Pharmacokinetics and Biodegradation Performance of a Hydroxypropyl Chitosan Derivative
SHAO Kai, HAN Baoqin, DONG Wen, SONG Fulai, LIU Weizhi, and LIU Wanshun
College of Marine Life Science, Ocean University of China, Qingdao 266003, P. R. China
Pharmacokinetics and Biodegradation Performance of a Hydroxypropyl Chitosan Derivative
SHAO Kai, HAN Baoqin*, DONG Wen, SONG Fulai, LIU Weizhi, and LIU Wanshun
College of Marine Life Science, Ocean University of China, Qingdao 266003, P. R. China
? Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
Hydroxypropyl chitosan (HP-chitosan) has been shown to have promising applications in a wide range of areas due to its biocompatibility, biodegradability and various biological activities, especially in the biomedical and pharmaceutical fields. However, it is not yet known about its pharmacokinetics and biodegradation performance, which are crucial for its clinical applications. In order to lay a foundation for its further applications and exploitations, here we carried out fluorescence intensity and GPC analyses to determine the pharmacokinetics mode of fluorescein isothiocyanate-labeled HP-chitosan (FITC-HP-chitosan) and its biodegradability. The results showed that after intraperitoneal administration at a dose of 10 mg per rat, FITC-HP-chitosan could be absorbed rapidly and distributed to liver, kidney and spleen through blood. It was indicated that FITC-HP-chitosan could be utilized effectively, and 88.47% of the FITC-HP-chitosan could be excreted by urine within 11 days with a molecular weight less than 10 kDa. Moreover, our data indicated that there was an obvious degradation process occurred in liver (< 10 kDa at 24 h). In summary, HP-chitosan has excellent bioavailability and biodegradability, suggesting the potential applications of hydroxypropyl-modified chitosan as materials in drug delivery, tissue engineering and biomedical area.
hydroxypropyl chitosan; fluorescein isothiocyanate; pharmacokinetics; biodegradation performance; biomedical material
1 Introduction
Chitosan, composed of D-glucosamine and N-acetyl D-glucosamine units, is an amino cationic polysaccharide derived from chitin by deacetylation. Due to its biocompatibility and biodegradability, chitosan has been widely used in a broad range of areas, such as material, medicine, food and agriculture (Dutta et al., 2009; Honarkar and Barikani, 2009; Pillai et al., 2009; Carreira et al., 2010). Nevertheless, poor solubility of chitosan in neutral solutions greatly limited its applications (Nishimura et al., 1991; Park et al., 2003). Therefore, many chemical modifications had been successively carried out to improve its water solubility (Xie et al., 2007; Dash et al., 2011). Carboxymethyl chitosan (CM-chitosan) and HP-chitosan are two important derivatives of chitosan. To meet different application requirements, many kinds of modifications have been developed based on CM-chitosan and HP-chitosan (Rinaudo, 2006; Jayakumar et al., 2010). Carboxymethyl derivatives of chitosan have been well characterized and applied in drug delivery, wound healing and tissue engineering (Khor and Lim, 2003; Chien et al., 2012; Shi et al., 2012). Early studies have showedthat HP-chitosan is a multifunctional water-soluble chitosan derivative once hydrophilic moiety to the OH groups at C-6 and C-3 and the NH2group of chitosan were introduced (Dong et al., 2001; Peng et al., 2005). Indeed, introduction of hydroxypropyl group to chitosan endow chitosan with new features like significant increase of solubility and moisturizing capability (Zhu et al., 2001; Wan et al., 2004; Peng et al., 2005), extending its applications as biomedical material, pharmaceutical excipient and bioactive reagent (Xie et al., 2002a; Liu et al., 2003; Prabaharan and Mano, 2005). Using HP-chitosan, Wang et al. (2010) developed an efficient targeted delivery of antisense oligodeoxynucleotides, a potential approach to overcome tumor drug resistance. It was also found that the grafted HP-chitosan copolymer killed 99.9% of S. aureus and E. coli within 30 min, and had relatively higher superoxide anion scavenging ability owing to the incorporation of hydroxyl groups (Xie et al., 2002b; Sun et al., 2004). In addition, HP-chitosan derivatives have been employed as composite hydrogel for corneal endothelium reconstruction and adhesion scaffold of mesenchymal stem cells in tissue engineering (Gao et al., 2008; Wang et al., 2009; Liang et al., 2011). Recently, more and more experimental results have been reported on the potential applications and exploitations of CM-chitosan and HP-chitosan in absorbable biomaterial field (Ling et al., 2008; Bayramoglu et al., 2011; Rivaet al., 2011).
Pharmacokinetics and degradation performance in vivo is critical for the medical application of biomaterials. CM-chitosan and HP-chitosan can be absorbed and degraded in vivo. Thus, to apply CM-chitosan and HP-chitosan as biological materials, it is important to ascertain the routes (Ravi Kumar, 2000). In order to gain clear insights into the pharmacokinetics mode of the modified-chitosan in vivo and its biodegradation performance, FITC was used as a fluorescent tracer and control. We previously evaluated the tissue distribution, degradation behavior and excretion of fluorescein isothiocyanate (FITC)-labeled CM-chitosan (FITC-CM-chitosan) through intraperitoneally administrating it to rats (Dong et al., 2010). We also compared the effects of molecular weight on the degradation performance of FITC-CM-chitosan. The results showed that molecular weight significantly influenced the pharmacokinetics and degradation performance of CM-chitosan after intraperitoneal administration in rats (Dong et al., 2012). However, the relevant studies on HP-chitosan have not been investigated yet.
In this study, we first synthesized hydroxypropylmodified chitosan and then verified such synthesis by FTIR and1H NMR. Through fluorescence intensity and GPC analysis on a rat model, we calculated the molecular weights of the body-distributed and urinary-excreted FITC-HP-chitosan. The absorption, distribution, pharmacokinetics, degradation and excretion of FITC-HP-chitosan were investigated. Moreover, the degradability of HP-chitosan in abdominal dropsy, plasma and homogenate of liver were also evaluated in vitro to confirm the acquired results in vivo. These findings provided important reference for the further exploitation and application of HP-chitosan in drug delivery, tissue engineering and other biomedical treatments.
2 Materials and Methods
2.1 Materials
Chitosan with a deacetylation degree (DD) of 93% and molecular weight of 310 kDa (GPC) was supplied by Biotemed Co., Ltd. (Ghorbani et al., 2013). Dextran standards were purchased from the National Institute for the Control of Pharmaceutical and Biological Products. Sprague-Dawley rats (200 ± 10 g body weight) were obtained from Qingdao Institute for Drug Control. All other reagents used were reagent grade.
2.2 Synthesis of HP-Chitosan
HP-chitosan was prepared as described previously (Xie et al., 2002b; Peng et al., 2005) from chitosan and propylene oxide under alkali condition with minor modifications. The brief processes were as follows: Chitosan (20.0 g) was added into 40% NaOH (20.0 mL) at -20℃for 72 h for alkalization. Next, isopropyl alcohol (25.0 mL) was mixed with the alkali chitosan with continuous stirring for 2 h, and then added propylene oxide (25.0 mL) and stirred at 45℃ for 10 h. Finally, the mixture was neutralized with 50% HCI, filtrated, purified with 95% alcohol, and dried for further use.
2.3 Characterizations of HP-Chitosan
2.3.1 Fourier transforms infrared spectroscopy (FTIR)
To verify the chemical structure of HP-chitosan, FTIR spectra of chitosan and HP-chitosan were recorded on a Nicolet Nexus-470 Fourier Transform Infrared Spectrometer (US). KBr method was adopted and data analysis was carried out on Jwstda-32 (Sashiwa et al., 1990; Shigemasa et al., 1996).
2.3.21H NMR spectrum
To verify the chemical structure and calculate substitution degree (DS) of HP-chitosan,1H NMR spectra of chitosan and HP-chitosan were recorded on a Bruker DPX300 spectrometer (Germany), using acetone as internal standard and 1% DCl D2O and D2O as solvent, respectively, at 25℃ (Zheng et al., 2011).
2.4 Preparation of FITC-HP-Chitosan
Preparation of FITC-HP-chitosan was as follows: HP-chitosan was dissolved in 0.02 mol L-1PBS (pH 8.0) with the addition of FITC, and the mixture was stirred at 25℃for 24 h. After removal of free FITC by dialysis, labeled HP-chitosan was precipitated in dehydrated methanol. Then, final FITC-HP-chitosan product was freeze-dried. To avoid the attenuation in fluorescence intensity, whole process was conducted in darkness (Dong et al., 2010). The labeling ratio of FITC to D-glucosamine residue was determined according to the concentration of FITC and HP-chitosan in the reaction medium.
2.5 Animal Experiment
The experiments were carried out in compliance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. Sprague-Dawley rats were housed individually and kept under standard laboratory conditions with free access to food and water for 5 d. After treated with intraperitoneal administration of 1 mL FITC-HP-chitosan (10 mg mL-1) in distilled water, the rats were randomly divided into metabolism cages. Treatment with 1 mL FITC (400 μg mL-1) in distilled water was used as control.
2.6 Extraction of FITC-HP-Chitosan in Rats
The samples of FITC-HP-chitosan in various tissues (liver, kidney and spleen) and body fluids (abdominal dropsy and plasma) of each rat were collected and investigated at different time after administration, respectively. Urine was diluted with distilled water to a volume of 25 mL after collection in brown volumetric flask. Then it was centrifuged at 5000 rpm for 20 min at 4℃ and 7 mL of the supernatant urine was stored in darkness at -20℃. Each testing time, four rats from each group were sacrificed, the tissues and body fluids were collected andquantified on weight or volume. Abdominal dropsy (1 mL) was collected by washing the abdominal cavity with 5 mL distilled water. Blood (1 mL) was gained by abdominal aortic method and stored in anticoagulation tubes (3.8% sodium citrate). On the other hand, liver, kidney and spleen were weighed and 1.0 g of the tissues were homogenized for 20 s with a Tissue Tearor (IKA, Germany), separately. All the homogenate and body fluid samples were diluted to 7 mL with distilled water, then centrifuged at 8000 r min-1for 20 min at 4℃ and stored in darkness at -20℃ until use.
2.7 Fluorescence Intensity Assay
Since the concentrations of FITC and FITC-HP-chitosan were positively correlated with fluorescence intensity, the concentrations of FITC and FITC moiety were determined according to the standard curves. By analyzing the fluorescence intensity, weight and volume, the content of FITC and FITC-labeled HP-chitosan in different tissues and body fluids were obtained. The FITC group was set up as a reference substance when the pharmacokinetics biological behavior of HP-chitosan was evaluated. The assay was carried out on a Shimadzu RF-530PC spectrofluorophotometer (Japan), and the intensity was determined at 520 nm with the excitation wavelength of 490 nm (Onishi and Machida, 1999; Dong et al., 2012). Statistical analyses of all data were performed by one-way ANOVA on SPSS (version 20.0).
2.8 Determination of Peak Molecular Weight
Peak molecular weights (Mp) of FITC-HP-chitosan were determined by GPC using HPLC on Breeze1525 system, assembled with the refractive index and fluorescence detector at 520 nm and with the excitation wavelength of 490 nm. Separations were achieved with Two Shodex OHPak SB-806 M columns in series. The mobile phase was 0.02 mol L-1PBS (pH 8.0), containing 0.1 mol L-1Na2SO4at a flow rate of 1.0 mL min-1at 35℃. Standard curve was carried out by dextran standards with molecular weights of 180, 2500, 10000, 41100, 133800 and 2000000 Da. Data analyses were carried out on GPCW32 (version 6.11). The molecular weights of all samples were determined based on the standard curves (Dong et al., 2012).
2.9 Biodegradation of HP-Chitosan in vitro
For in vitro study of degradation mechanism of HP-chitosan, we adopted FITC as a fluorescent tracer as well to overcome the interference of autologous proteins and polysaccharides in body fluids in the GPC analysis. By creating an environment to mimic abdominal dropsy, plasma and homogenate of liver in vitro, the biodegradation of HP-chitosan was evaluated. The assay was performed as follows: FITC-HP-chitosan was dissolved in normal saline at a concentration of 10 mg mL-1. Then 4 mL normal saline, fresh abdominal dropsy, freshly prepared plasma or homogenate of liver were mixed with equal volume of FITC-HP-chitosan in saline solution, respectively. After incubated at 37℃ for 6 or 24 h, molecular weights of the FITC-HP-chitosan from different media were determined based on the standard curves developed by GPC analysis.
3 Results
3.1 Characterization of HP-Chitosan
The chemical structures of chitosan and HP-chitosan was determined by FTIR and1H NMR spectroscopy. The FTIR spectrum of chitosan (Fig.1a) showed the basic characteristic absorption peaks at 1030 cm-1(C-O stretching vibration of 3-OH), 1160 cm-1(C-O stretching vibration of 6-OH) and 1599 cm-1(-NH2). Whereas in the FTIR spectrum of HP-chitosan (Fig.1b), the characteristic absorption peaks of chitosan were at 1030 cm-1, and the peaks of 1160 cm-1and 1599 cm-1almost disappeared, indicating the hydroxypropylation reaction occurred at 6-OH and 3-OH, as well as NH2groups of chitosan. Furthermore, new characteristic peaks at 2970 cm-1and 1376 cm-1, which attributed to the C-H stretching and bending of the CH3group, revealed the hydroxypropylation of chitosan (Peng et al., 2005; Prabaharan and Mano, 2005).
Fig.2 presented the1H NMR spectra of chitosan and HP-chitosan. The proton assignment of chitosan was as follows: 1.91 ppm (CH3, acetamido group), 3.04 ppm (CH, C-2 of glucosamine ring), 3.61 ppm (CH, C-2 of glucosamine ring with the substituted amino group), 3.7–3.9 ppm (CH, C-3, 4, 5 and 6 of glucosamine ring), 4.75 ppm (CH, C-1 of glucosamine ring) (Zheng et al., 2011). The characteristic proton assignment of HP-chitosan appeared at 0.99–1.02 ppm (CH3, C-9), indicating the hydroxypropylation of chitosan (Xie et al., 2002b; Peng et al., 2005). DS designated as the average number of hydroxypropyl groups on each sugar residue was calculated by1H NMR analysis (Xie et al., 2002b; Peng et al., 2005). The DS value was 2.7, which was calculated by the following formula:
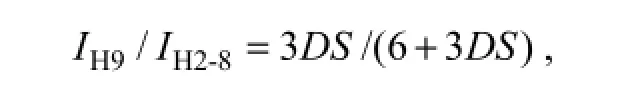
IH9and IH2-8represented the absorption intensity of hydrogen at C-9 and C-2 to C-8 (Fig.2). HP-chitosan was synthetized successfully with characteristic features and the GPC analysis confirmed the molecular weight was about 214 kDa.
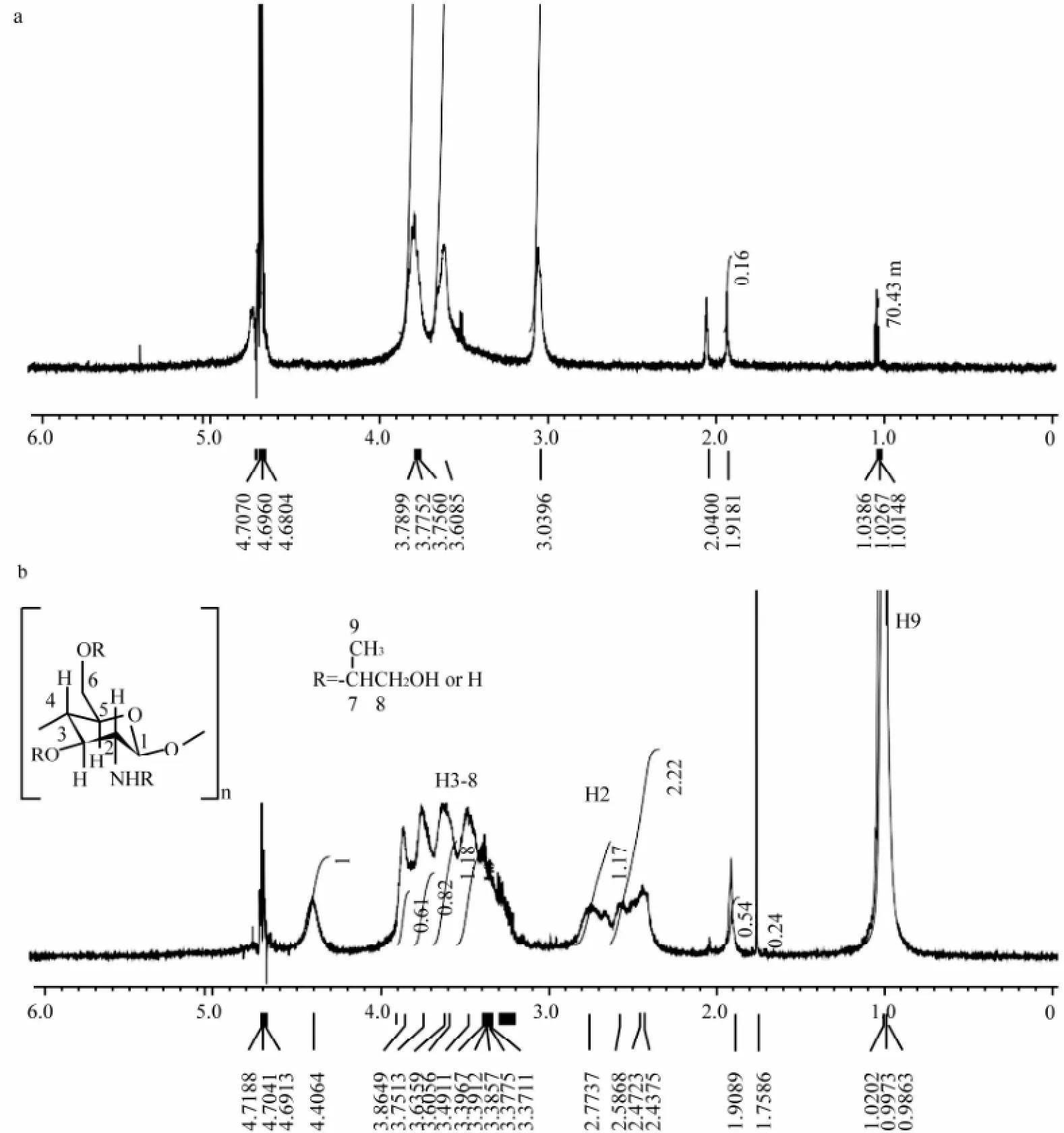
Fig.21H NMR spectra of chitosan (a) and HP-chitosan (b).
3.2 Labeling Efficiency of FITC-HP-Chitosan
To minimize the fluorescence intensity attenuation of FITC and FITC moiety, all the FITC and FITC-HP-chitosan samples were stored in darkness at -20℃ before analysis. By analysis of the standard curves of FITC and FITC-HP-chitosan, the content of FITC in FITC-HP-chitosan was 4.24%, and the labeling efficiency of FITCHP-chitosan was 1:40, one FITC moiety per 40 D-glucosamine residues.

Fig.3 FITC remains (a) and percentage (b) of FITC and FITC-HP-chitosan in abdominal dropsy at each sampling time points after intraperitoneal administration in rats; Means ± SD, n = 4.
3.3 Absorption of FITC-HP-Chitosan
The results indicated that the FITC-HP-chitosan could be absorbed rapidly and efficiently by the abdominal cavity in 6 h, after the administration of intraperitoneal injection in rats at a dose of 10000 μg per rat. As shown in Fig.3, for the FITC-HP-chitosan group, 91.9% of the original dose was absorbed at 6 h and 8.1% remained in the abdominal dropsy. After 6 h, a rapid decline was represented and the remains of FITC-HP-chitosan reduced to less than 1% at 72 h. In comparison to FITC-HP-chitosan, FITC showed a similar absorption curve and was absorbed more rapidly.
3.4 Distribution of FITC-HP-Chitosan in vivo
Following the intraperitoneal injection at a dose of 10000 μg per rat, FITC-HP-chitosan could be detected in blood and in several different tissues after 6 h. The concentration-time profile of FITC-HP-chitosan and FITC after intraperitoneal injection in plasma, and the distribution of labeled HP-chitosan in liver, kidney and spleen were shown in Fig.4.
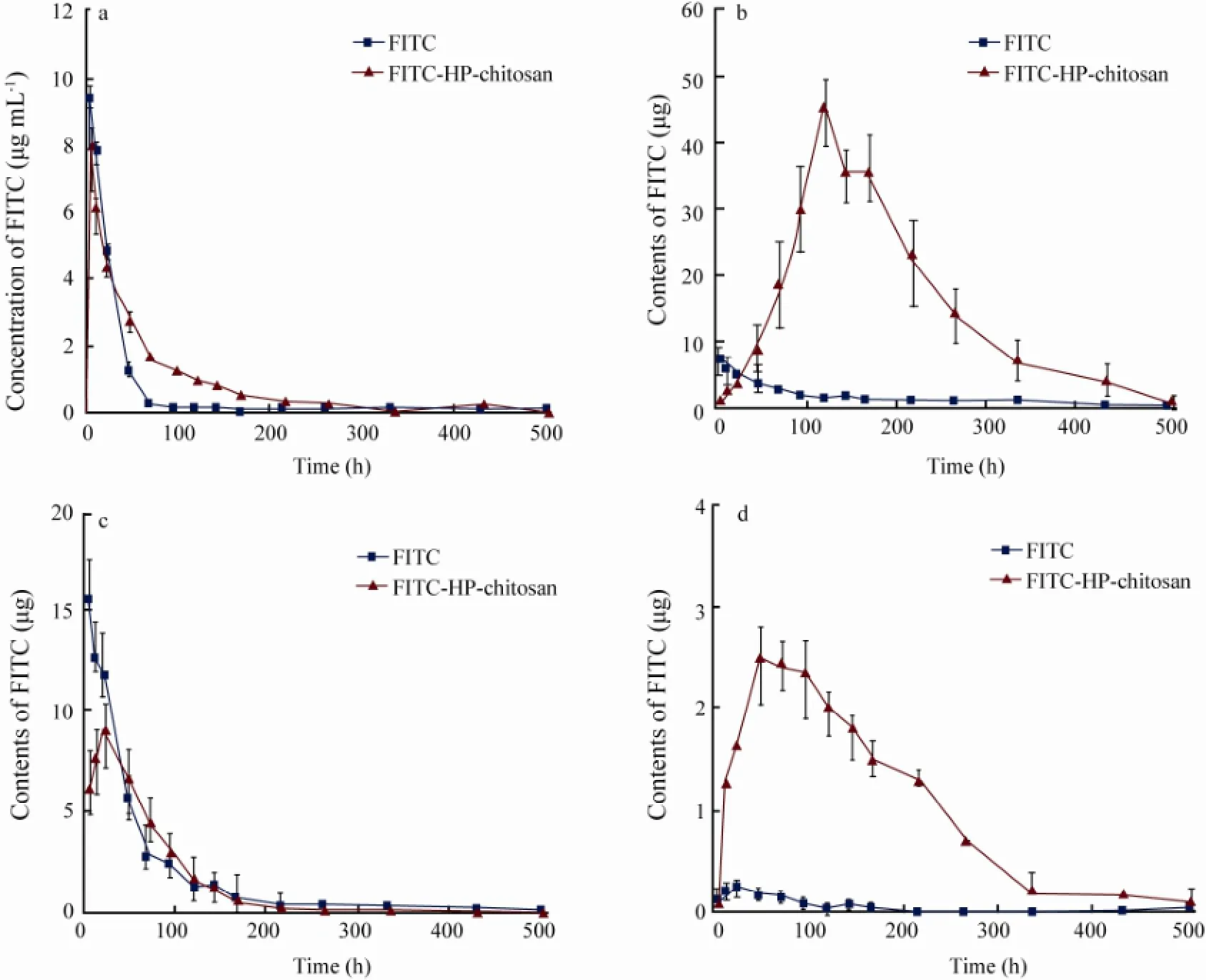
Fig.4 Concentration of the label of FITC and FITC-HP-chitosan in plasma (a); Tissue distribution of the label in liver (b), kidney (c) and spleen (d) at each sampling time points after intraperitoneal administration in rats; Means ± SD, n = 4.
The results in blood (Fig.4a) showed a rapid growth in contents of FITC-HP-chitosan in the first 6 h. At 6 h the content of remained FITC-HP-chitosan reached the top, 2631.25 μg mL-1, i.e., the FITC moiety of FITC-HP-chitosan was 7.97 μg mL-1, and then gradually declined to 97.00 μg mL-1(FITC moiety 0.29 μg mL-1) on day 9. In comparison to FITC-HP-chitosan, FITC showed a similar distribution curve. Thus FITC-HP-chitosan was transported mainly by blood system. The results in liver (Fig.4b) suggested that the amount of FITC-HP-chitosan increased continuously in the first 5 d. The peak FITC moiety of FITC-HP-chitosan was about 45.87 μg on day 5 and then gradually reduced to 4.12 μg on day 18. While for the FITC control, the average amount maintained at a lower level. On the whole, liver was a major organ where FITC-HP-chitosan distributed. The data in kidney (Fig.4c) showed that the content-time profile of FITC-HP-chitosan after intraperitoneal injection appeared to increase in the first 24 h and then decreased rapidly. And the peak FITC moiety was about 9.08 μg at 24 h. It was demonstrated that kidney played a certain role in FITC-HP-chitosan pharmacokinetics process. The results in spleen (Fig.4d) seemed to be intriguing that FITC scarcely distributed to spleen after intraperitoneal administration in rats at a dose of 400 μg per rat, while FITC-HP-chitosan kept a relative higher and noteworthy content in the first 11 d even though the peak FITC moiety was only 2.55 μg at 48 h. It has long been known that spleen is the major organ of the immune system, and HP-chitosan may contribute to the immune capacity of the body.
3.5 Excretion of FITC-HP-Chitosan in Rats
By fluorescence intensity analysis of the urine samples, it was demonstrated that urinary excretion was the major pathway for FITC and FITC-HP-chitosan (Onishi and Machida, 1999). The data in Fig.5 showed that 38.49% of the original dose of FITC-HP-chitosan was excreted within 24 h, and 88.47% was excreted in 11 d after intraperitoneal administration. As a control, FITC was found to be more rapid and efficient in urinary excretion process, as 62.35% of the original dose of FITC was excreted within 24 h, and 91.93% was excreted in 60 h after intraperitoneal administration.
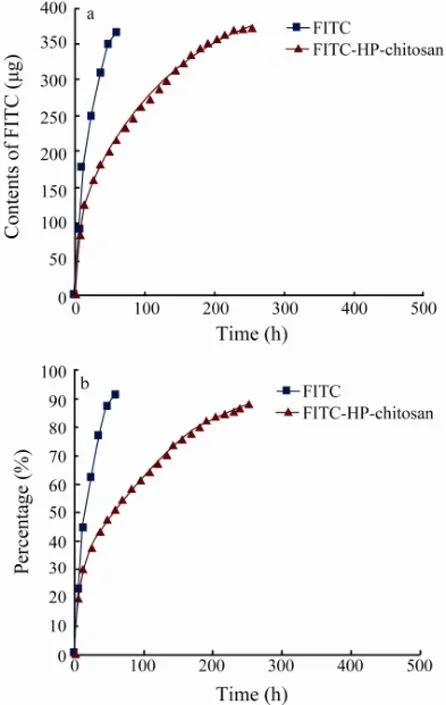
Fig.5 Cumulative amount (a) and percentage (b) of the label by urinary excretion of FITC and FITC-HP-chitosan at each sampling time points.
3.6 Degradation of FITC-HP-Chitosan in vivo
After intraperitoneal administration, the degradation performance of FITC-HP-chitosan remains unclear so far. GPC result showed that the degradation of FITC-HP-chitosan was related to abdominal dropsy, plasma and liver in particular. The GPC curves of FITC-HP-chitosan of non-treated and extracted samples of abdominal dropsy, plasma and urine 6 h after intraperitoneal injection was showed in Fig.6. From the analyses of the standard curve and retention times, the average peak molecular weights of FITC-HP-chitosan of non-treated and extracts of abdominal dropsy, plasma, and urine at 6 h was 213.80, 143.21, 126.76 and 0.72 kDa, respectively. Moreover, the molecular weight of degradation products in abdominal dropsy, plasma, liver and urine at 6 h and 24 h were investigated by GPC analysis (Fig.7). As was shown, molecular weight of the urinary excreted FITCHP-chitosan was less than 10 kDa. It was interesting to note that an objective degradation process (< 10 kDa at 24 h) occurred in liver, demonstrating that liver played a key role in degradation of FITC-HP-chitosan. On the other hand, the mechanism of degradation was related to abdominal dropsy (100 kDa–200 kDa at 6 h) and plasma (50 kDa–100 kDa at 24 h) to some degree. Additionally, HP-chitosan showed excellent biodegradability as 88.47% could be excreted in urine within 11 d with a molecular weight less than 10 kDa.
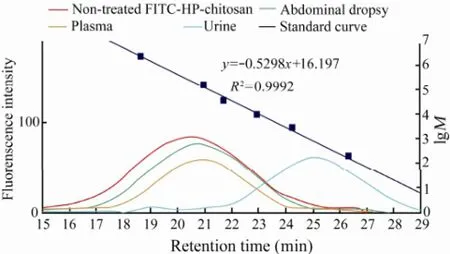
Fig.6 The GPC curves of FITC-HP-chitosan of nontreated and extracted samples of abdominal dropsy, plasma, and urine 6 h after intraperitoneal injection. The dextran standard was made up of 2000000, 133800, 41100, 10000, 2500, and 180.
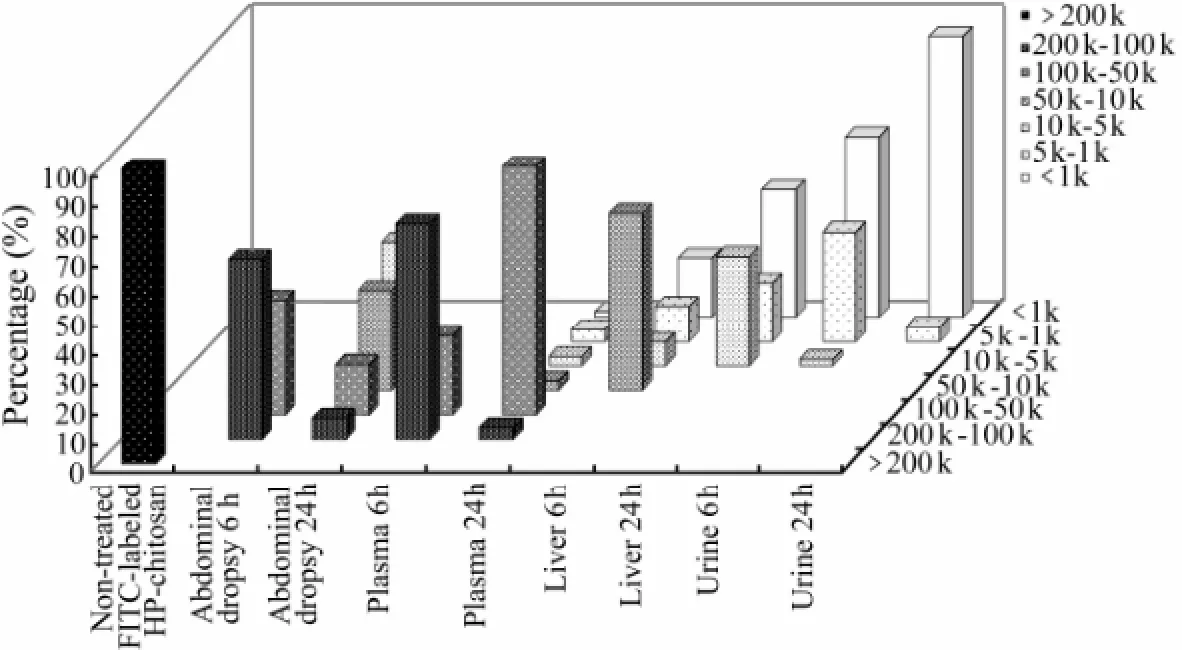
Fig.7 Percentages of degradation products of FITC-HP-chitosan of different molecular weights in abdominal dropsy, plasma, liver and urine in rats at 6 h and 24 h, calculated by GPC analysis.
3.7 Degradation of FITC-HP-Chitosan in vitro
To gain more insights into the actual metabolic pathway and biodegradation performance of this derivative, in vitro degradation of FITC-HP-chitosan by abdominal dropsy, plasma and homogenate of liver was investigated as well. Fig.8 showed the average peak molecular weights of non-treated and degradation products of FITCHP-chitosan treated with abdominal dropsy, plasma and homogenate of liver for 6 h and 24 h, respectively. Corresponding to the retention times, the average molecular weights of FITC-HP-chitosan of non-treated, 6 h with abdominal dropsy, 24 h with abdominal dropsy, 6 h with plasma and 24 with plasma were 214.19, 187.30, 119.26, 87.91, 82.71 kDa (Figs.8a, b), respectively. Additionally, the average molecular weight of FITC-HP-chitosan 6 h with homogenate of liver (Fig.8c) was made up of two parts: 37.43 kDa (41.23%) and 2.56 kDa (56.18%), and the molecular weight at 24 h became 2.41 kDa (95.28%). The results in vivo and in vitro were consistent that liver played a key role in degradation of HP-chitosan.
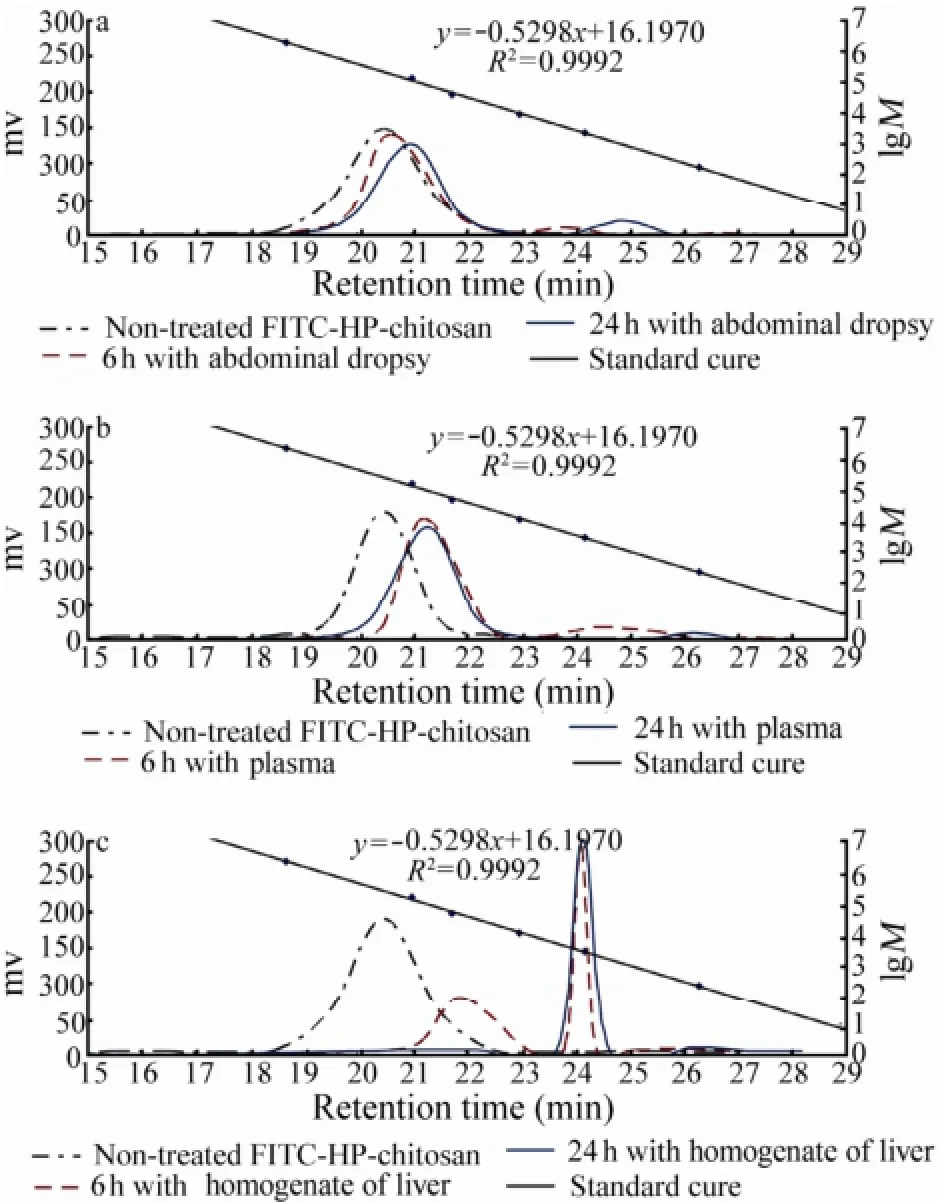
Fig.8 The GPC analysis of average peak molecular weights of FITC-HP-chitosan of non-treated and degradation products of FITC-HP-chitosan treated with abdominal dropsy (a), plasma (b) and homogenate (c) of liver at 37℃ in vitro for 6 h and 24 h, respectively.
4 Discussion
HP-chitosan has attracted large amount of attention in a broad range of scientific areas especially in drug delivery, biomedical material, pharmaceutical excipient and bioactive reagent (Wan et al., 2004; Peng et al., 2005). The introduction of hydroxyethyl group enables it potential and attractive as absorbable biomaterial applied in vivo (Xie et al., 2002a; Liu et al., 2003). Previous studies suggest that lysozyme and chitinase are able to degrade chitosan, which is affected by many factors including water-solubility, degree of acetylation and molecular weight (Muzzarelli, 1997; V?rum et al., 1997; Mao et al., 2005; Dong et al., 2012). Our previous works indicate that liver plays a vital role in biodegradation of CM-chitosan and urinary excretion is the major pathway for the degraded products with molecular weight less than 45 kDa (Dong et al., 2010). In this study, the absorption, distribution, pharmacokinetics, degradation and excretion of the FITC labeled HP-chitosan in rats were investigated. In addition, the reliability and stability of FITC as a fluorescent tracer were also confirmed by a comparison between FITC and FTC-HP-chitosan.
It is known that chitosan is not to be absorbable by orally administration on account of only chitobiose or chitotriose can be absorbed by the digestive tract among various chitooligosaccharides (Chen et al., 2005). In previous study, 85% of FITC-CM-chitosan was excreted in urine within 11 d. Our present work showed that through the intraperitoneal injection of FITC-HP-chitosan at a dose of 10 mg per rat, 98.62% of the content was rapidly absorbed in 24 h, 88.47% was excreted in urine within 11 d. The GPC analysis in vivo and in vitro also indicated that a significant degradation process occurred in abdominal cavity, plasma and liver. Thus intraperitoneal administration is an effective method for HP-chitosan. This result is consistent with our previous finding (Dong et al., 2010).
The results showed that the blood system was a main method of transporting degradation products of FITCHP-chitosan, and also played a certain role in the degradation of FITC-HP-chitosan (from 200 kDa to 80 kDa in vitro). A time-related elimination of the label with a wide molecular weight distribution range (less than 1 kDa–200 kDa) occurred in blood during 9 days. This is consistent with the metabolic cycle of FITC-HP-chitosan in other organs and urinary excretion. The degradation products of FITC-HP-chitosan were transported and distributed in liver, kidney and spleen. It was indicated that live played a key role in the degradation of FITC-HP-chitosan. As was shown, the peak in liver at 5 d reached 10.82% of the original. The GPC analysis also showed that an objective degradation process occurred in vivo (<10 kDa at 24 h) and the degradation process with homogenate of liver in vitro was related with time (from 200 kDa to 2 kDa), suggesting the important role of liver for the biodegradation of HP-chitosan. Kidney is the vital organ of the urinary system. A sharp decline in accumulation of FITCHP-chitosan appeared in first 3 d (from 3.84% to less than 1%). It was interesting that urinary excretion undertook 88.47% of the label excreted during an 11-day period and presented a similar distribution curve as in kidney. Overall, 40.14% of the FITC-HP-chitosan movedfast to urine and kidney and only 0.81% of the original distributed in liver in the first 24 h. However, there were minor differences between our findings and the previous studies. Onishi and Machida (1999) found that 50% of FITC-chitin moved fast to the kidney and urine, and the label almost did not distribute in liver and spleen in the first 24 h. In addition, previous investigation on CM-chitosan suggested the molecular weight of the urinary excreted FITC-CM-chitosan was less than 45 kDa (Dong et al., 2010). In this study, it was demonstrated that the average molecular weight of the urinary-excreted FITCHP-chitosan was less than 10 kDa. The inconsistent results could be attributed to the differences of materials, molecular weights or analysis methods. In fact, the average molecular weight of CM-chotosan in previous study was more than 400 kDa (Dong et al., 2010), while the HP-chitosan adopted in this study was about 210 kDa, which might cause the difference.
Regarding to the degradation behavior of HP-chitosan in vitro, FITC was also used as a fluorescent tracer in order to overcome the interference of autologous proteins and polysaccharides in body fluids in the GPC analysis. It was demonstrated that homogenate of liver degraded FITC-HP-chitosan to a lower molecular weight level (about 2 kDa) than abdominal dropsy and plasma (about 120 kDa–80 kDa), but it took a longer time. The degradation process in liver was complicated and persistent due to the presence of a variety of active enzymes. Above all, the results observed in vivo and in vitro were consistent, and both of them indicated that liver played a key role in degradation of HP-chitosan.
Compared to the previous study (Dong et al., 2010), a similar remarkable distribution curve of FITC-HP-chitosan was observed in spleen after the intraperitoneal administration. It had been reported that oligosaccharides released by chitosan-based polysaccharides might have impact on the immune system (Pangburn et al., 1982; Bland et al., 2004). In addition, Gorzelanny et al. (2010) found that the recognition of chitosan by human macrophages was triggered by the enzyme chitotriosidase due to the production of chitosan oligosaccharides, which in turn stimulated further chitotriosidase secretion and oligosaccharides production. As the major organ, spleen has a crucial influence on the immune capacity of the body. However, how the degradation products of FITC-HP-chitosan affect the immune capacity remains unknown, which needs more study.
5 Conclusions
The pharmacokinetics mode and biodegradation performance of HP-chitosan, a multifunctional water-soluble derivative of chitosan, were investigated in this study. After intraperitoneal administration at a dose of 10 mg per rat, FITC-HP-chitosan was absorbed rapidly and distributed to liver, kidney and spleen through the blood system. Through GPC analysis, the degradation behavior of HP-chitosan was related to abdominal dropsy, plasma and liver, while liver played a main role during the biodegradation of HP-chitosan. Urinary excretion was the leading pathway of excretion of FITC-HP-chitosan, while about 88.47% of the dose was excreted within 11 days with a molecular weight less than 10 kDa. In general, these findings will guide potential applications and exploitations of this versatile and absorbable derivative in the fields of drug delivery, tissue engineering and other biomedical materials.
Acknowledgements
This work was financially supported by National High Technology Research and Development Program of China (863 Program, Grant No. 2007AA091603).
Bayramoglu, G., Akcal?, K. C., Gultekin, S., Bengu, E., and Arica, M. Y., 2011. Preparation and characterization of poly (hydroxyethyl methacrylate-co-poly (ethyleneglycol-methacrylate)/hydroxypropyl-chitosan) hydrogel films: Adhesion of rat mesenchymal stem cells. Macromolecular Research, 19: 385-395.
Bland, E. J., Keshavarz, T., and Bucke, C., 2004. The influence of small oligosaccharides on the immune system. Carbohydrate Research, 339: 1673-1678.
Carreira, A., Gon?alves, F., Mendon?a, P., Gil, M., and Coelho, J., 2010. Temperature and pH responsive polymers based on chitosan: Applications and new graft copolymerization strategies based on living radical polymerization. Carbohydrate Polymers, 80: 618-630.
Chen, A.-S., Taguchi, T., Sakai, K., Matahira, Y., Wang, M.-W., and Miwa, I., 2005. Effect of chitobiose and chitotriose on carbon tetrachloride-induced acute hepatotoxicity in rats. Biological and Pharmaceutical Bulletin, 28: 1971-1973.
Chien, Y., Liao, Y.-W., Liu, D.-M., Lin, H.-L., Chen, S.-J., Chen, H.-L., Peng, C.-H., Liang, C.-M., Mou, C.-Y., and Chiou, S.-H., 2012. Corneal repair by human corneal keratocytereprogrammed iPSCs and amphiphatic carboxymethylhexanoyl chitosan hydrogel. Biomaterials, 33: 8003-8016.
Dash, M., Chiellini, F., Ottenbrite, R., and Chiellini, E., 2011. Chitosan-A versatile semi-synthetic polymer in biomedical applications. Progress in Polymer Science, 36: 981-1014.
Dong, W., Han, B., Feng, Y., Song, F., Chang, J., Jiang, H., Tang, Y., and Liu, W., 2010. Pharmacokinetics and biodegradation mechanisms of a versatile carboxymethyl derivative of chitosan in rats: In vivo and in vitro evaluation. Biomacromolecules, 11: 1527-1533.
Dong, W., Han, B., Shao, K., Yang, Z., Peng, Y., Yang, Y., and Liu, W., 2012. Effects of molecular weights on the absorption, distribution and urinary excretion of intraperitoneally administrated carboxymethyl chitosan in rats. Journal of Materials Science: Materials in Medicine, 23: 2945-2952.
Dong, Y., Wu, Y., Wang, J., and Wang, M., 2001. Influence of degree of molar etherification on critical liquid crystal behavior of hydroxypropyl chitosan. European Polymer Journal, 37: 1713-1720.
Dutta, P., Tripathi, S., Mehrotra, G., and Dutta, J., 2009. Perspectives for chitosan based antimicrobial films in food applications. Food Chemistry, 114: 1173-1182.
Gao, X., Liu, W., Han, B., Wei, X., and Yang, C., 2008. Preparation and properties of a chitosan-based carrier of corneal endothelial cells. Journal of Materials Science: Ma-terials in Medicine, 19: 3611-3619.
Ghorbani, R., Emamzadeh, A., Khazaie, Y., Dormiani, K., Ghaedi, K., Rabbani, M., Foruzanfar, M., Karbalaie, K., Karamali, F., Lachinani, L., Kiani-Esfahani, A., Nematollahi, M., and Esfahani, M. H., 2013. Constructing a Mouse Oct4 Promoter/EGFP Vector, as a Whole-Cellular Reporter to Monitor the Pluripotent State of Cells. Avicenna Journal of Medical Biotechnology, 5: 2-9.
Gorzelanny, C., P?ppelmann, B., Pappelbaum, K., Moerschbacher, B. M., and Schneider, S. W., 2010. Human macrophage activation triggered by chitotriosidase-mediated chitin and chitosan degradation. Biomaterials, 31: 8556- 8563.
Honarkar, H., and Barikani, M., 2009. Applications of biopolymers I: Chitosan. Monatshefte für Chemie-Chemical Monthly, 140: 1403-1420.
Jayakumar, R., Prabaharan, M., Nair, S., Tokura, S., Tamura, H., and Selvamurugan, N., 2010. Novel carboxymethyl derivatives of chitin and chitosan materials and their biomedical applications. Progress in Materials Science, 55: 675-709.
Khor, E., and Lim, L. Y., 2003. Implantable applications of chitin and chitosan. Biomaterials, 24: 2339-2349.
Liang, Y., Liu, W., Han, B., Yang, C., Ma, Q., Song, F., and Bi, Q., 2011. An in situ formed biodegradable hydrogel for reconstruction of the corneal endothelium. Colloids and Surfaces B: Biointerfaces, 82: 1-7.
Ling, K., Zheng, F., Li, J., Tang, R., Huang, J., Xu, Y., Zheng, H., and Chen, J., 2008. Effects of substitution degree of photoreactive groups on the properties of UV-fabricated chitosan scaffold. Journal of Biomedical Materials Research Part A, 87: 52-61.
Liu, Y., Chen, G., and Hu, K.-A., 2003. Synthesis, characterization and structural analysis of polylactide grafted onto water-soluble hydroxypropyl chitin as backbone. Journal of Materials Science Letters, 22: 1303-1305.
Mao, S., Shuai, X., Unger, F., Wittmar, M., Xie, X., and Kissel, T., 2005. Synthesis, characterization and cytotoxicity of poly (ethylene glycol)-graft-trimethyl chitosan block copolymers. Biomaterials, 26: 6343-6356.
Muzzarelli, R., 1997. Human enzymatic activities related to the therapeutic administration of chitin derivatives. Cellular and Molecular Life Sciences CMLS, 53: 131-140.
Nishimura, S., Kohgo, O., Kurita, K., and Kuzuhara, H., 1991. Chemospecific manipulations of a rigid polysaccharide: Syntheses of novel chitosan derivatives with excellent solubility in common organic solvents by regioselective chemical modifications. Macromolecules, 24: 4745-4748.
Onishi, H., and Machida, Y., 1999. Biodegradation and distribution of water-soluble chitosan in mice. Biomaterials, 20: 175-182.
Pangburn, S., Trescony, P., and Heller, J., 1982. Lysozyme degradation of partially deacetylated chitin, its films and hydrogels. Biomaterials, 3: 105.
Park, J. H., Cho, Y. W., Chung, H., Kwon, I. C., and Jeong, S. Y., 2003. Synthesis and characterization of sugar-bearing chitosan derivatives: Aqueous solubility and biodegradability. Biomacromolecules, 4: 1087-1091.
Peng, Y., Han, B., Liu, W., and Xu, X., 2005. Preparation and antimicrobial activity of hydroxypropyl chitosan. Carbohydrate Research, 340: 1846-1851.
Pillai, C., Paul, W., and Sharma, C. P., 2009. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Progress in Polymer Science, 34: 641-678.
Prabaharan, M., and Mano, J. F., 2005. Hydroxypropyl chitosan bearing β-cyclodextrin cavities: Synthesis and slow release of its inclusion complex with a model hydrophobic drug. Macromolecular Bioscience, 5: 965-973.
Ravi Kumar, M. N., 2000. A review of chitin and chitosan applications. Reactive and Functional Polymers, 46: 1-27.
Rinaudo, M., 2006. Chitin and chitosan: Properties and applications. Progress in Polymer Science, 31: 603-632.
Riva, R., Ragelle, H., des Rieux, A., Duhem, N., Jér?me, C., and Préat, V., 2011. Chitosan and Chitosan Derivatives in Drug Delivery and Tissue Engineering, Chitosan for Biomaterials II. Springer, 19-44.
Sashiwa, H., Saimoto, H., Shigemasa, Y., Ogawa, R., and Tokura, S., 1990. Lysozyme susceptibility of partially deacetylated chitin. International Journal of Biological Macromolecules, 12: 295-296.
Shi, L., Tang, C., and Yin, C., 2012. Glycyrrhizin-modified O-carboxymethyl chitosan nanoparticles as drug vehicles targeting hepatocellular carcinoma. Biomaterials, 33: 7594-7604.
Shigemasa, Y., Matsuura, H., Sashiwa, H., and Saimoto, H., 1996. Evaluation of different absorbance ratios from infrared spectroscopy for analyzing the degree of deacetylation in chitin. International Journal of Biological Macromolecules, 18: 237-242.
Sun, T., Xie, W., and Xu, P., 2004. Superoxide anion scavenging activity of graft chitosan derivatives. Carbohydrate Polymers, 58: 379-382.
V?rum, K. M., Myhr, M. M., Hjerde, R. J., and Smidsr?d, O., 1997. In vitro degradation rates of partially N-acetylated chitosans in human serum. Carbohydrate Research, 299: 99-101.
Wan, Y., Creber, K. A., Peppley, B., and Tam Bui, V., 2004. Ionic conductivity and tensile properties of hydroxyethyl and hydroxypropyl chitosan membranes. Journal of Polymer Science Part B: Polymer Physics, 42: 1379-1397.
Wang, J., Tao, X., Zhang, Y., Wei, D., and Ren, Y., 2010. Reversion of multidrug resistance by tumor targeted delivery of antisense oligodeoxynucleotides in hydroxypropyl-chitosan nanoparticles. Biomaterials, 31: 4426-4433.
Wang, S., Liu, W., Han, B., and Yang, L., 2009. Study on a hydroxypropyl chitosan-gelatin based scaffold for corneal stroma tissue engineering. Applied Surface Science, 255: 8701-8705.
Xie, W., Xu, P., Liu, Q., and Xue, J., 2002a. Graft-copolymerization of methylacrylic acid onto hydroxypropyl chitosan. Polymer Bulletin, 49: 47-54.
Xie, W., Xu, P., Wang, W., and Liu, Q., 2002b. Preparation and antibacterial activity of a water-soluble chitosan derivative. Carbohydrate Polymers, 50: 35-40.
Xie, Y., Liu, X., and Chen, Q., 2007. Synthesis and characterization of water-soluble chitosan derivate and its antibacterial activity. Carbohydrate Polymers, 69: 142-147.
Zheng, M., Han, B., Yang, Y., and Liu, W., 2011. Synthesis, characterization and biological safety of O-carboxymethyl chitosan used to treat Sarcoma 180 tumor. Carbohydrate Polymers, 86: 231-238.
Zhu, H., Mizugaki, T., Ebitani, K., and Kaneda, K., 2001. Dimethylaminoethylated hydroxypropyl-chitosan: Preparation and application as polymeric ligand to form Rh6 cluster complexes for the reduction of benzaldehyde and nitrobenzene. Journal of Applied Polymer Science, 80: 447-453.
(Edited by Qiu Yantao)
(Received February 20, 2014; revised April 28, 2014; accepted June 6, 2015)
J. Ocean Univ. China (Oceanic and Coastal Sea Research)
DOI 10.1007/s11802-015-2600-6
ISSN 1672-5182, 2015 14 (5): 888-896
http://www.ouc.edu.cn/xbywb/
E-mail:xbywb@ouc.edu.cn
* Corresponding author. Tel: 0086-532-82032105
E-mail: baoqinh@ouc.edu.cn
 Journal of Ocean University of China2015年5期
Journal of Ocean University of China2015年5期
- Journal of Ocean University of China的其它文章
- The Mechanism of the Acclimation of Nannochloropsis oceanica to Freshwater Deduced from Its Transcriptome Profiles
- A Carboxymethyl Cellulase from a Marine Yeast (Aureobasidium pullulans 98): Its Purification, Characterization, Gene Cloning and Carboxymethyl Cellulose Digestion
- Effects of Dietary Stachyose on Growth Performance, Digestive Enzyme Activities and Intestinal Morphology of Juvenile Turbot (Scophthalmus maximus L)
- Pharmacokinetics and Biodegradation of Chitosan in Rats
- Changes in Plasma Osmolality, Cortisol and Amino Acid Levels of Tongue Sole (Cynoglossus semilaevis) at Different Salinities
- Effects of Different Microbes on Fermenting Feed for Sea Cucumber (Apostichopus japonicus)
