-H was 64.3% (18/28) in HNPCC adenoma,significantly higher than that in sporadic adenoma [3.1% (1/32),χ2=10.67,P<0.01];a similar difference was revealed in the comparison between HNPCC carcinoma and sporadic carcinoma [71.4% (10/14)vs.12.5% (3/24),χ2=13.64,P<0.01](Table 2).
Relationship of COX-2 with MMR protein expression and MSI status
Among the 14 patients of HNPCC carcinoma,COX-2 expression was significantly higher in MMR positive cases than in MMR negative cases (4/4vs.2/10,χ2=7.47,P<0.05).The similar difference was noticed in sporadic carcinoma patients (21/21vs.1/3,χ2=15.27,P<0.05) and HNPCC adenoma patients as well (8/9vs.7/19,χ2=6.65,P<0.05).However,the difference in COX-2 expression between MMR positive and MMR negative sporadic adenomas was hard to determine due to the small amount of MMR negative cases (only 1) (Table 1).
Similarly,among HNPCC carcinoma,HNPCC adenoma,and sporadic carcinoma patients,COX-2 expression was significantly higher in non-MSI-H (MSI-L plus MSS) cases than in MSI-H cases (4/4vs.2/10,χ2=7.47;9/10vs.6/18,χ2=8.29;21/21vs.1/3,χ2=15.27;allP<0.05).But the difference in sporadic adenoma was hard to determine as only 1 case was found with MSI-H (Table 2).

Table 1.The relationship between cyclooxygenase-2 (COX-2)expression and mismatch repair (MMR) protein expression in colorectal adenoma and carcinoma
Relationship between MSI and MMR expression
Among the four groups of HNPCC adenoma,HNPCC carcinoma,sporadic adenoma,and sporadic carcinoma,the detection of MSI-H was noticed to be largely consistent with MMR-deletion.To be specific,nearly all the patients with MMR deficiency manifested MSI-H at the same time,with the only one case of exception in HNPCC adenoma group (Table 3).
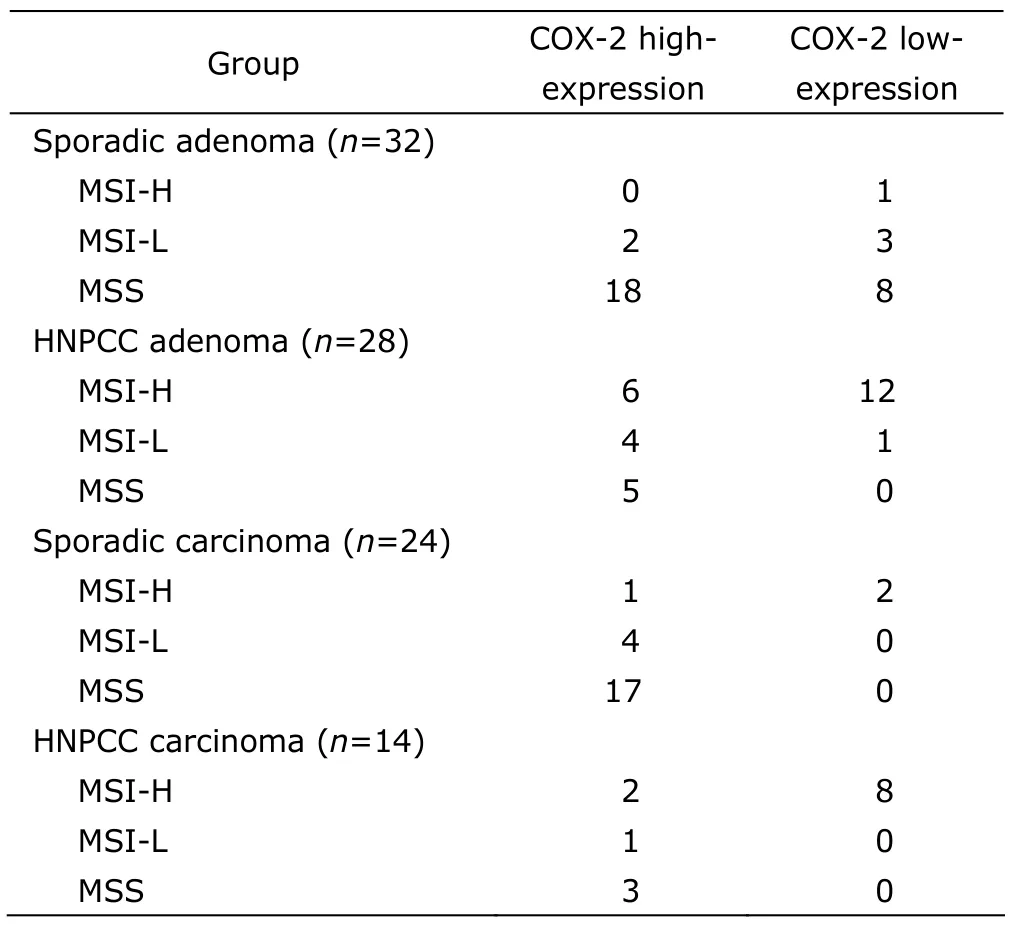
Table 2.The relationship between COX-2 expression and microsatellite instability (MSI) in colorectal adenoma and carcinoma
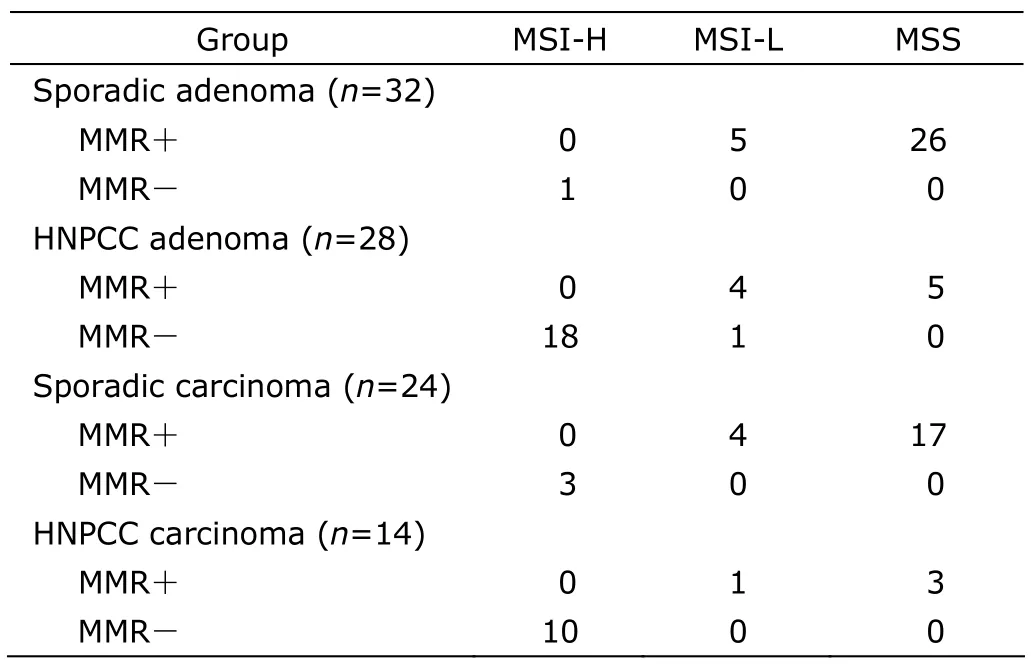
Table 3.The relationship between MMR protein expression and MSI in colorectal adenoma and carcinoma
DISCUSSION
It is generally believed that elevation of COX-2 mRNA and COX-2 protein levels could be found in 85%-95% patients with colorectal carcinoma and in 40%-50% patients with colorectal adenoma.4COX-2 is an effective therapeutic target for adenomatous polyps or carcinoma induced by the mutation of adenomatous polyposis coli (APC) gene,and it plays a crucial role in prophylaxis of colorectal tumor.9,10Selective COX-2 inhibitor can effectively prevent bowel adenoma and significantly suppress pre-existing adenoma.It could also inhibit the development of colorectal carcinoma.11
We had performed a small-sample observation to investigate the effect of COX-2 inhibitor celecoxib and aspirin in FAP adenoma and HNPCC adenoma,and the result demonstrated that celecoxib inhibited the growth of FAP adenoma but its interventional effect on HNPCC adenoma was not ideal.12We speculated that COX-2 inhibitor was effective for treating FAP adenoma because COX-2 was expressed at a high level in that disease.For that reason,the determination of COX-2 expression level in HNPCC adenoma and carcinoma is very important for understanding of the therapeutic effect of COX-2 inhibitor in those diseases.The present study demonstrated that the rate of COX-2 high-expression was significantly lower in HNPCC carcinoma than in sporadic carcinoma.The low expression of COX-2 in HNPCC adenoma and carcinoma might explain why COX-2 inhibitor could not effectively prevent the progress of those two types of colorectal cancer even though COX-2 is a key component in tumor pathogenesis and an effective target for tumor prophylaxis.
In the present study,COX-2 low-expression was demonstrated to be frequent in sporadic carcinoma with MMR-deficiency or MSI-H genotype as well as in HNPCC adenoma and HNPCC carcinoma,while COX-2 high-expression was present in most cells with positive MMR expression or MSS,in agreement with the result in the study of Karnes et al.13Some investigators presumed that the balance of genomic methylation and demethylation in organisms might be involved in the relationship among MMR protein expression,MSI,and COX-2 expression in colorectal adenoma and carcinoma.When the effect of methyltransferase is strengthened by some factors,so is methylation of MMR promoter,followed by non-expression of MMR protein,MMR function deficiency,and MSI,finally leading to one type of tumorigenesis.14When demethylase is strengthened by some other factors,genomic methylation is decreased.Demethylation of COX-2 gene promoter results in overexpression of COX-2 protein,which might induce another type of tumorigenesis.4The results of our present study suggested that COX-2 or MMR protein expression status might help in the classification of colorectal cancer for appropriate clinical prophylaxis and treatment.If increased expression of COX-2 is detected,COX-2 inhibitors such as celecoxib could be recommended for treatment.If the patient is diagnosed as MMR-deficient colorectal carcinoma,treatment should target at methylation of MMR promoter,employing demethylating agent or genetic therapy.For better guided clinical practice,the role of COX-2 expression in tumorigenesis and prophylaxis of HNPCC,FAP,and sporadic colorectal carcinoma,and the relationship of COX-2 with MMR protein and MSI in the process of colorectal tumorigenesis remain to be further explored.
1.Bonis PA,Trikalinos TA,Chung M,et al.Hereditary nonpolyposis colorectal cancer∶diagnostic strategies and their implications.Evid Rep Technol Assess (Full Rep)2007;150∶1-180.
2.Zhang L.Immunohistochemistry versus microsatellite instability testing for screening colorectal cancer patients at risk for hereditary nonpolyposis colorectal cancer syndrome.Part II.The utility of microsatellite instability testing.J Mol Diagn 2008;10∶301-7.
3.Oba M,Miwa K,Fujimura T,et al.Chemoprevention of glandular stomach carcinogenesis through duodenogastric reflux in rats by a COX-2 inhibitor.Int J Cancer 2008;123∶1491-8.
4.Bresalier RS.Chemoprevention of colorectal neoplasia∶advances and controversies (the COX-2 story).Curr Opin Gastroenterology 2007;23∶44-7.
5.Chew MH,Koh PK,Ng KH,et al.Phenotypic characteristics of hereditary non-polyposis colorectal cancer by the Amsterdam criteria∶an Asian perspective.ANZ J Surg 2008;78∶556-60.
6.Vasen HF,Watson P,Mecklin JP,et al.New clinical criteria for hereditary nonpolyposis colorectal cancer (HNPCC,Lynch syndrome) proposed by the International Collaborative group on HNPCC.Gastroenterology 1999;116∶1453-6.
7.Sheng JQ,Chan TL,Chan YW,et al.Microsatellite instability and novel mismatch repair gene mutations in northern Chinese population with hereditary non-polyposis colorectal cancer.Chin J Dig Dis 2006;7∶197-205.
8.Lim SC,Lee TB,Choi CH,et al.Expression of cyclooxygenase-2 and its relationship to p53 accumulation in colorectal cancers.Yonsei Med J 2007;48∶495-501.
9.Ferrario A,Fisher AM,Rucker N,et al.Celecoxib and NS-398 enhance photodynamic therapy by increasingin vitroapoptosis and decreasingin vivoinflammatory and angiogenic factors.Cancer Res 2005;65∶9473-8.
10.Yim HW,Jong HS,Kim TY,et al.Cyclooxygenase-2 inhibits novel ginseng metabolite-mediated apoptosis.Cancer Res 2005;65∶1952-60.
11.Brown JR,DuBois RN.COX-2∶a molecular target for colorectal cancer prevention.J Clin Oncol 2005;23∶2840-55.
12.Sheng JQ,Li SR,Yang XY,et al.Clinical management of adenomatous polyposis in patients with hereditary non-polyposis colorectal cancer and familial adenomatous polyposis.Zhonghua Yi Xue Za Zhi 2006;86∶526-9.
13.Karnes WE Jr,Shattuck-Brandt R,Burgart LJ,et al.Reduced COX-2 protein in colorectal cancer with defective mismatch repair.Cancer Res 1998;58∶5473-7.
14.Song WQ,Han CL,Zhou BJ,et al.Relationship between COX-2 expression and hMLH1,hMSH2 expression in colorectal carcinoma.World Chin J Digestol 2005;13∶105-9.
 Chinese Medical Sciences Journal2010年4期
Chinese Medical Sciences Journal2010年4期
- Chinese Medical Sciences Journal的其它文章
- Endothelial Nitric Oxide Synthase Gene Polymorphisms Associated with Susceptibility to High Altitude Pulmonary Edema in Chinese Railway Construction Workers at Qinghai-Tibet over 4 500 Meters above Sea Level△
- Thoraco-abdominal Aorta Revascularization through a Retroperitoneal Approach
- Clinicopathological Features of Non-familial Colorectal Cancer with High-frequency Microsatellite Instability△
- Epigenetic Repression of SATB1 by Polycomb Group Protein EZH2 in Epithelial Cells△
- NF-E2:a Novel Regulator of Alpha-hemoglobin Stabilizing Protein Gene Expression△
- Giant Hydroureteronephrosis Associated with Ipsilateral Inguinal Hernia and Contralateral Hydronephrosis:a Case Study
