Endothelial Nitric Oxide Synthase Gene Polymorphisms Associated with Susceptibility to High Altitude Pulmonary Edema in Chinese Railway Construction Workers at Qinghai-Tibet over 4 500 Meters above Sea Level△
Yu-jing Sun,Ming-wu Fang,Wen-quanNiu,Guang-pingLi,Jing-liangLiu,
Shou-quanDing2,Ying Xu2,Guo-shuYu2,Jian-qunDong1,Yun-junPan2,
Wei-ya Dong2,Tian Wang2,Jing-wen Cao2,Xiao-bo Li2,Zhong-xiang Wang2,
Guang-xue Yu2,Hui-cheng Sun2,Zhong-hou Jia2,Jun Liu2,Xiao-ming Wang1,
Qin Si1,Qi-xia Wu 1,Wen-yu Zhou1,Tong-chun Zhu2,and Chang-chun Qiu1 *
1National Laboratory of Medical Molecular Biology,Institute of Basic Medical Sciences,Chinese Academy of Medical Sciences &Peking Union Medical College,Beijing 100005,China
2Hospital of China Railway Construction Corporation,Beijing 100039,China
HIGH altitude pulmonary edema (HAPE) is a rare and potentially fatal acute respiratory disease that occurs in healthy people when exposed to altitude exceeding 2 500 m.1It is characterized by high pressure in pulmonary arteries,with edema in pulmonary interstitial tissue and alveoli.Although hypoxia is a major trigger factor,some individuals are more susceptible to HAPE than others when exposed to the same hypoxia conditions.The existence of genetic predisposition to HAPE has been suggested as the possible explanation.2Some candidate genes have already been reported,3-7but the precise genetic basis of the development of HAPE remains unclear.Therefore,it is particularly important to identify the candidate genes and determine the effects of these genes on HAPE in a large cohort.
Recent experiments and clinical studies have found that nitric oxide (NO) production is reduced in HAPE patients and NO inhalation could improve the conditions.8,9These studies have also demonstrated that NO deficiency may involve in the etiology of HAPE.First identified as“the endothelium-derived relaxing factor”in 1987,10NO has been implicated in neuronal transmission,immunological response,and vasodilatation.11,12NO is synthesized from the precursor L-arginine by NO synthase,which exists in human body in three distinct isoforms:inducible NO synthase (iNOS),constitutive neuronal NO synthase (nNOS),and endothelial NO synthase (eNOS or NOS3).13-15As the name indicates,eNOS is expressed in the endothelium,and is responsible for the most part of NO production in body tissues.The gene coding eNOS is located on chromosome 7 q35-36,containing 26 exons,spanning 21kb,and occuring as a single-copy gene in the haploid human genome.15
To date,three clinically relevant polymorphisms of eNOS gene have been associated with cardiovascular disease,including T-786C with a mutation in the promoter region,a 27bp variable number tandem repeat (VNTR)polymorphism in intron 4,and 894G/T with a mutation in exon 7,the third one resulting in a Glu-298-Asp amino acid substitution in the mature eNOS protein.11,12Studies on eNOS,as a potential candidate gene responsible for susceptibility to HAPE,have come to inconsistent results that are difficult to confirm.A study in a Japanese population concluded that the 894G/T and the 27bp VNTR polymorphisms of eNOS gene have a positive association with HAPE susceptibility.16However,a study in a European population and another one in a three-generation Han Chinese family failed to confirm this conclusion.17,18Apart from the different genetic backgrounds of the studied populations,such inconsistencies might stem primarily from experimental designs,including the use of slightly small cohorts,and sampling variability in the case-control studies.To provide more convincing evidence,we conducted a casecontrol association analysis in a large population consisting of Han Chinese railway construction workers working in Qinghai-Tibet at an altitude over 4 500 m above sea level to determine whether the polymorphisms of eNOS gene play a role in the genetic etiology of HAPE.
SUBJECTS AND METHODS
Study population
Our study protocol was approved by the Ethics Committee of the Institute of Basic Medical Sciences (Chinese Academy of Medical Sciences &Peking Union Medical College).Written informed consents have been obtained from all the individuals enrolled in this study.The subjects were workers participating for the first time in the Qinghai-Tibet railway construction during the period from July 2001 to December 2005.Before entering that construction project,they were born and had been living at low altitude.They all received standard physical and clinical examinations before ascending to the construction sites to exclude those with hypertension,diabetes,cardiopulmonary disease,asthma,inflammation,and any metabolic diseases.Qualified ones then spent 3-6 days in Golmud at 2 860 m above sea level for primary acclimatization to high-altitude environment.After that,the workers were assigned to different sites of construction located at 4 500-5 072 m above sea level,but with the life routine similarly scheduled,including the same food,same living condition (8 workers sharing a 40-m2room),and same working hour.Moreover,they were all exposed to similar environmental conditions characterized by high altitude,hypoxia,and extreme cold.
Selection of HAPE patients and healthy controls
The onset of HAPE is typically present within 1-6 days after ascending to an altitude of 4 500-5 072 m above sea level.5,7The HAPE group consisted of 149 male workers with an average age of 31.3 years (range,18-48 years),who were diagnosed on the basis of standard diagnostic criteria.1,2All the HAPE patients met specific criteria,that is,onset of typical symptoms at high altitude,including cough and dyspnea at rest,absence of infection,presence of pulmonary rales and cyanosis,and patchy shadows in chest X-ray.By giving oxygen,bed rest,or descending to lower altitudes (<2 000 m),the condition of these patients was reversed after 2-3 days of treatment,with symptoms and signs of HAPE disappeared.
A total of 160 healthy controls were randomly selected from the co-workers of those HAPE patients,matching the patients in age,sex,ethnicity,lifestyle (for instance,smokers or non-smokers),and working conditions.These subjects remained healthy after working at Qinghai-Tibet railway construction sites for at least 3 months,without suffering from HAPE or high altitude cerebral edema.
Sample collection
With informed consents from all the subjects,doctors at a hospital near the Qinghai-Tibet construction setting collected 5 mL venous blood samples from each HAPE patient and control.The whole blood was immediately separated to blood cells and plasma by centrifuging at 2 000 rpm (radius not available) for 10 minutes at 4°C,and then stored at-20°C.The blood cells and plasma were transported to Beijing for the following biochemical assays and genetic polymorphism analysis.
Determination of plasma NO
Since eNOS is responsible for most of the NO produced in the body tissues,we could determine eNOS activity by monitoring changes in NO concentration.NO concentrations were measured using a colorimetric assay kit (Roche Applied Science,Indianapolis,IN,USA) following the manufacturer’s instructions,19calculating with a nitrate standard curve by summing up the total nitrate and nitrite.The NO concentration of each plasma sample was measured twice and the average value was recorded.
Determination of genotypes
Genomic DNA was isolated from peripheral white blood cells with phenol-chloroform extraction.7Genotyping was performed using polymerase chain reaction (PCR) with a PTC-200TM thermal cycler (Bio-Rad Laboratories,Hercules,CA,USA).Primers for PCR were designed based on the published sequence of eNOS (http://www.ncbi.nlm.nih.gov/Genbank,accession number X76307.1) (Table 1).
The fragment containing the T-786C variation in the promoter was amplified by PCR using its specific primers(Table 1).The PCR products were digested with MspI enzyme for 3 hours at 37°C.The digested fragments were separated by means of electrophoresis in 10% polyacryamide gel and were visualized with silver staining.20
The 894G/T polymorphism in exon 7 was detected as previously described.20The 457bp-fragment was generated by PCR amplification using primers specific for the region containing 894G/T variant.The PCR fragments,after being digested with MboI enzyme for 3 hours at 37°C,were electrophoresed on 3% agarose gel and visualized with ethidium bromide staining.20,21
The length polymorphism of the 27bp NVTR in intron 4 of eNOS gene was identified by PCR using the specific primers (Table 1) as previously described.20,22PCR fragments were separated with electrophoresis in 10% polyacrylamide gels and visualized with silver staining.22We determined the length of allele c by electrophoresis in 12%polyacrylamide gel,visualized the fragments with silver staining,and confirmed the structure of allele c by means of direct sequencing (Fig.1) (ABI 377 DNA sequencing,PE Applied Biosystems,Carlsbad,CA,USA).
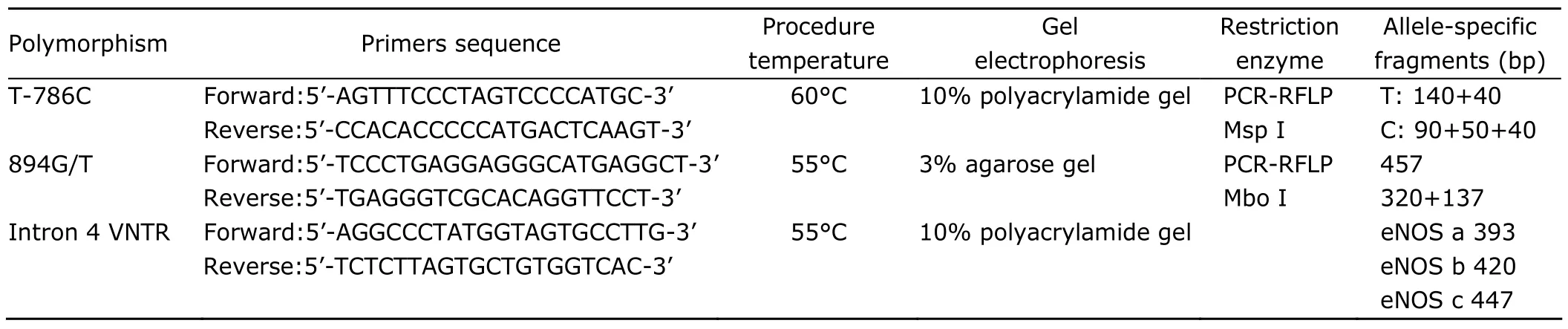
Table 1.Primers sequence and PCR amplification conditions used for genotyping of eNOS polymorphisms
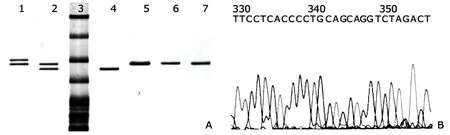
Figure 1.Allele-specific electrophoresis patterns of the eNOS variant containing 27bp VNTR in intron 4 (A) and the sequence of the 27bp repeat (B).
Statistical analysis
Statistical analyses in the present study were performed using SAS9.1.3 (SAS Institute Inc.,Cary,NC,USA).Continuous values are expressed as means±SD.Deviation of genotype frequency from Hardy-Weinberg equilibrium was assessed by chi-square test with 1 degree of freedom.Allele frequencies were calculated based on genotype frequencies in HAPE and control groups,and the intergroup difference was estimated with chi-square test and Fisher’s exact test.Haplotype frequencies were calculated using the EH/EH+ program (version 1.20).Pvalues lower than 0.05 were considered statistically significant.
RESULTS
Clinical characteristics
The baseline clinical characteristics of the two groups are summarized in Table 2.We found statistically significant differences between HAPE patients and healthy controls in all the seven recorded items.The levels of blood urea nitrogen (BUN),C-reactive protein (CRP),and hemoglobin were significantly increased in the plasma of HAPE patients compared with the healthy controls,while systolic blood pressure (SBP),diastolic blood pressure (DBP),pulmonary artery pressure (PAP),and creatinine (CRE) were decreased in HAPE subjects compared to controls.
Distribution of the three polymorphisms of eNOS gene
As shown in Table 3,the frequency distribution of the three polymorphisms of eNOS was in Hardy-Weinberg equilibrium in both HAPE and healthy control groups.The frequency of the T allele in the 894G/T polymorphism was significantly higher in the patients with HAPE than in healthy controls (6.71%vs.1.88%,P=0.0028),while no difference in the frequency of T-786C polymorphism was found between the two groups.There are three alleles of the 27bp VNTR within intron 4 in the present study,among which allele c composed of six 27bp repeats was detected only in control subjects (it was calculated together with allele b for its low frequency).
Plasma NO concentration
In order to determine whether the polymorphisms of eNOS influence the activity of eNOS enzyme,we measured and compared the plasma NO level in HAPE patients and healthy controls,also the NO level in the subjects with different genotypes.We did not find a significant difference in NO generation between HAPE group and control group.Although 894G/T polymorphism was found to be associated with HAPE susceptibility (P<0.01),we did not detect any difference in NO level between individual carriers of the T894 or G894 variants in either patient or control groups(bothP>0.05,data not shown).
Haplotype analysis
Haplotype analysis revealed a significant association between the haplotypes of eNOS and susceptibility or resistance to HAPE.As shown in Table 4,there are seven possible haplotypes in the Han Chinese in our study groups (the haplotype C-T-b was excluded from this study for its rare occurrence with a frequency less than 0.05‰).Three of these haplotypes were found common (>50‰ in frequency),and also with significant inter-group differences in their frequencies (allP<0.001).Interestingly,although the allele frequencies did not always show significant difference between the two groups,we found significant difference in the overall distribution of eNOS haplotypes.In particular,the H1(T-G-b) and H2(T-G-a) haplotypes were found more common in the control group than in the HAPE group (862.20‰vs.802.74‰,P<0.001;37.89‰vs.3.47‰,P<0.0001),suggesting that the two haplotypes may provide some protection against the development of HAPE.In contrast,the H3(T-T-b) and H6(C-G-a) haplotypes were more common in the HAPE group than in control group (65.17‰vs.15.15‰,P<0.0001,odd ratio=4.54;102.02‰vs.46.96‰,P<0.0001,odd ratio=2.31),indicating that H3and H6haplotypes possibly increase susceptibility to HAPE.These two haplotypes account for 16.7% of all the detected haplotypes.
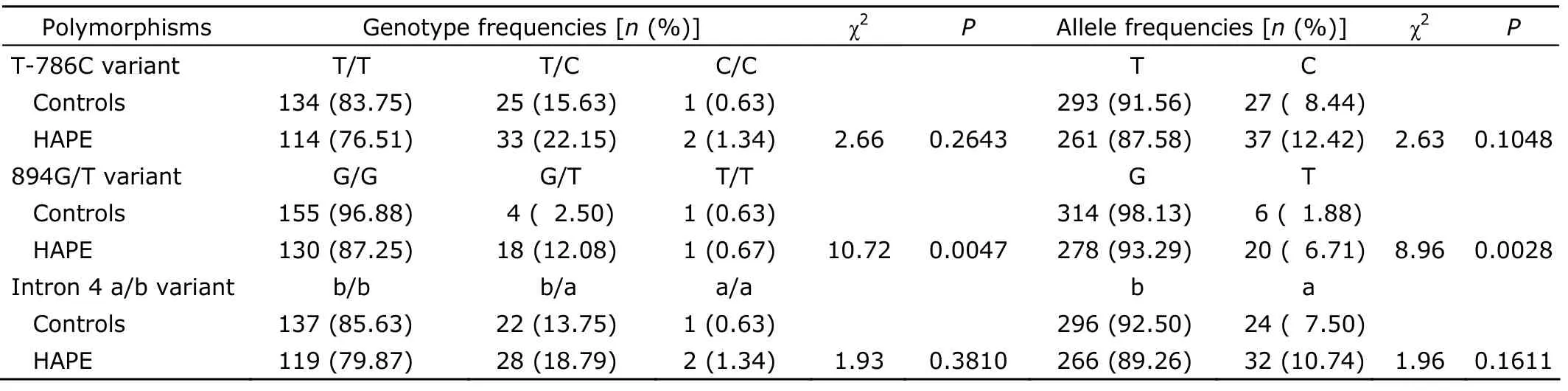
Table 3.Genotypes and alleles frequencies of the three polymorphisms of eNOS among HAPE patients and healthy controls
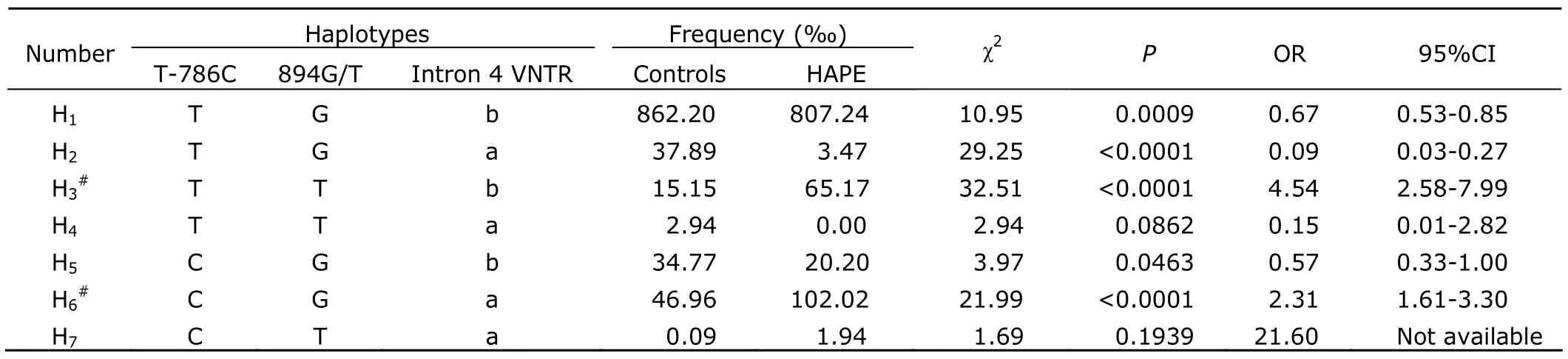
Table 4.Estimated haplotype frequencies of eNOS gene in HAPE patients and healthy controls
DISCUSSION
The present case-control study investigated the association of eNOS gene,at the allelic,genotypic,and haplotypic levels,with the susceptibility to HAPE in a large group.The most noteworthy finding of this study is the significantly positive association of the H3(T-T-b) and H6(C-G-a) haplotypes of eNOS gene with HAPE in the studied Chinese railway construction workers at Qinghai-Tibet construction sites over 4 500 m above sea level.The data from haplotype frequency analysis may suggest the possibility to screen out high risk population susceptible to HAPE at genetic level.
Association of the polymorphisms of eNOS with susceptibility to HAPE has not been generally acknowledged,well illustrated by the contradictory findings about the association of 894G/T substitution and 27bp VNTR with HAPE from three studies with cohorts of three different nationalities.17-19The conflict among these studies may be attributed to various factors,the most important of which was genetic background,because the distribution of the 894G/T allele,27bp VNTR,and T-786C allele of eNOS gene in normal control population varies among different reports involving different populations.21,23-25
In the present study,the HAPE patients and healthy controls match in demographical characteristics,lifestyle,and working conditions,allowing an unbiased estimation of the relative risk parameters.In addition,the size of the cohort avoids some limitations encountered in studies in small groups of subjects.4,5,18-20These designs add more reliability to our findings on association of eNOS alleles and haplotypes with susceptibility or resistance to HAPE.26
Of the three polymorphisms studied,only the 894G/T polymorphism was found statistically associated with susceptibility to HAPE,implying that this missense mutation might either affect the function of the eNOS enzyme,or be closely linked to some undetected locus that affects HAPE susceptibility.Since the 894G/T variant is located in exon 7 of the eNOS gene,it may induce changes in eNOS activity,leading to an increase or decrease in NO production.However,no significant difference associated with G894 or T894 variants was found in NO level,leaving the involvement of 894G/T polymorphism in eNOS regulatory activity an open possibility.It is also likely that there is a polymorphism in another gene in linkage disequilibrium with the 894G/T variant.Nagassaki et al27once reported that eNOS genotype and allele have no effect on circulating NO levels in healthy males,hence the 894G/T polymorphism is probably only a genetic marker associated with some undetected locus.On the other hand,the positive association of the two eNOS haplotypes with HAPE susceptibility emphasizes the possibility that interaction of multiple genetic markers in a haplotype may be a major determinant of HAPE susceptibility.28,29Therefore,we postulate that 894G/T polymorphism is a genetic marker associated with an unidentified functional variant.Further studies are necessary to confirm this postulation.
In conclusion,our findings suggest that the haplotypes H1(T-G-b) and H2(T-G-a) in eNOS gene might have a protective effect against the development of HAPE,while the haplotypes H3(T-T-b) and H6(C-G-a) might increase susceptibility to HAPE.These findings may provide helpful information for singling out individuals susceptible to HAPE.
ACKNOWLEDGEMENT
We thank the 309 railway construction workers for participating in this study,and all the doctors working at the railway worksite hospitals for their kind assistance.
1.Hackett PH,Roach RC.High-altitude illness.N Engl J Med 2001;345:107-14.
2.Mortimer H,Patel S,Peacock AJ.The genetic basis of high-altitude pulmonary oedema.Pharmacol Ther 2004;101:183-92
3.Hotta J,Hanaoka M,Droma Y,et al.Polymorphisms of renin-angiotensin system genes with high-altitude pulmonary edema in Japanese subjects.Chest 2004;126:825-30.
4.Qi Y,Niu W,Zhu T,et al.Synergistic effect of the genetic polymorphisms of the renin-angiotensin-aldosterone system on high-altitude pulmonary edema:a study from Qinghai-Tibet altitude.Eur J Epidemiol 2008;23:143-52.
5.Saxena S,Kumar R,Madan T,et al.Association of polymorphisms in pulmonary surfactant protein A1 and A2 genes with high-altitude pulmonary edema.Chest 2005;128:1611-9.
6.Qi Y,Niu WQ,Zhu TC,et al.Genetic interaction of Hsp70 family genes polymorphisms with high-altitude pulmonary edema among Chinese railway constructor at altitudes exceeding 4000 meters.Clin Chim Acta 2009;405:17-22.
7.Stobdan T,Kumar R,Mohammad G,et al.Probable role of beta2-adrenergic receptor gene haplotype in high-altitude pulmonary oedema.Respirology 2010;15:651-8.
8.Anand IS,Prasad BA,Chugh SS,et al.Effects of inhaled nitric oxide and oxygen in high-altitude pulmonary edema.Circulation 1998;98:2441-5.
9.Ahsan A,Mohd G,Norboo T,et al.Heterozygotes of NOS3 polymorphisms contribute to reduced nitrogen oxides in high-altitude pulmonary edema.Chest 2006;130:1511-9.
10.Palmer RM,Ferrige AG,Moncada S.Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor.Nature 1987;327:524-6.
11.Palmer LA,Semenza GL,Stoler MH,et al.Hypoxia induces type II NOS gene expression in pulmonary artery endothelial cellsviaHIF-1.Am J Physiol 1998;274:L212-9.
12.Moncada S,Palmer RM,Higgs EA.Nitric oxide:physiology,pathophysiology,and pharmacology.Pharmacol Rev 1991;43:109-42.
13.Chartrain NA,Geller DA,Koty PP,et al.Molecular cloning,structure,and chromosomal localization of the human inducible nitric oxide synthase gene.J Biol Chem 1994;269:6765-72.
14.Grasemann H,Yandava CN,Storm van’s Gravesande K,et al.A neuronal NO synthase (NOS1) gene polymorphism is associated with asthma.Biochem Biophys Res Commun 2000;272:391-4.
15.Marsden PA,Heng HH,Scherer SW,et al.Structure and chromosomal localization of the human constitutive endothelial nitric oxide synthase gene.J Biol Chem 1993;268:17478-88.
16.Droma Y,Hanaoka M,Ota M,et al.Positive association of the endothelial nitric oxide synthase gene polymorphisms with high-altitude pulmonary edema.Circulation 2002;106:826-30.
17.Weiss J,Haefeli WE,Gasse C,et al.Lack of evidence for association of high altitude pulmonary edema and polymorphisms of the NO pathway.High Alt Med Biol 2003;4:355-66.
18.Lorenzo VF,Yang Y,Simonson TS,et al.Genetic adaptation to extreme hypoxia:Study of high-altitude pulmonary edema in a three-generation Han Chinese family.Blood Cells Mol Dis 2009;43:221-5.
19.Dhillom SS,Mahadevan K,Bandi V,et al.Neutrophils,nitric oxide and microvascular permeability in severe sepsis.Chest 2005;128:1706-12.
20.Yoshimura M,Yasue H,Nakayama M,et al.Genetic risk factors for coronary artery spasm:significance of endothelial nitric oxide synthase gene t-786-->c and missense glu298asp variants.J Investig Med 2000;48:367-74.
21.Lamblin N,Cuilleret FJ,Helbecque N,et al.A common variant of endothelial nitric oxide synthase (Glu298Asp) is associated with collateral development in patients with chronic coronary occlusions.BMC Cardiovasc Disord 2005;5:27.
22.Ahsan A,Charu R,Pasha MA,et al.eNOS allelic variants at the same locus associate with HAPE and adaptation.Thorax 2004;59:1000-2.
23.Hingorani AD,Liang CF,Fatibene J,et al.A common variant of the endothelial nitric oxide synthase (Glu298→Asp) is a major risk factor for coronary artery disease in the UK.Circulation 1999;100:1515-20.
24.Tanus-Santos JE,Desai M,Flockhart DA.Effects of ethnicity on the distribution of clinically relevant endothelial nitric oxide variants.Pharmacogenetics 2001;11:719-25.
25.Hoffmann IS,Tavares-Mordwinkin R,Castejon AM,et al.Endothelial nitric oxide synthase polymorphism,nitric oxide production,salt sensitivity and cardiovascular risk factors in Hispanics.J Hum Hypertens 2005;19:233-40.
26.Cox NJ,Bell GI.Disease associations.Chance,artifact,or susceptibility genes? Diabetes 1989;38:947-50.
27.Nagassaki S,Metzger IF,Souza-Costa DC,et al.eNOS genotype is without effect on circulating nitrite/nitrate level in healthy male population.Thromb Res 2005;115:375-9.
28.Crawford DC,Nickerson DA.Definition and clinical importance of haplotypes.Annu Rev Med 2005;56:303-20.
29.Johnson GC,Esposito L,Barratt BJ,et al.Haplotype tagging for the identification of common disease genes.Nat Genet 2001;29:233-7.
 Chinese Medical Sciences Journal2010年4期
Chinese Medical Sciences Journal2010年4期
- Chinese Medical Sciences Journal的其它文章
- Thoraco-abdominal Aorta Revascularization through a Retroperitoneal Approach
- Clinicopathological Features of Non-familial Colorectal Cancer with High-frequency Microsatellite Instability△
- Epigenetic Repression of SATB1 by Polycomb Group Protein EZH2 in Epithelial Cells△
- NF-E2:a Novel Regulator of Alpha-hemoglobin Stabilizing Protein Gene Expression△
- Expression of Cyclooxygenase-2 and Its Relationship with Mismatch Repair and Microsatellite Instability in Hereditary Nonpolyposis Colorectal Cancer△
- Giant Hydroureteronephrosis Associated with Ipsilateral Inguinal Hernia and Contralateral Hydronephrosis:a Case Study
