Changes of Nerve Growth Factor in Amniotic Fluid and Correlation with Ventriculomegaly△
Xiao-yan Xia ,Xing-hua Huang ,Yi-xin Xia *,and Wei-hua Zhang
1Department of Obstetrics,Beijing Obstetrics and Gynecology Hospital,Capital Medical University,Beijing 100026,China
2Department of Obstetrics and Gynecology,General Hospital of Armed Police Forces,Beijing 100039,China
NEUROTROPHINS (NTs) constitute a family of structurally related molecules,including nerve growth factor (NGF),brain-derived neurotrophic factor,glial cell line-derived neurotrophic factor,NT-3,and NT-4/5.1NTs support the survival and maintenance of specific populations of neurons both in peripheral and central nervous system.2As a member of this family,NGF is an important neurotrophic factor in cerebral cortical development for its ability to stimulate neuronal precursor cell proliferation.NGF plays a role in promoting the growth and differentiation of neurons,regulating the survival of motor neurons,exciting and stimulating the growth of axons and dendrites of neurons,and inducing the destining growth of neuronal fiber.3
Ventriculomegaly (VM) is defined as the presence of an excess of fluid in the lateral ventricles of the developing brain.4Fetal VM is one of the most common findings in ultrasound screening for fetal malformation during pregnancy,with an incidence of 1-2/1000.5,6The diagnosis is usually made when the diameter of one or both ventricles is ≥10 mm from 14 weeks of gestational age to term.VM is severe when the width of the ventricular is over 15 mm.7However,the sensitivity of ultrasound is low when the gestational age is less than 24 weeks.7Moreover,VM is among the most common false positive diagnoses at ultrasound screening for fetal malformation.8A novel sensitive approach is therefore necessary for timely detection of VM.In this study,we detected the change of NGF in amniotic fluid during pregnancy,and explored the correlation of NGF in amniotic fluid with fetal VM.
PATIENTS AND METHODS
Experimental model
The subjects (collected in the period of 2004-2007) were divided into four groups.The first was second-trimester pregnancy group (n=113).The second was third-trimester pregnancy group (n=110).The third group was fetal VM or choroid plexus cyst (CPS) (n=12),detected by ultrasound performed at 20-24 weeks.The subjects in the forth group,healthy controls (n=12),were detected with neither VM nor other abnormalities in ultrasonography,and matched with VM group in gestational age.All the general clinical data were recorded including maternal age,gestational weeks,complications,and abnormal history of pregnancy.
Sample collection
In the second-trimester pregnancy group,amniotic fluid was obtained during amniocentesis.Indications for amniocentesis included advanced maternal age,high risk result of triple screen test,and abnormal history of pregnancy.The amniotic fluid in the third-trimester pregnancy group was collected during cesarean section,including one case of twin gestation with two amniotic fluid samples and one triplet with three amniotic fluid samples.In VM group,the amniotic fluid was collected during amniocentesis for chromosomal karyotype or pregnant termination.In control group,the amniotic fluid was obtained during amniocentesis for advanced maternal age.Each sample was sterilized and collected into two cups in order to duplicate the experiment.The samples were centrifuged for 10 minutes at 3000 rpm (relative centrifugal force 10 ×g)within 4-6 hours after collection,and frozen at-80°C until assayed.
Enzyme-linked immunosorbent assay (ELISA)
Two-site ELISA was performed according to the manufacturer’s instructions (Promega ImmunoAssay System,Beijing,China).Briefly,100 μL of coating buffer containing the capture antibody (anti-NGF polyclonal antibody) was added to 96-well ELISA plates and incubated for 14-18 hours at 4°C.The plates were then washed and blocked overnight at 4°C with blocking buffer.Subsequently,100 μL NGF at different concentrations (original solution and serial dilutions gradually diluted in the ratio of 1∶2,until reaching the ratio of 1∶2000) and samples were added to the wells,and the plates were incubated at room temperature for 6 hours with shaking.Then the wells were incubated with anti-NGF monoclonal antibody (Promega,Madison,WI,USA) at 4°C for 16-20 hours.After washing,100 μL detection antibody (anti-rat IgG-horseradish peroxidase conjugate,Promega) was added into the plates and incubated at room temperature for 2.5 hours with shaking.Following multiple washing,the wells were treated with 100 μL of tetramethylbenzidine solution and incubated in the dark at room temperature for 5-10 minutes.Development was stopped by adding 50 μL of 1N hydrochloric acid.The plates were read at OD 450 nm.9
Statistical analysis
Statistical analysis was performed using SAS V 9.1 software.The data are expressed as means±SD.Normal distribution of the data was tested.Inter-group differences were tested with the Kruskal-Wallis analysis of variance(ANOVA).An analysis of covariance was performed between the VM group and control group in order to rule out the impact of gestational ages.P<0.05 was considered statistically significant.
RESULTS
Characteristics of the four groups were described in Table 1.Twelve cases with VM or CPS were determined by ultrasonography (Table 2).
According to the results in second-trimester pregnancy group and third-trimester pregnancy group,the NGF levels in amniotic fluid were found decreased along with the increase of gestational age (Fig.1).There was a strong negative correlation between the NGF levels and gestational age (r=-0.6149,P<0.0001).The NGF level in amniotic fluid in fetal VM group was significantly lower than that in healthy controls (33.95±29.24 pg/mLvs.64.73±16.21 pg/mL,P=0.024,Table 3).The NGF level in amniotic fluid was 56.60±26.73 pg/mL in second-trimester pregnancy group,and 12.62± 13.95 pg/mL in third-trimester pregnancy group,significantly higher in the former group(P<0.0001,Table 3).
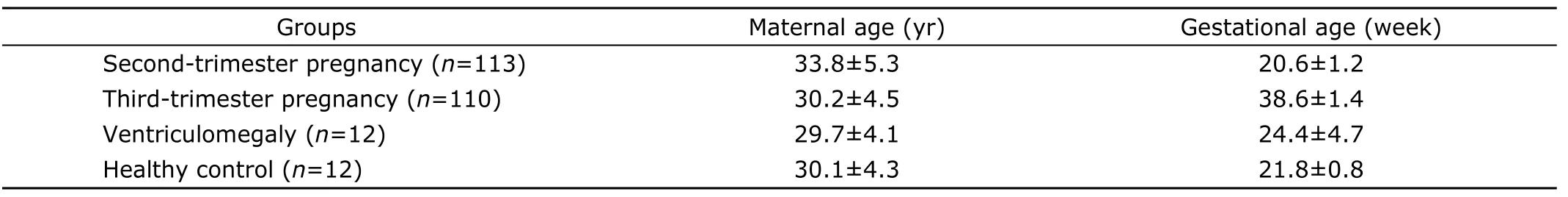
Table 1.Demographic and clinical information of the four studied groups§
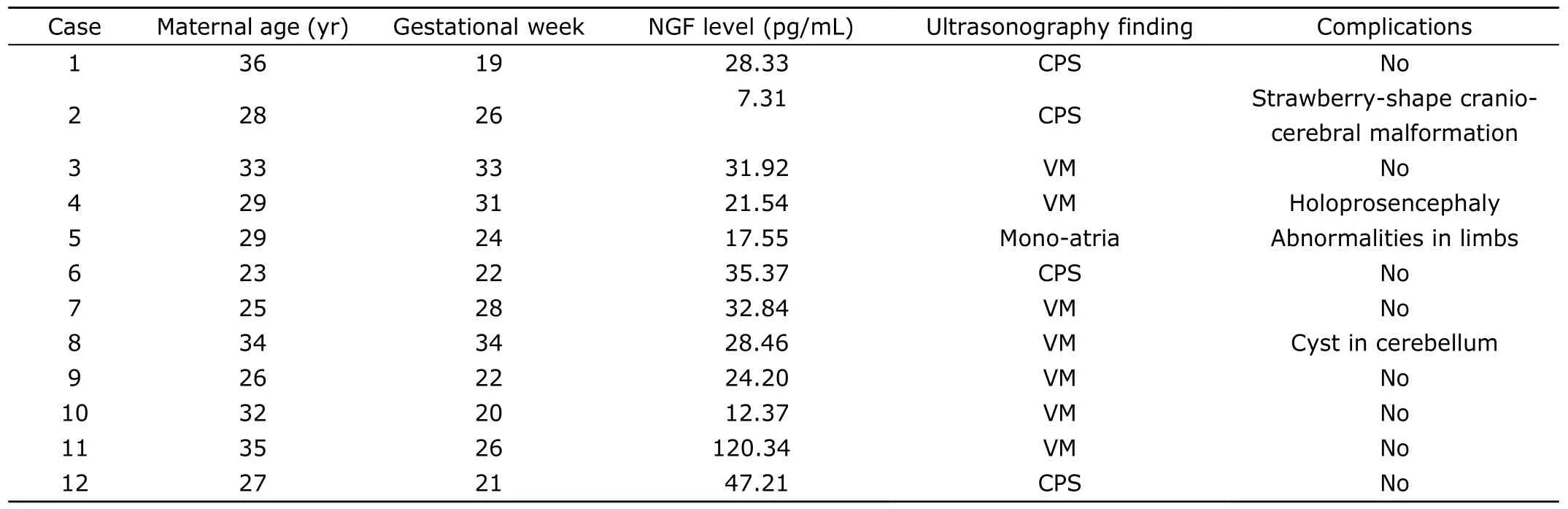
Table 2.Ultrasonography description of fetal nerve system abnormalities in VM group (n=12)
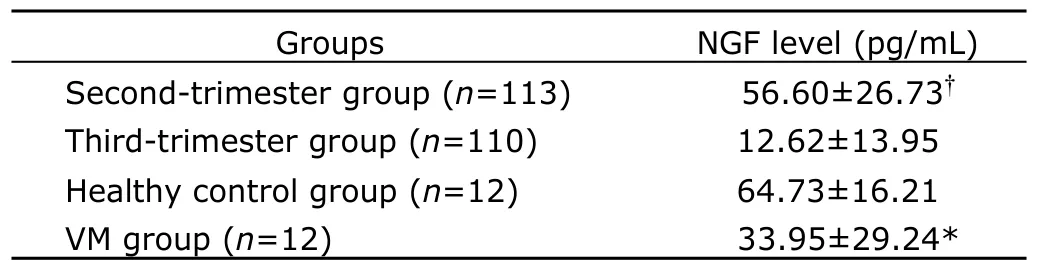
Table 3.Comparison of NGF level between ventriculomegaly group and healthy controls;comparison of secondtrimester group with third-trimester group using analysis of covariance§
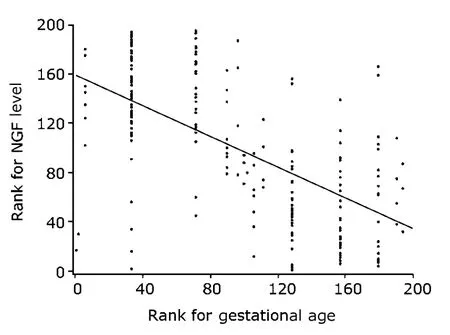
Figure 1.Relationship between the NGF level in amniotic fluid and gestational age in second-trimester pregnancy group (n=113) and third-trimester pregnancy group(n=110).The scatter diagram shows a significantly negative correlation between NGF level and gestational age (r=-0.6149,P<0.0001).
DISCUSSION
In this study,we first found that NGF level decreased from second-trimester to third-trimester,indicating that NGF level in human amniotic fluid declines as gestational age increases.This change of NGF in amniotic fluid corresponds with the development of fetal nerve system.10A study by Mashayekhi et al shown that the total NGF level in cerebrospinal fluid drops in the process of chick embryo development.11These findings suggest that NGF levels might increase in the early stage of embryonic development,and reach a peak at a specific time,then decrease while development proceeds.Further studies in human are necessary to confirm that hypothesis.
Hydrocephaly means VM with increased cerebrospinal fluid pressure and usually an abnormally large head circumference.VM can result from different conditions,such as abnormal turnover of cerebrospinal fluid,abnormal migration and proliferation of neurons,agenesis of the corpus callosum,and holoprosencephaly.12Since it is impossible to measure the cerebrospinal fluid pressure of fetus in uterus,the finding of VM in prenatal ultrasound screening is relied upon to indicate abnormal accumulation of cerebrospinal fluid in cerebral ventricles.4However,the sensitivity is low at gestational ages shorter than 24 weeks,with only 57% of severe VM cases diagnosed before 24 weeks.7The low sensitivity before 24 weeks probably reflects the natural history of the disease,not a measurement error.Because cerebrospinal fluid accumulation in the ventricular system develops over time and VM becomes evident only later in gestation.4In order to rule out the influence of gestational weeks,we selected the healthy control group matched with VM group in gestational age.Inter-group comparison revealed significantly lower NGF level in VM group than that in healthy control group.A negative correlation between NGF levels in amniotic fluid and fetal VM was found,consistent with the finding by Marx et al.12Although the relationship between NGF level in amniotic fluid and the development of fetal nerve system has not been clarified yet,decreased NGF levels in amniotic fluid may be a marker for the presence of fetal VM and other central nervous system abnormalities.12
As mentioned above,fetal VM was mainly detected with ultrasound screening and magnetic resonance imaging for fetal malformation.However,VM is among the most common false positive diagnoses at ultrasound screening for fetal malformation.8Our findings suggest that the NGF level in amniotic fluid may help in diagnosing VM prenatally with high accuracy.However,our sample size was not large enough.Besides,amniocentesis in second-trimester pregnancy may have the risk of fetal loss at the incidence of about 0.5%.13Further research is required to confirm the finding of the present study,and to identify the change of NGF level in blood during pregnancy and its correlation with the NGF level in amniotic fluid.We hope a safer method could be developed in earlier detecting fetal VM and other cerebral abnormalities.
In conclusion,our study showed that during pregnancy,the NGF level in amniotic fluid decreases while gestation proceeds.The NGF level is significantly lower in fetal VM than in healthy controls.A significantly negative correlation has been found between the NGF level and fetal VM.
We thank Prof.Ying-yu Chen for direction in ELISA test,Sha-sha Li and Li Li for assistance in collecting amniotic fluid samples during amniocentesis,and Xin Gao for assistance with statistical analysis.
1.Tyler WJ,Perrett SP,Pozzo-Miller LD.The role of neurotrophins in neurotransmitter release.Neuroscientist 2002;8∶524-31.
2.Huang EJ,Reichardt LF.Neurotrophins∶roles in neuronal development and function.Annu Rev Neurosci 2001;24∶677-736.
3.Mashayekhi F,Salehi Z.Infusion of anti-nerve growth factor into the cisternum magnum of chick embryo leads to decrease cell production in the cerebral cortical germinal epithelium.Eur J Neurol 2007;14∶181-6.
4.Gaglioti P,Oberto M,Todros T.The significance of fetal ventriculomegaly∶etiology,short-and long-term outcomes.Prenat Diagn 2009;29∶381-8.
5.Garel C,Luton D,Oury JF,et al.Ventricular dilatations.Childs Nerv Syst 2003;19∶517-23.
6.Leitner Y,Stolar O,Rotstein M,et al.The neurocognitive outcome of mild isolated fetal ventriculomegaly verified by prenatal magnetic resonance imaging.Am J Obstet Gynecol 2009;201∶215.e1-6.
7.Gaglioti P,Danelon D,Bontempo S,et al.Fetal cerebral ventriculomegaly∶outcome in 176 cases.Ultrasound Obstet Gynecol 2005;25∶372-7.
8.Martinez-Zamora MA,Borrell A,Borobio V,et al.False positives in the prenatal ultrasound screening of fetal structural anomalies.Prenat Diagn 2007;27∶18-22.
9.Xing J,Lu J,Li J.Contribution of nerve growth factor to augmented TRPV1 responses of muscle sensory neurons by femoral artery occlusion.Am J Physiol Heart Circ Physiol 2009;296∶H1380-7.
10.Cunningham FG,Leveno KJ,Bloom SL,et al.Fetal growth and development.In∶Cunningham FG,Leveno KJ,Bloom SL,et al,editors.Williams obstetrics.22nd ed.New York∶McGraw-Hill;2005.p.91-120.
11.Mashayekhi F,Azari M,Moghadam LM,et al.Changes in cerebrospinal fluid nerve growth factor levels during chick embryonic development.J Clin Neurosci 2009;16∶1334-7.
12.Marx CE,Vance BJ,Jarskog LF,et al.Nerve growth factor,brain-derived neurotrophic factor,and neurotrophin-3 levels in human amniotic fluid.Am J Obstet Gynecol 1999;181∶1225-30.
13.Cunningham FG,Leveno KJ,Bloon SL,et al.Prenatal diagnosis and fetal therapy.In∶Cunningham FG,Leveno KJ,Bloom SL,et al,editors.Williams obstetrics.22nd ed.New York∶McGraw-Hill;2005.p.313-39.
 Chinese Medical Sciences Journal2011年2期
Chinese Medical Sciences Journal2011年2期
- Chinese Medical Sciences Journal的其它文章
- Risk Factors Analysis on Traumatic Brain Injury Prognosis
- Erythropoietin Receptor Positive Circulating Progenitor Cells and Endothelial Progenitor Cells in Patients with Different Stages of Diabetic Retinopathy△
- Inhibition of SIRT1 Increases EZH2 Protein Level and Enhances the Repression of EZH2 on Target Gene Expression△
- Immediate Surgical Intervention for Penile Fracture:a Case Report and Literature Review
- Clinical Treatment and Anatomy Study of Maxillary First Molars with Five Root Canals
- Magnetic Resonance Urography and X-ray Urography Findings of Congenital Megaureter
