Pharmacokinetics and Biodegradation of Chitosan in Rats
LI Hui, JIANG Zhiwen, HAN Baoqin, NIU Shuyi, DONG Wen, and LIU Wanshun
College of Marine Life Sciences, Ocean University of China, Qingdao 266003, P. R. China
Pharmacokinetics and Biodegradation of Chitosan in Rats
LI Hui, JIANG Zhiwen, HAN Baoqin, NIU Shuyi, DONG Wen, and LIU Wanshun*
College of Marine Life Sciences, Ocean University of China, Qingdao 266003, P. R. China
? Ocean University of China, Science Press and Spring-Verlag Berlin Heidelberg 2015
Chitosan, an excellent biomedical material, has received a widespread in vivo application. In contrast, its metabolism and distribution once being implanted were less documented. In this study, the pharmacokinetics and biodegradation of fluorescein isothiocyanate (FITC) labeled and muscle implantation administrated chitosan in rats were investigated with fluorescence spectrophotometry, histological assay and gel chromatography. After implantation, chitosan was degraded gradually during its distribution to diverse organs. Among the tested organs, liver and kidney were found to be the first two highest in chitosan content, which was followed by heart, brain and spleen. Urinary excretion was believed to be the major pathway of chitosan elimination, yet 80% of chitosan administered to rats was not trackable in their urine. This indicated that the majority of chitosan was degraded in tissues. In average, the molecular weight of the degradation products of chitosan in diverse organs and urine was found to be <65 kDa. This further confirmed the in vivo degradation of chitosan. Our findings provided new evidences for the intensive and safe application of chitosan as a biomedical material.
chitosan; fluorescein isothiocyanate; pharmacokinetics; biodegradation; fluorescence spectrophotometry; muscle implantation
1 Introduction
Chitosan, a linear and semi-crystalline polysaccharide, is composed of (1→4)-2-acetamido-2-deoxy-β-D-glucan (N-acetyl D-glucosamine) and (1→4)-2-amino-2-deoxyβ-D-glucan (D-glucosamine). The grade of chitosan depends on the content of acetylated component, N-acetyl D-glucosamine (Dong et al., 2010; Hoppe-Seiler, 1994; Rinaudo, 2006). Non toxic chitosan is derived from chitin, the major component of the shells of crustaceans (e.g., crab, shrimp, prawn, lobster, and crawfish), the cuticle of insect and the cell wall of fungi by alkaline deacetylating (Sinha et al., 2004; Xia et al., 2011).
Although the difference of N-deacetylation degree between chitosan and chitin is not clearly defined (Jikakis, 2004; Muzzarelli, 1973), some researchers persist that the deacetylation degree of chitosan should reach at least 60% (Acosta et al., 1993; Madihally and Matthew, 1999) while others persist that the degree of the N-deacetylation of chitosan should range from 40% to 98% (Hejazi et al., 2003). The molecular weight (Mw) of chitosan ranges from 3.8 kDa to 2000 kDa. In addition, its particle size, density and viscosity are crucial to its physico-chemical property as they significantly affect the biological activity of chitosan (Hejazi et al., 2003; Sinha et al., 2004).
Because of its special structure and biochemical prop-erty, chitosan has received widespread applications in biomedicine, agriculture and environmental field (Park et al., 2003). More attractively, chitosan promises for being a perfect biological material and pharmaceutical formulation due to its excellent biological activity such as high biocompatibility, biodegradability, immunogenicity and low toxicity (Di Martino et al., 2005; Dong et al., 2010; Xia et al., 2011). Chitosan is also filmogenic, fiber forming and gel forming (Notin et al., 2006). Materials made of chitosan, for example, absorbable surgical suture, artificial skin, tampon, bandage and medicine cloth, have been widely used in medicine. Some researchers even use chitosan as the supporting part of vascular prosthesis (Albanna et al., 2012; Kong et al., 2012) and the skeleton of various biodegradable medical materials (Ikada and Tsuji, 2000).
To date, chitosan has been widely used in human body as an excellent medical degradable material. The pharmacokinetics and body distribution of water-soluble chitosan (50% of deacetylation degree) administrated to mice intraperitoneally have been studied (Onishi and Machida, 1999). However, the metabolic pathway and degradation mechanism of water-insoluble chitosan in vivo after muscle implantation have not been documented yet. This scenario is widely concerned as it relates to the security of chitosan application in vivo.
Muscle implantation, a route of chitosan administration, is feasible in evaluating the organizational security of medical biomaterials (Gad, 2008). This route meets therequirements for studying the metabolism of chitosan in artificial vascular prosthesis (Moon et al., 2008). In addition, fluorescence labeling is one of the important methods of investigating the distribution, degradation and absorption of polysaccharides in vivo (Aicher et al., 2003). In the present study, water insoluble chitosan was labeled with fluorescein isothiocyanate (FITC) and implanted in the muscle of rats. The pharmacokinetics, tissue distribution, and unary excretion of FITC-labeled chitosan (hereinafter FITC-chitosan) in rats were studied through fluorescence spectrophotometry and histological assay. The Mwof chitosan degradation products in various organs and urine was determined with gel chromatography. Our findings should aid to the safe use of chitosan in vivo.
2 Materials and Methods
2.1 Materials
Water-insoluble chitosan (93% deacetylation degree) was supplied by Biotemed (Qingdao, Shandong, China). FITC was purchased from Sigma-Aldrich (St. Louis, MO, USA). The Mwcalibration standard for aqueous scanning electron microscopy in gel chromatography (Pullulan standards) and water-soluble gel filtration column of Shodex OHpak SB800 was purchased from Showa Denko (Tokyo, Japan). All reagents and chemicals were at analytical grade (Shanghai, China). Regenerated cellulose tubular dialysis bags with MWCO 8–14 kDa were purchased from Soledad (Beijing, China).
2.2 Synthesis of FITC-Chitosan
The 1% chitosan solution was prepared by dissolving chitosan in 0.1 mol L-1acetic acid, which was muddied with 0.5 mol L-1NaOH after raising the acidity to pH9.0. The solution was mixed with a half volume of methanol first and then with FITC drop by drop to a final concentration of 2 mg mL-1according to preselected labeling ratio of FITC to D-glucosamine residue. The labeling was carried out at room temperature in dark for 24 h. By adjusting the acidity to pH11, FITC-chitosan was precipitated. After dialysis, the precipitate was dissolved in alcohol and repeatedly precipitated by centrifuging until fluorescence was not detectable. The prepared FITC-chitosan was repeatedly washed with distilled water followed by vacuum drying. The labeling efficiency was calculated using the standard curve, which was prepared by plotting the absorbance at 490 nm of a series of FITC methanol solutions.
2.3 Animal Experiments
Sprague-Dawley rat (100 individuals, male, 150 g ± 8 g in body weight) were purchased from Qingdao Institute for Drug Control (Shandong, China). All operations were carried out following the Guide for the Care and Use of Laboratory Animals (USA), which had been approved by the Animal Care and Use Committee of Ocean University of China (Qingdao, Shandong, China).
The rats were randomly divided into 14 groups, 3 each. The rats were anesthetized with pentobarbital and fixed on operating table. After removing the hair and exposing the muscle, FITC-chitosan powder (10 mg per rat) was implanted into the muscle. Immediately after implantation, the rats were randomly divided into 14 cages, 3 each. Six rats were implanted with FITC, which were used as positive control. Other six rats were kept untreated, which were fed in two cages and used as the blank control. All the rats were allowed to access food and water freely during the whole observation period.
Urine in each cage was collected, combined with cage salver washings (phosphate buffered saline, PBS; pH7.2), and diluted with PBS to a volume of 30 mL per 6 h. The mixture was centrifuged at 5000 r min-1for 20 min to remove the contaminants. Starting at 6 h, the urine was collected every 12 h. All the urines were kept at -20℃ till use.
From day 1 to day 120, the blood, 1 mL each rat, was collected from the abdominal aortic and diluted to 7 mL with PBS (pH7.2) in heparin tubes. On day 120, the rats were killed with their organs, including liver, spleen, kidney, heart and brain weighed, and homogenized using a tissue homogenizer (Tekmar Tissumizer, USA). Homogenized organs, 1 g each, were mixed with PBS (pH7.2) and diluted to 7 mL. The organ homogenates and the blood were centrifuged at 5000 r min-1for 20 min with the supernatant collected for further tests.
FITC fluorescence intensity of urines, organ homogenates and sera were measured with fluorescence spectrophotometry (emission wavelength, 520 nm; excitation wavelength, 490 nm) and calculated according to net fluorescence intensity obtained by subtracting that of blank control from that of sample. The content of FITC-chitosan in tissues and urines was calculated from fluorescence intensity, tissue weight and urine volume. To more intuitively confirm tissue distribution of FITC-chitosan, fluorescence observation was carried out on various organs using quick frozen sections and histocompatibility evaluation (Glinsky et al., 2004).
2.4 Tissue Section and Fluorescence Analysis
The muscle where FITC-chitosan was implanted was cut and placed in 10% formaldehyde. After 48 h, the muscle was embedded in paraffin blocks, sectioned, stained and observed under a microscope (Artandi et al., 2000). Liver, kidney, spleen, heart and brain of all groups were fast frozen in liquid nitrogen. Consecutive sections, 8 μm in thickness, were immediately examined under a fluorescence microscope. According the standard curve of FITC, the FITC concentration was measured. All differences were checked for significance with t-testing.
2.5 Determination of Peak Mw(Mp) of FITC-Chitosan

Fig.1 The structure of chitosan (A), fluorescein isothiocyanate (FITC, B), and FITC-labeled chitosan (C).
The Mpof FITC-chitosan was determined by gel permeation chromatography (GPC) using high performance liquid chromatograph (HPLC) on a Breeze 1525 system equipped with a fluorescence and refractive index detector (emission wavelength, 520 nm; excitation wavelength, 490 nm) (Waters, USA). A Shodex OHPak SB-806 M column and a Shodex OHPak SB-804 M column were used in tandem as the HPLC medium. The columns were eluted at a flow rate of 1.0 mL min-1at 30℃ using an elution solvent consisting of 0.02 mol L-1PBS (pH7.2) and 0.1 mol L-1Na2SO4. Pullulan with Mwof 180, 2500, 5900, 9600, 21100, 47100, 107000 and 200000 were used as Mwstandards. Data from the fluorescence and differential refractive index detector were integrated and analyzed using GPCW32 (LONGZIDA, Beijing), and the Mwof chitosan was calculated based on the standard curve.
3 Results
3.1 Preparation of FITC-Chitosan
The FITC part in FITC-chitosan was 12.4%, and the labeling efficiency was approximately one FITC moiety per 17 D-glucosamine residues. The spontaneous fluorescence decay caused by fluorescence quenching cannot be avoided in vitro and in vivo. Thus, the fluorescence intensity of all samples should be synchronously measured, and FITC used for the preparation of fluorescence standard curve should be stored in dark at room temperature after muscle implantation.
3.2 Serum Concentration and Organ Distribution of FITC and FITC-Chitosan
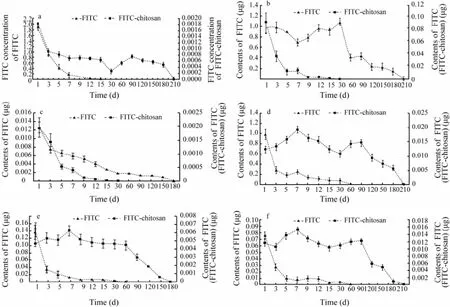
Fig.2 Serum concentration (a) and organ distribution of FITC and FITC-chitosan in liver (b), spleen (c), kidney (d), heart (e) and brains (f) of rats on different days after muscle implantation. The data were expressed as mean ± SD (n=3).
FITC was found to distribute widely and quickly in serum and various organs after muscle implantation. However, FITC-chitosan was not absorbed as fast as FITC as thimbleful fluorescence was detectable in serum and various organs after muscle implantation. The con-centration of FITC in serum was 1.86 μg mL-1on day 1 after implantation, which declined rapidly in following days, and fluorescence was not detectable on day 15 (Fig.2a). The FITC moiety in FITC-chitosan was 1.83 × 10-3μg mL-1(equivalent to 0.015 μg mL-1FITC-chitosan in serum) on day 1 after implantation, and fluorescence was constantly detectable in serum during the whole experimental period (Fig.2a).
FITC and FITC-chitosan existed in the liver, spleen, kidney, heart and brain of rats after muscle implantation. The content of FITC moiety reached the maximum, 0.093 ± 0.012 μg (equivalent to approx. 0.75 ± 0.1 μg FITC-chitosan) in liver on day 30 after implantation while the fluorescence of FITC was not detectable in positive control during this period (Fig.2b). The FITC curve sharply declined from day 1 to day 5, and the fluorescence was not detectable on day 15. In comparison, the FITC-chitosan curve of liver was relatively flat, and the fluorescence was detectable in 210 d (Fig.2b). Among the tested organs, the content of FITC and FITC-chitosan in spleen was extremely low, which was only approximately onetenth of that in other organs (Fig.2c). Similarly, FITC-chitosan in kidney had lower FITC moiety and longer excretion time than FITC. It sharply declined from day 1 to day 3, and the fluorescence was not detectable on day 6. The accumulative amount of FITC moiety of FITC-chitosan in kidney peaked at 0.019 ± 0.009 μg on day 7 after implantation (equivalent to approximately 0.15 ± 0.07 μg FITC-chitosan, Fig.2d).
In compared with liver and kidney, brain and heart were relatively low in the content of FITC and FITC-chitosan (Figs.2e, f). The FITC content in rat brain and heart decreased rapidly on day 1 after implantation, and the fluorescence was not detectable on day 6. The accumulative amount of FITC moiety in brain and heart both reached their maximum of 0.015 ± 0.007 and 0.006 ± 0.002 μg, respectively (equivalent to approx. 0.12 ± 0.056 and 0.05 ± 0.016 μg FITC-chitosan, respectively) on day 7 after implantation. Thereafter, the fluorescence was gradually weakened and completely disappeared on day 210.
The distribution of FITC-chitosan in different organs early after implantation was more intuitively confirmed by fluorescence microscopy analysis of quick frozen sections (Fig.3). Hematoxylin-eosin (HE) staining and anatomy pictures of rat thigh muscle showed that FITC-chitosan was completely degraded in 210 d, whereas inflammation was gradually reduced from day 30 to day 120, and completely disappeared on day 210 (Fig.4).
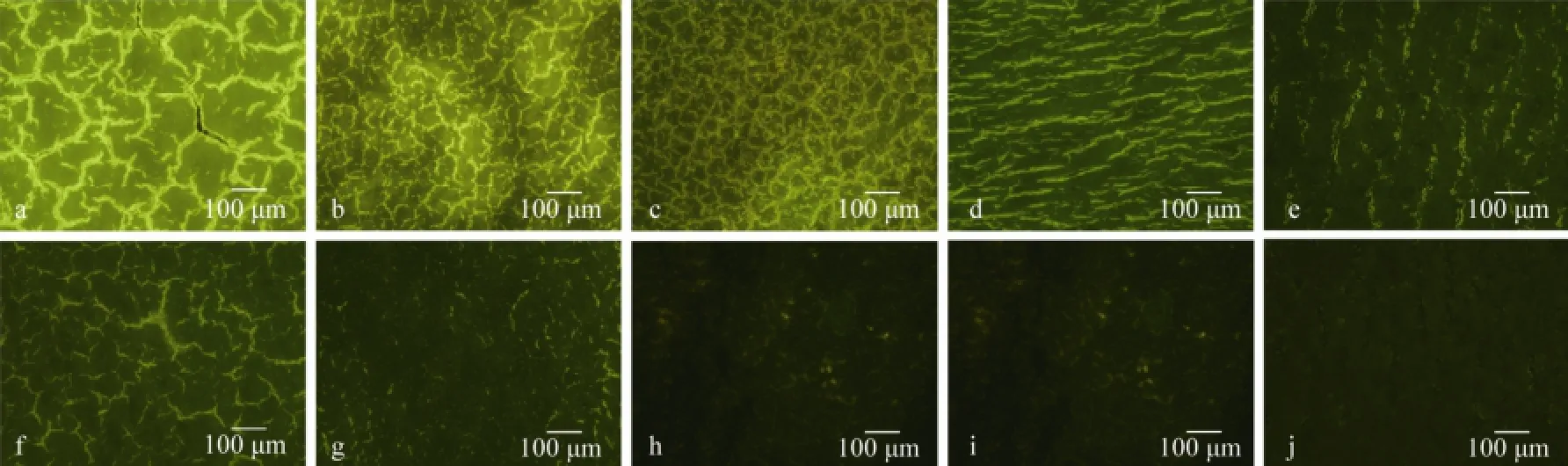
Fig.3 Fluorescence microphotograph of the quick frozen sections of various rat organs. a–e, liver, kidney, spleen, heart and brain, respectively, which were sampled from the experimental rats on day 30 after muscle implantation of FITC-chitosan; f–j, liver, kidney, spleen, heart and brain, respectively, which were sampled from blank control.
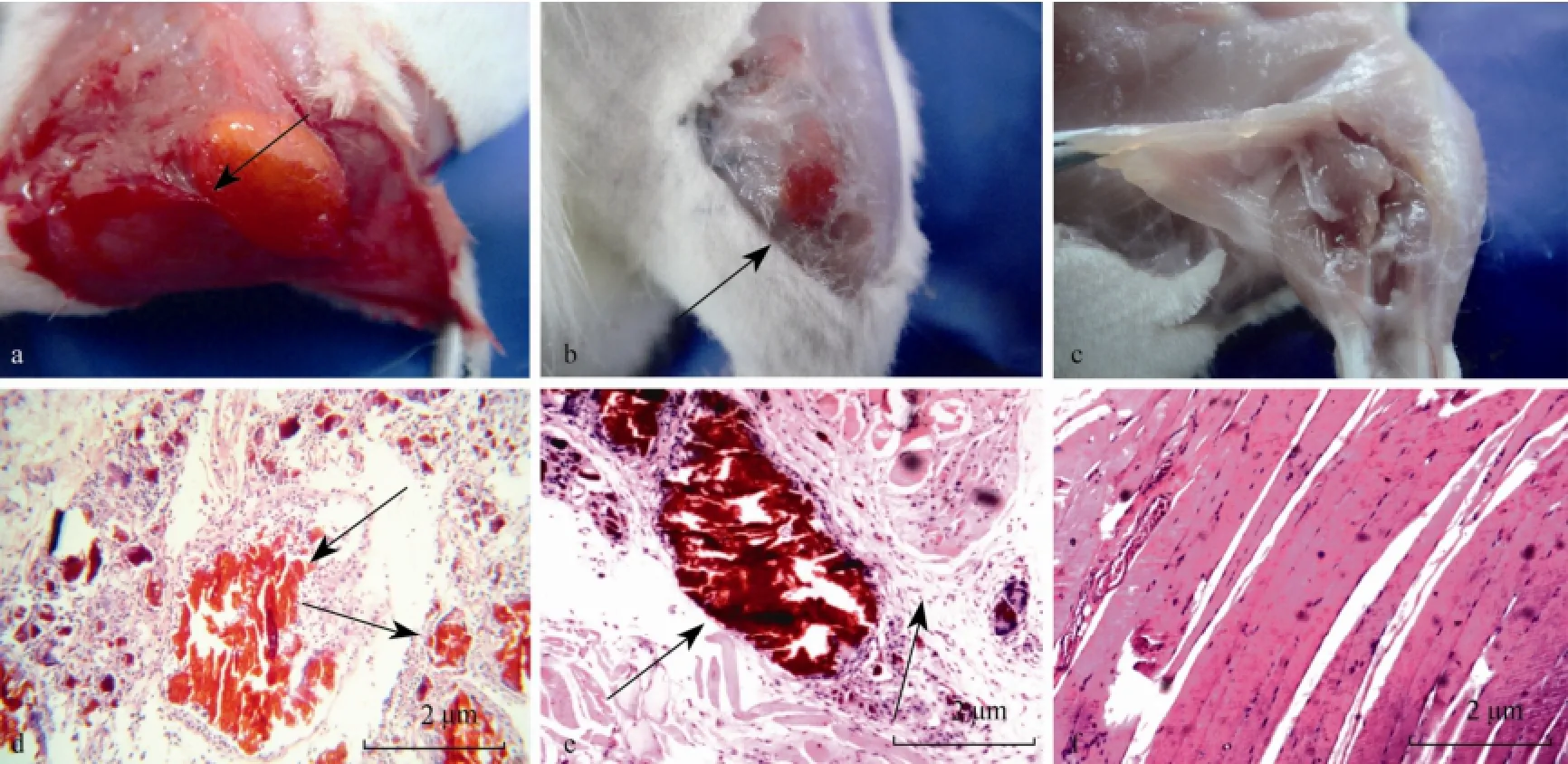
Fig.4 Hematoxylin-eosin staining (a–c) and anatomy picture (d–f) of rat thigh muscle sampled on day 30, 120 and 210 after implantation of FITC-chitosan, respectively. Arrows point to the tissue with unabsorbed FITC-chitosan and severe inflammation.
3.3 Urinary FITC and FITC-Chitosan
Approximately, 0.19% of FITC-chitosan administered was excreted into urine in 1 d, 3.12% in 13 d, 10.34% in 120 d and 13.9% in 210 d after implantation. In positive control, excretion of FITC was more rapid than that of FITC-chitosan, 2.53% in 1 d and 5.56% in 60 d after muscle implantation (Figs.5a, b). By observing the anatomic site, FITC and FITC-chitosan were completely degraded and absorbed in 60 and 210 d after implantation, respectively (Fig.4). Therefore, approximately 80% FITC-chitosan and 90% of FITC administered in rats were untraceable.
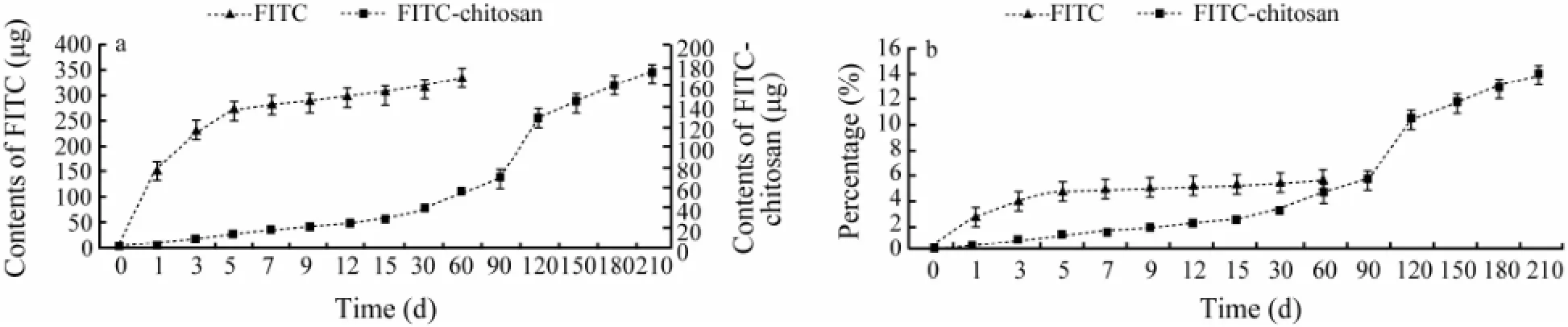
Fig.5 Time curve of the cumulative content (a) and percentage (b) of FITC and FITC-chitosan in urine after implantation.
3.4 Molecular Weight of FITC-Chitosan in Organs and Urine
The Mpand Mwdistribution of FITC-chitosan and its degradation products in different organs and urine were determined. The GPC curve of the extracts from liver, spleen, kidney, heart, brain and serum collected at different times after muscle implantation are shown in Fig.6. The average Mwof liver, kidney and urine at the peak position was calculated by comparing with a dextran standard (Table 1). The Mwdistribution of chitosan degradation products was described with the percentage of different Mw. As shown in Fig.7, > half (68.25%) of FITC-chitosan degradation products had an average Mwless than 10 kDa, which accounted for the largest part in organs in a given time of metabolism.
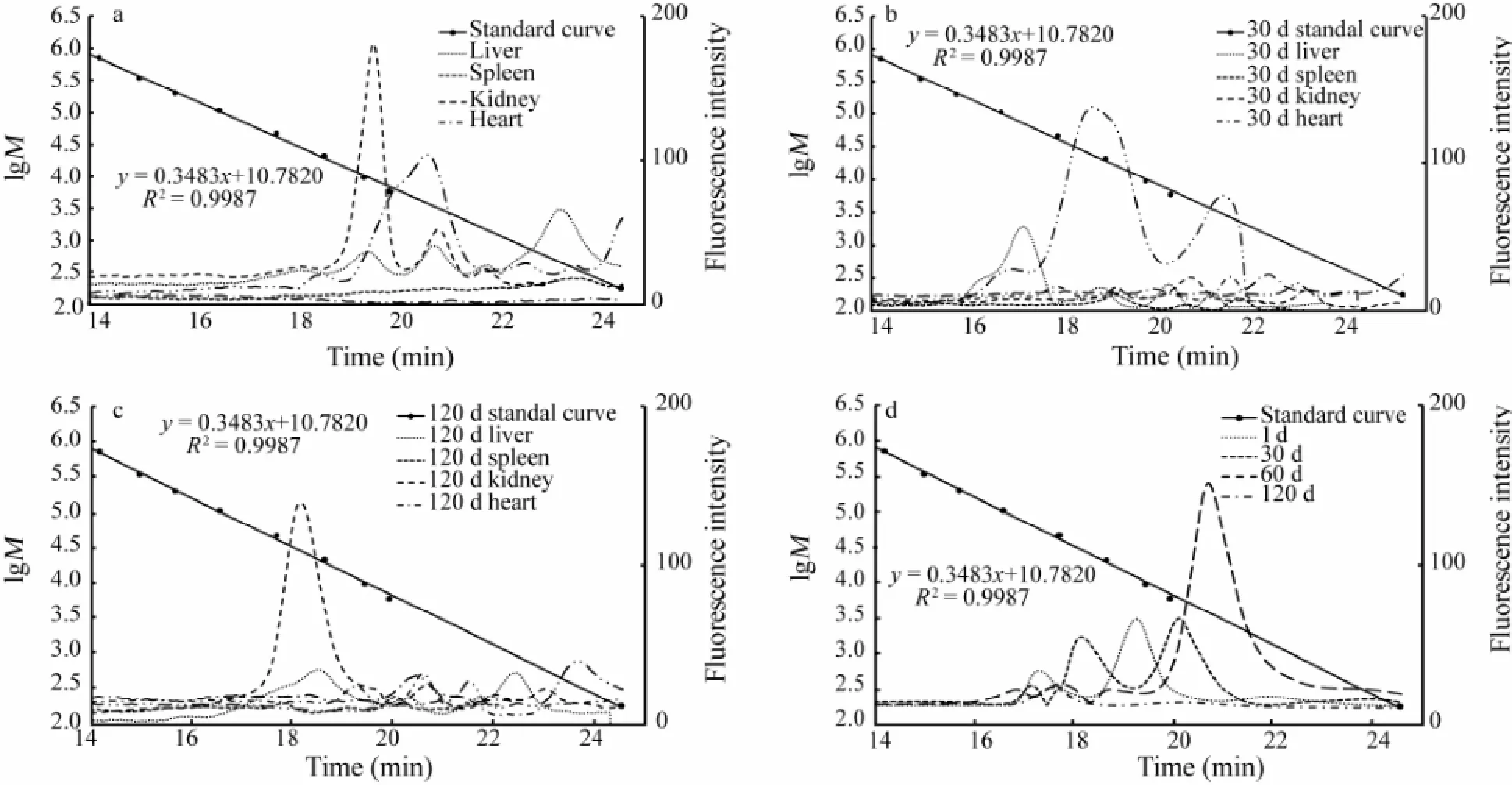
Fig.6 Gel permeation chromatography (GPC) curve of FITC-chitosan in the extract of serum, liver, spleen, kidney, heart and brain on day 1 (a), 30 (b) and 120 (c) after muscle implantation, and the GPC curve of FITC-chitosan in the extract of urine (d) on day 1, 30, 60 and 120 after muscle implantation. Phosphate-buffer saline (0.02 mol L-1, pH7.2) containing 0.1 mol L-1Na2SO4was used as elution solvent. The molecular weight of dextran standards is 708000, 344000, 200000, 107000, 47100, 21100, 9600, 5900 and 180, respectivekly, with a retention time of 14.19, 14.98, 15.69, 16.57, 17.70, 18.65, 19.44, 19.93 and 24.53 min, respectively.
4 Discussion
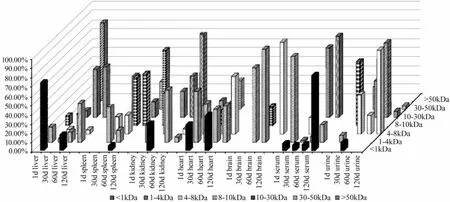
Fig.7 Change in the molecular weight and percentage of fluorescein isothiocyanate labeled chitosan in different organs and urine after muscle implantation (50 mg kg-1body weight).
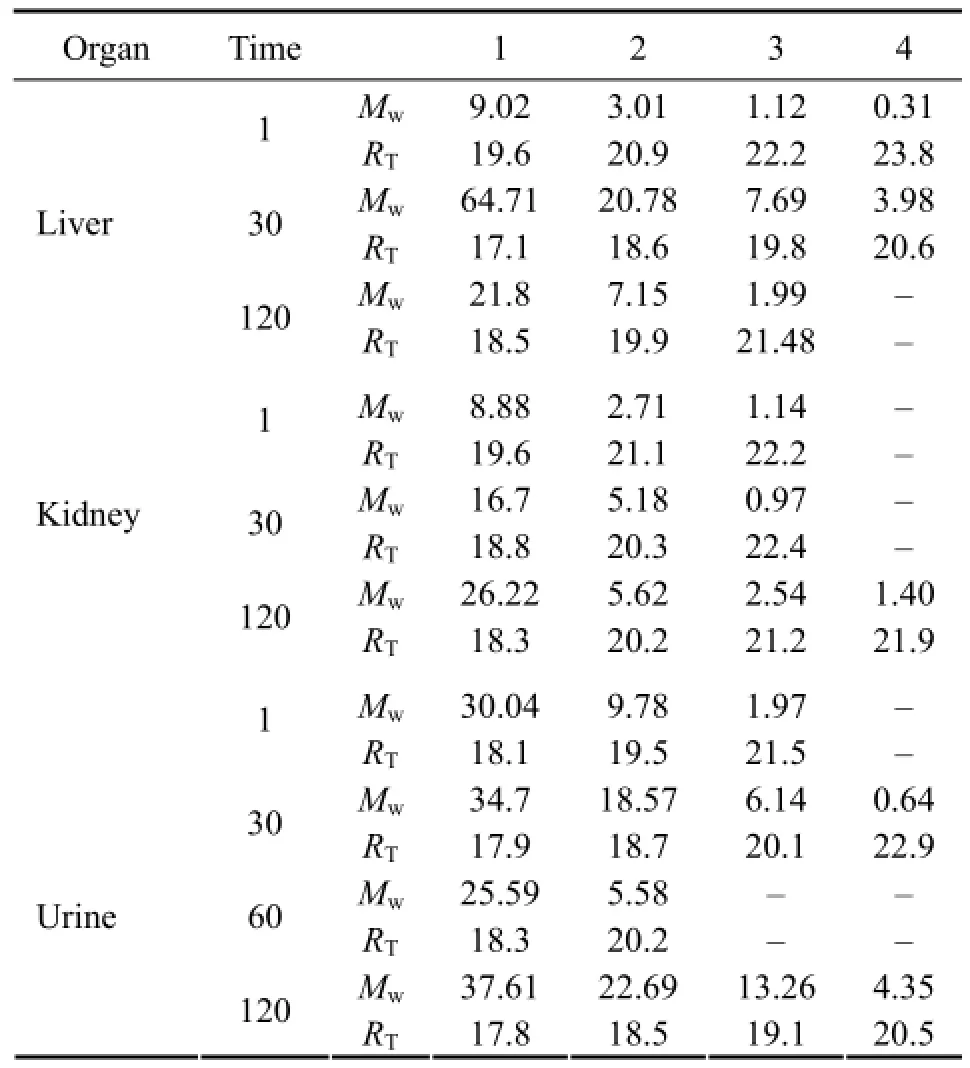
Table 1 Average molecular weights (Mw, kDa) and retention time (RT, min) of chitosan degradation products in rat liver, kidney, and urine at selected time points (d) after muscle implantation
Chitosan is widely applied in various biomedical and pharmaceutical areas owing to its non-toxicity and high biocompatibility and biodegradablity (Tsaih et al., 2004). Although it can be degraded in vitro in enzymatic, chemical or physical ways (Dong et al., 2010; Huang et al., 2013), the degradation of chitosan in vivo mainly relies on the catalysis of enzymes, such as chitinase, lysozyme and those of bacteria in vertebrates (Kean and Thanou, 2010). In the present study, the pharmacokinetics and biodegradability of chitosan implanted in the muscle of rats were investigated in vivo.
The difference in the pharmacokinetics between FITC and FITC-chitosan proved the authenticity of the former as a fluorescent tracer of chitosan. Chitosan labeled with FITC was chosen to study the distribution of chitosan degradation products in rat serum and virous organs. The selection of chitosan administration route depends on different applications and research purposes (Cheng et al., 2008). Oral or intraperitoneal administration is suitbale for water-soluble chitosan (e.g., carboxymethyl chitosan and 50% deacetylation degree chitosan), which can be rapidly absorbed and completely degraded (Varia et al., 1984). As for vascular prosthesis or bone stents, however, water-insoluble chitosan must be used to maintain the mechanical strength for a long time (Gogolewski, 2000). Obviously, muscle implantation is the most suitable administration route for evaluating the performamnce of water-insoluble chitosan in vivo.
In the present study, the pharmacokinetics of waterinsoluble chitosan after muscle implantation was focused on. In contrast to oral or intraperitoneal administration of water-soluble chitosan, our results showed that only approximately 20% of muscle implanted chitosan was eliminated through urine within 210 d after muscle implantation, 50 mg kg-1body weight of rat (Fig.5). This indicated that the selection of administration route markedly influenced the pharmacokinetics of chitosan in vivo. Over 80% of muscle-implanted chitosan in rats was untrackable, possibly because the observation time was so long that the fluorescene of the labeled FITC on chitosan quenched (Fig.5). GPC analysis revealed that a timerelated, unstable and unhomogeneous degradation of FITC-chitosan took place after muscle administration.
The results of fluorescence spectrophotometry analysis and their confirmation by GPC indicated that FITC-chitosan gradually distributed to serum and major organs including liver, spleen, kidney, heart and brain (Figs.2 and 6). According to a comprehensive analysis of fluorescence spectrophotometry and GPC, there was no degraded chitosan in spleen or brain in 1 d after muscle implantation, and the FITC detected in spleen and brain might be attributed to the contanimant in FITC-chitosan (Figs.2, 6 and 7). Additionally, a low Mwdistribution of degraded chitosan (<30 kDa) in serum and other organs tested was found. However, on day 30 after implantation and on thefollwing days, a low content of chitosan was constantly detectable in spleen and brain, as well as in serum and other organs. It should be noted that the Mwof degraded chitosan did not show regular distribution patterns (neither increasing nor decreasing gradually) when the observation time was extended. According to statistics, 61.6% of chitosan degradation products were oligochitosan (<5 kDa), and Mwof the rest part varied between 5 and 65 kDa.
Urinary excretion is an important pathway of chitosan elimination in vivo. However, only 20% of the administered FITC-chitosan was excreted during a 210-d period, whereas the rest (80%) was not effectively trackable (Fig.5). The Mwof urinary excreted FITC-chitosan was less than 65 kDa (Table 1). Apparently, our data were inconsistent with those presented previously. Such an inconsistency may be attributed to the variation of the shape, site and test time of implanted chitosan in different research fields.
The test cycle reported early was far lower than ours (Dong et al., 2010; Onishi and Machida, 1999; Zeng et al., 2008). Onishi found that chitosan with 50% deacetylation degree was barely distributed in organs other than kidney (Onishi and Machida, 1999). However, we found that chitosan degradation products widely distributed in various organs of rats, for example, liver, spleen, kidneys, heart and brain. Among the five organs tested, the content of degraded chitosan was much higher in liver and kidney than in spleen, heart and brain.
After intraperitoneal administration, the retention time of chitosan with 50% deacetylation degree in vivo was as short as 24 h (Onishi and Machida, 1999) while that of chitosan with 93% deacetylation degree used in the present study was up to 210 d. This difference may result from the administration route and solublity of chitosan. However, the true reasons need to be deciphored further. Due to the long test cycle, the fluorescence of FITC- chitosan may quench to a certain extent, thus most of the chitosan administered was not trackable.
Spleen and brain are the major organs of immune and nerve system, respectively, in which time-related accumulation of oligochitosan and other low Mwchitosan degradation products are worthy of paying attention. As reported early, oligochitosan plays a significant role in strengthening immune system and repairing nervous damages (Huang et al., 2007; Jiang et al., 2009). However, further investigatiojns are needed to verify if the oligochitosan and other chitosan degradation products are beneficial for immune and nerve system.
5 Conclusions
The pharmacokinetics and biodegradation of muscle implanted chitosan in rats were investigated in vivo. After implantation, chitosan was gradually degraded, distributing to liver, kidney, heart, brain, spleen and serum. Urinary excretion was the major elimination way of chitosan degradation products less than 65 kDa. Our results evidenced that chitosan was apllicable in vivo as a biomedical material.
Acknowledgements
This work was supported funancialy by Qingdao Biotemed Biomaterial Co., Ltd. and the National ‘Twelfth Five-Year’ Support Plan for Science & Technology of Chinia (2012BAI18B06).
Acosta, N., Jimenez, C., Borau, V., and Heras, A., 1993. Extraction and characterization of chitin from crustaceans. Biomass Bioenergy, 5: 145-53.
Aicher, A., Brenner, W., Zuhayra, M., Badorff, C., Massoudi, S., Assmus, B., and Dimmeler, S., 2003. Assessment of the tissue distribution of transplanted human endothelial progenitor cells by radioactive labeling. Circulation, 107 (16): 2134-2139.
Albanna, M. Z., Bou-Akl, T., Walters, H. L., and Matthew, H. W. T., 2012. Improving the mechanical properties of chitosanbased heart valve scaffolds using chitosan fibers. Journal of the Mechanical Behavior of Biomedical Materials, 5 (1): 171-180.
Artandi, S. E., Chang, S., Lee, S. L., Alson, S., Gottlieb, G. J., Chin, L., and DePinho, R. A., 2000. Telomere dysfunction promotes non-reciprocal translocations and epithelial cancers in mice. Nature, 406 (6796): 641-645.
Chang, W., Chen, C., Ji, D., Lee, S., Tsai, T., Wu, H., and Yang, J., 2011. Development of a chitosan-polyglutamate based injectable polyelectrolyte complex scaffold. Carbohydrate Polymers, 85 (2): 318-324.
Cheng, Y., Xu, Z., Ma, M., and Xu, T., 2008. Dendrimers as drug carriers: Applications in different routes of drug administration. Journal of Pharmaceutical Sciences, 97 (1): 123-143.
Di Martino, A., Sittinger, M., and Risbud, M. V., 2005. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials, 26 (30): 5983-5990.
Dong, W., Han, B., Feng, Y., Song, F., Chang, J., Jiang, H., Tang, Y., and Liu, W., 2010. Pharmacokinetics and biodegradation mechanisms of a versatile carboxymethyl erivative of chitosan in rats: In vivo and in vitro evaluation. Biomacromolecules, 11 (6): 1527-1533.
Gad, S. C., 2008. Safety Evaluation of Medical Devices. CRC Press, 576pp.
Glinsky, G. V., Glinskii, A. B., Stephenson, A. J., Hoffman, R. M., and Gerald, W. L., 2004. Gene expression profiling predicts clinical outcome of prostate cancer. Journal of Clinical Investigation, 113 (6): 913-923.
Gogolewski, S., 2000. Bioresorbable polymers in trauma and bone surgery. Injury, 31 (Suppl 4): 28-32.
Hejazi, M., and Amiji, R., 2003. Chitosan-based gastrointestinal delivery systems. Journal of Controlled Release, 89 (2): 151-165.
Hoppe-Seiler, F., 1994. Chitin and chitosan. Berichte der Deutschen Chemischen Gesellschaft, 27: 3329-31.
Huang, R., Deng, Z., Yang, C., Yin, Y., Xie, M., Wu, G., Li, T., Li, L., Tang, Z., Kang, P., Hou, Z., Deng, D., Xiang, H., Kong, X., and Guo, Y., 2007. Dietary oligochitosan supplementation enhances immune status of broilers. Journal of the Science of Food and Agriculture, 87 (1): 153-159.
Huang, Y., Huang, W., Ren, X. E., Wu, Y., and Yang, F., 2013. Degradation of chitosan by hydrodynamic cavitation. Poly-mer Degradation and Stability, 98 (1): 37-43.
Ikada, Y., and Tsuji, H., 2000. Biodegradable polyesters for medical and ecological applications. Macromolecular Rapid Communications, 21 (3): 117-132.
Jiang, M., Zhuge, X., Yang, Y., Gu, X., and Ding, F., 2009. The promotion of peripheral nerve regeneration by chitooligosaccharides in the rat nerve crush injury model. Neuroscience Letters, 454 (3): 239-243.
Jikakis, J. P., (ed.), 1984. Chitin, Chitosan and Related Enzymes. Academic Press, New York, 423pp.
Kean, T., and Thanou, M., 2010. Biodegradation, biodistribution and toxicity of chitosan. Advanced Drug Delivery Reviews, 62 (1): 3-11.
Kong, X., Han, B., Wang, H., Li, H., Xu, W., and Liu, W., 2012. New biodegradable small-diameter artificial vascular prosthesis: A feasibility study. Journal of Biomedical Materials Research Part A, 100A (6): 1494-1504.
Madihally, S. V., and Matthew, H. W., 1999. Porous chitosan scaffolds for tissue engineering. Biomaterials, 20 (12): 1133-1142.
Moon, D. G., Christ, G., Stitzel, J. D., Atala, A., and Yoo, J. J., 2008. Cyclic mechanical preconditioning improves engineered muscle contraction. Tissue Engineering Part A, 14 (4): 473-482.
Muzzarelli, R. A. A., 1973. Natural Chelating Polymers. Pergamon Press, New York, 83-227.
Notin, L., Viton, C., David, L., Alcouffe, P., Rochas, C., and Domard, A., 2006. Morphology and mechanical properties of chitosan fibers obtained by gel-spinning: Influence of the dryjet-stretching step and ageing. Acta Biomaterialia, 2 (4): 387-402.
Onishi, H., and Machida, Y., 1999. Biodegradation and distribution of water-soluble chitosan in mice. Biomaterials, 20: 175-182.
Park, J. H., Cho, Y. W., Chung, H., Kwon, I. C., and Jeong, S. Y., 2003. Synthesis and characterization of sugar-bearing chitosan derivatives: Aqueous solubility and biodegradability. Biomacromolecules, 4 (4): 1087-1091.
Rinaudo, M., 2006. Chitin and chitosan: Properties and applications. Progress in Polymer Science, 31 (7): 603-632.
Sinha, V. R., Singla, A. K., Wadhawan, S., Kaushik, R., Kumria, R., Bansal, K., and Dhawan, S., 2004. Chitosan microspheres as a potential carrier for drugs. International Journal of Pharmaceutics, 274 (1-2): 1-33.
Tsaih, M. L., Tseng, L. Z., and Chen, R. H., 2004. Effects of removing small fragments with ultrafiltration treatment and ultrasonic conditions on the degradation kinetics of chitosan. Polymer Degradation and Stability, 86 (1): 25-32.
Varia, S. A., Schuller, S., Sloan, K. B., and Stella, V. J., 1984. Phenytoin prodrugs III: Water-soluble prodrugs for oral and/ or parenteral use. Journal of Pharmaceutical Sciences, 73 (8): 1068-1073.
Xia, W., Liu, P., Zhang, J., and Chen, J., 2011. Biological activities of chitosan and chitooligosaccharides. Food Hydrocolloids, 25 (2): 170-179.
Zeng, L., Qin, C., Wang, W., Chi, W., and Li, W., 2008. Absorption and distribution of chitosan in mice after oral administration. Carbohydrate Polymers, 71 (3): 435-440.
(Edited by Qiu Yantao)
(Received January 3, 2014; revised March 27, 2014; accepted May 26, 2015)
J. Ocean Univ. China (Oceanic and Coastal Sea Research)
DOI 10.1007/s11802-015-2573-5
ISSN 1672-5182, 2015 14 (5): 897-904
http://www.ouc.edu.cn/xbywb/
E-mail:xbywb@ouc.edu.cn
* Corresponding author. Tel: 0086-532-82032105
E-mail: wanshunliu@hotmail.com.
 Journal of Ocean University of China2015年5期
Journal of Ocean University of China2015年5期
- Journal of Ocean University of China的其它文章
- The Mechanism of the Acclimation of Nannochloropsis oceanica to Freshwater Deduced from Its Transcriptome Profiles
- A Carboxymethyl Cellulase from a Marine Yeast (Aureobasidium pullulans 98): Its Purification, Characterization, Gene Cloning and Carboxymethyl Cellulose Digestion
- Effects of Dietary Stachyose on Growth Performance, Digestive Enzyme Activities and Intestinal Morphology of Juvenile Turbot (Scophthalmus maximus L)
- Pharmacokinetics and Biodegradation Performance of a Hydroxypropyl Chitosan Derivative
- Changes in Plasma Osmolality, Cortisol and Amino Acid Levels of Tongue Sole (Cynoglossus semilaevis) at Different Salinities
- Effects of Different Microbes on Fermenting Feed for Sea Cucumber (Apostichopus japonicus)
