Morphometric studies of genus Placocheilus (Teleostei:Cypriniformes) from Red River, China
Wei ZHOU, Min-hui LI, Chun-ping WANG, Mi QI
Key Laboratory of Forest Disaster Warning & Control in Yunnan Province, Faculty of Forestry, Southwest Forestry University, Kunming Yunnan, 650224, China
INTRODUCTION
The genusPlacocheiluswas established by Wu (1977) on the basis of the description of type species DiscognathuscaudofasciatusPellegrin et Chevey.Placocheiluswas originally described as of order Cypriniformes, subfamily Barbininae and later re-classified into subfamily Labeoninae. The genusPlacocheilusis distributed in Dulong River, Nujiang River and Yuanjiang River-Honghe River (Red River) drainage, Yunnan Province, China; Nam Na Basin in Lai Chau, Vietnam and Nam Ma Basin in Laos (Chen et al, 2012; Chu & Cui, 1989; Cui & Li,1984; Kottelat, 2001; Wu, 1977; Zhang et al, 2002). The species of genusPlacocheilusis rheophilic and its lower lip has been modified into a mental adhesive disc (Chu & Cui, 1989).So far, the genus contains four species: P. caudofasciatus Pellegrin & Chevey,P. cryponemusCui & Li,P. robustusZhang et al and P. dulongensis Chen et al.
For quite some time, thePlacocheilusfrom Yuanjiang River-Honghe River drainage was deemed asP. caudofasciatus.Zhang et al (2002) re-evaluated the structures of scaleless midventral belly region, ratio of depth/length of caudal peduncle,and length/width of mental adhesive disc. Herein thePlacocheilusfrom Yuanjiang River-Honghe River drainage was re-classified into two independent species: P. caudofasciatus from Lixian River with its branches in Yunnan, China as well Tuojiang River (Heishui River or Black River, lower Lixian River Basin), Vietnam and Nam Ma Basin in Laos, all belong to the tributaries of Honghe drainage; and a new species,P. robustus,from Yuanjiang River and its tributaries of Red River Basin.However, due to the difficulties of discriminatingP. robustusandP. caudofasciatus, the classification has been usually based on collecting sites. As stated in Checklist of Fishes ofYunnan,P. robustuswas considered to be the synonym ofP.caudofasciatus without explanations (Chen, 2013). The taxonomic status of these two species should be further clarified.1
The conventional morphological measurements have limitations in comprehensiveness and accuracy. That is to say,the measuring distance extends along both horizontal and vertical coordinates and it is rather restricted to head and caudal peduncle areas. As a result, it is impossible to cover the entire body surface (Xie et al, 2003). The multivariate morphometry overcomes the above shortcomings (Bookstein et al, 1985) so that it has been successfully applied for population measurements. In other words, determining the validity of existing species or conjecture unknown species by evaluating the morphological differences among congeners (Cai et al,2001; Xie et al, 2003; Yang et al, 2003). Through multivariate morphometry, Li et al (2008) reported that one single fish species Pseudecheneis sulcata from different drainages actually belonged to several different species. The findings of Yang et al (2011) and Yang et al (2013) accorded with the previous results of Discogobio yunnanensis and Garra orientalis,and no intraspecies difference was detected. And Min et al (2009) revealed no morphological differentiations among different populations ofSinocyclocheilus grahamifrom the same drainage and confirmed the validity of the species.
In the present study, by adopting multivariate morphometry and principal component analysis, through measuring external morphological characters, including longitudinal, lateral and oblique distances, the major morphological differentiations and morphometric differences betweenP. robustusandP.caudofasciatus were compared. These findings provide evidence for clarifying the taxonomic status of these two species.
MATERIALS AND METHODS
Materials
The specimens (n=72) ofP. caudofasciatusandP. robustuswere deposited in Museum of Animal Section of Southwest Forestry University (SWFU) and Museum of Fish Section of Kunming Institute of Zoology (KIZ) (Table 1, Figure 1). The specimens Zhang et al (2002) used for describing P. robustus as a new species are currently conserved at KIZ. And 53 specimens at SWFU collected from Phona Tho at Lai Chau,Noire [Song Da] River at Lai Chau, Black River, Vietnam, were utilized for observing scale coverage only.
Study methods
Conventional measuring methods and multivariate morphometric studies were combined for specimen measurements. Multiple variable statistics (principal component analysis) was used for statistical analysis.
Morphological character measurements
A total of 10 anatomical coordinates were selected (Figure 2).With the left side of body taken as the bench mark, 23 frame characters and 15 general characters were measured. The measurements of countable and general characters were performed per Kottelat (2001); disc width and length per Zhang et al (2002). The scale coverage was observed under binocular microscope (Nikon, SMZ645). The length of scaleless region (distance from the origin line of pectoral fins to scaleless midventral belly region) was determined and its percentage in total length from the origin of pectoral fin to ventral fin calculated. Lineal distances between anatomical coordinates were obtained by an electronic digital caliper(accuracy=0.1 mm).
Statistical analysis
Logarithmic (log10) transformation of all morphological character data were processed with Microsoft Excel 2003 for eliminating the variations caused by size differences among the specimens (Xie et al, 2003; Li et al, 2008). Principal component analysis was conducted by SPSS 17.0 for Windows. Standarddata transformation was completed by the default settings for factorial analysis. Covariance matrix and Varimax were applied for factorial analysis. And scatter plots were constructed on the basis of the scores of principal components.
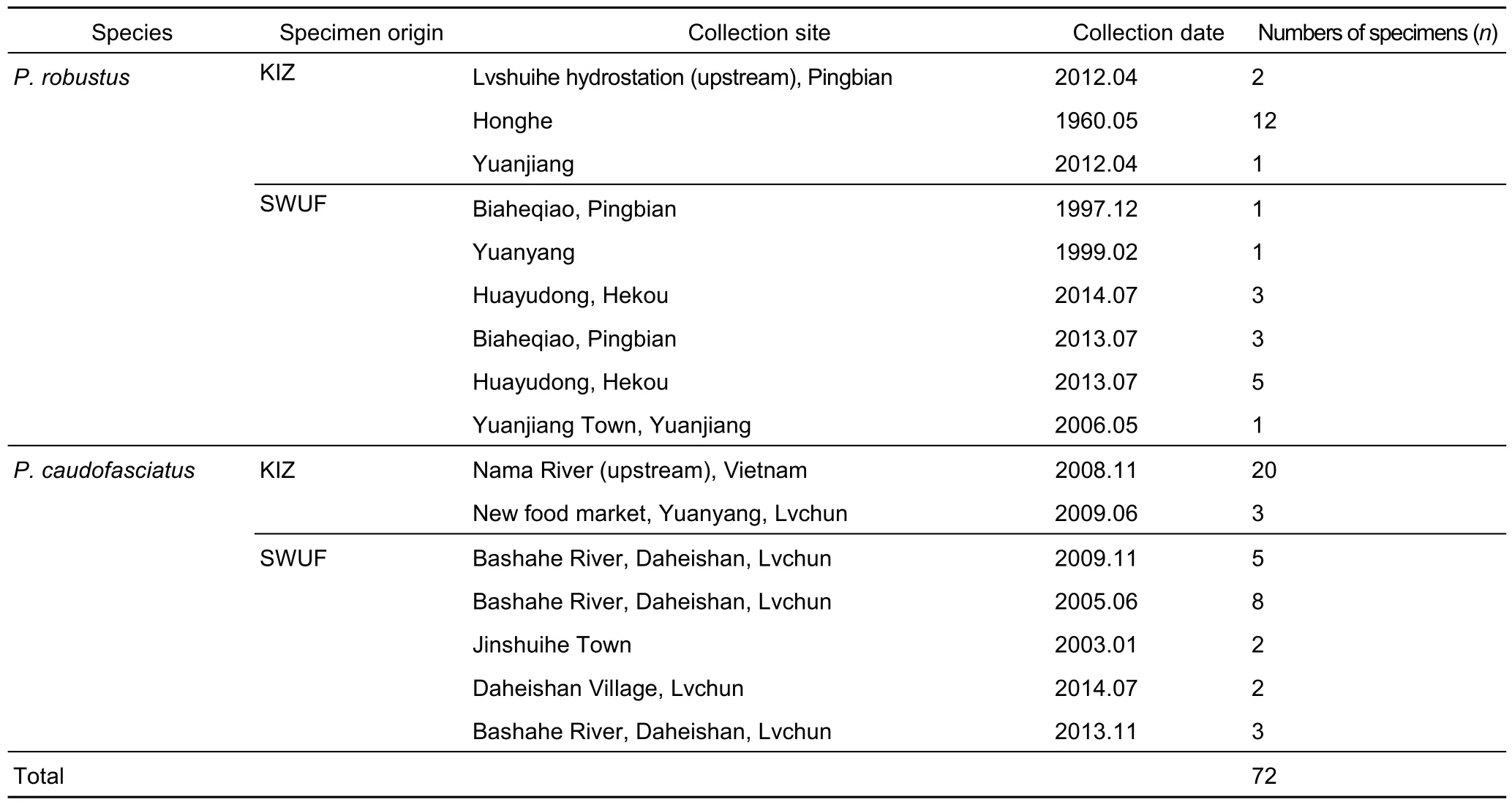
Table 1 List of examined specimens
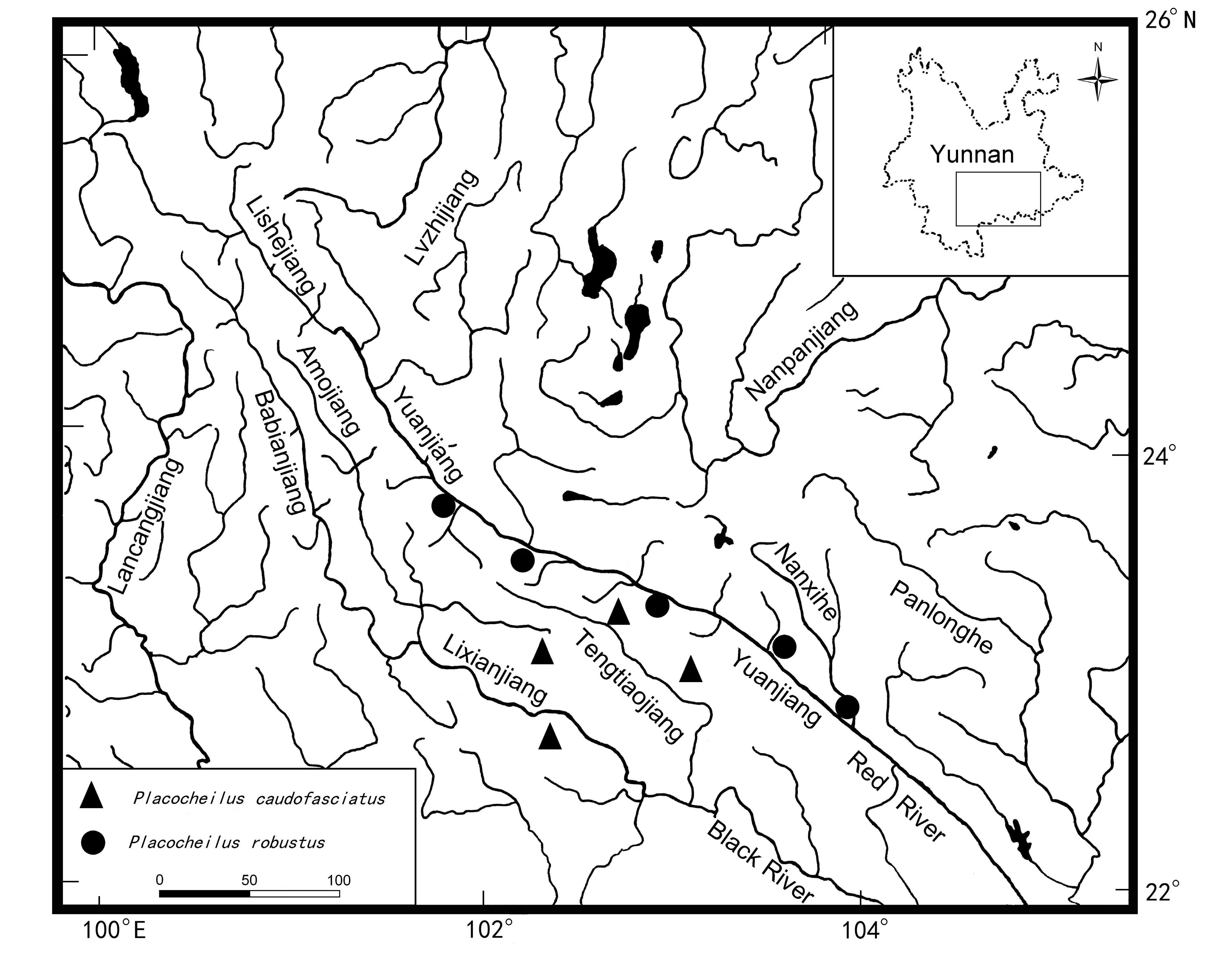
Figure 1 Distribution maps of Placocheilus caudofasciatus and Placocheilus robustus

Figure 2 Morphometric frame characters of Placocheilus caudofasciatus
RESULTS
Comparison of morphological characters
Besides scale coverage of midventral belly region, caudal peduncle and length/width of mental adhesive disc were listed(Zhang et al, 2002). In the present study, the rays of fins, scales in lateral line and scales in peduncle were also compared betweenP. caudofasciatusandP. robustus. Yet no significant differences existed in none of the relevant characters (Table 2,Table 3).
Principal component analysis
The specimens were divided into two groups according to their collection sites: group from Yuanjiang River Basin and its tributaries (P. robustus) and group from Lixian River Basin (P.caudofasciatus). The principal component analysis of 23 frame characters and 15 general characters showed that the variances of PC1, PC2 and PC3 were 48.093%, 29.866% and 12.297% respectively with an accumulative variance of 90.257% (Table 4). Scatter plots regarding PC1 vs PC2 and PC2 vs PC3 were constructed (Figure 3). The scatter plots indicated that these two Placocheilus species were nondistinguishable.
DISCUSSION
Pellegrin (1936) describedDiscognathus caudofasciatuson the basis of one holotype of MNHN 1935-0327. And it was collected from Noire (Song Da) River at Lai Chau, Black River, Vietnam.The specimens currently preserved at SWFU were also collected from Noire (Song Da) River at Lai Chau, Vietnam.Thus the above specimens are de facto topotypes.

Table 2 Morphological character comparisons between Placocheilus caudofasciatus and Placocheilus robustus
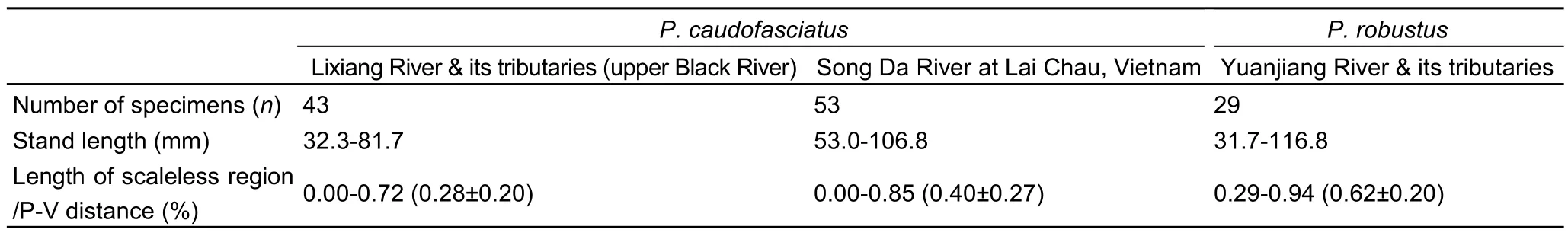
Table 3 Midventral region scale coverage of Placocheilus caudofasciatus v.s. Placocheilus robustus

Table 4 Principal component analyses of Placocheilus caudofasciatus v.s. Placocheilus robustus
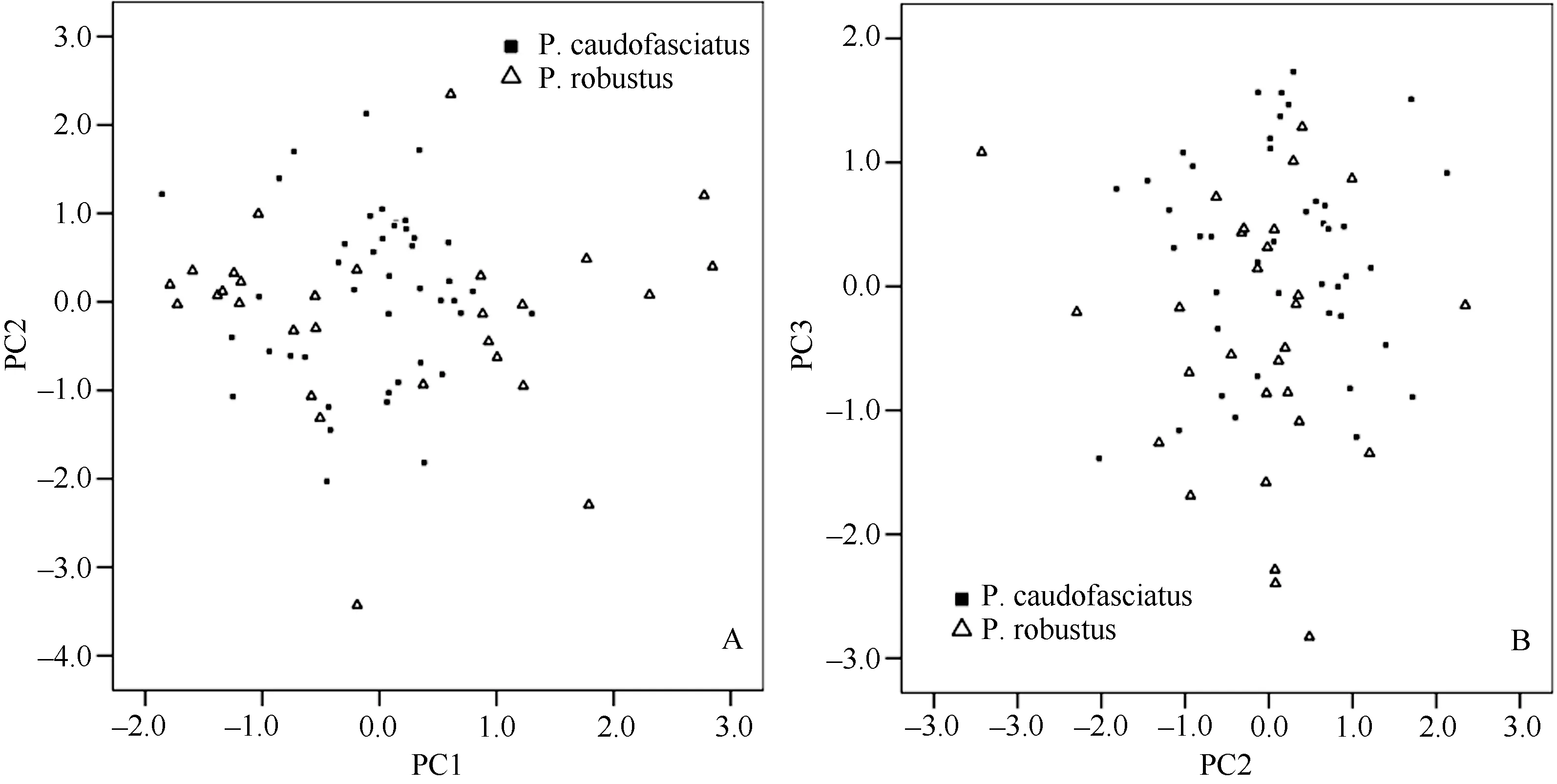
Figure 3 Scatter plots of principal component analysis
As reported by Zhang et al (2002), comparing withP.caudofasciatus, P. robustus was characterized by stouter caudal peduncle, smaller mental adhesive disc, medium-sized scaleless midventral belly region extends slightly beyond halfway from pectoral fin to ventral fin origin, whereas scaleless midventral region in P. caudofasciatus is limited to basal area of pectoral fin. Significant variations of scale coverage existed in specimens from Phona Tho at Lai Chau, Noire [Song Da] River at Lai Chau, Black River, Vietnam, in fact, these scale coverage variations include all the midventral belly region scale coverage status in P. caudofasciatus and P. robustus specimens from Vietnam and China. Here the character data were different from those in Zhang et al (2002). Other than inevitable measurement variations from different experiments, we assume that the major reason for these differences was the body lengths of specimens fell into a wide range. Therefore, among various-sized individuals, significant variations in some body parts were observed due to allometry. Based upon the data here and Zhang et al (2002), most diagnostic character measurements from Zhang et al (2002) were lined up end-to-end between two species. Therefore some inter-species differences were implicated. However there were significant overlaps with the data here (Table 3). It is concluded that the diagnostic characters of Zhang et al (2002) for distinguishingP. caudofasciatusandP. robustuswere not firmly supported.
Long-term geographic isolation blocks both genetic and unit communications among different populations. Thereafter,differentiations in morphology, ecology, physiology and biochemistry, or even reproductive isolation would occur as outcomes. When reproductive isolation happens, two populations are two independent species. However, in taxonomic studies, populations from different collection sites,but with similar or close physical forms are usually classified on the basis of their morphological characters. In the present study,the results of both external measurements and morphometry showed that no significant morphological differences existed between P. caudofasciatus and P. robustus, indicating that both were actually of one species and the latter was the synonym of the former. And it was in accordance with the results of Chen(2013). These conclusions are also supported by the results of muscular and skeletal anatomy (Li et al, 2016). The phylogenetic tree showed that P. caudofasciatus and P.robustusare gathered together. The genetic distance based on Cyt b showed that the distance among the effective specices of Garra and Placocheilus were 5% or higher; whereas, that betweenP. caudofasciatusandP. robustuswas only 4.2%,suggesting that P. robustus was the synonym of P.caudofasciatus(Wang, 2012). Even though the morphological and molecular results were mutually supportive, the genetic distance betweenP. caudofasciatusandP. robustus(4.2%) was still higher than the standard criteria (3%) for discriminating species in DNA barcoding studies. Thus for clarifying the species effectiveness, further molecular phylogenetic studies are warranted.
ACKNOWLEDGEMENTS
We greatly appreciate invaluable assistance of Prof. Jun-Xing YANG and Miss Li-Na DU during specimen examinations and insightful feedbacks of Dr. Xiao-Yong CHEN during manuscript preparations.
Bookstein FL, Chernoff B, Elder RL, Humphries JM, Smith GR, Strauss RE.1985. Morphometrics in Evolutionary Biology. Philadelphia: The Academy of Natural Science Special Publication.
Cai MJ, Zhang MY, Zeng QL, Liu HZ 2001. A study on morphometrics of the genusMeglobrama.Acta Hydrobilogica Sinica, 25(6): 631-635.(in Chinese)
Chen XY. 2013. Checklist of fishes of Yunnan.Zoological Research, 34(4):281-343. (in Chinese)
Chen ZM, Pan XF, Xiao H, Yang JX.2012. A new Cyprinidn species,Placocheilusdulongensis, from the upper Irrawaddy system in Northwastern Yunnan, China.Zoologischer Anzeiger, 251(3): 215-222.
Chu XL, Cui GH. 1989. Labeoninae. In: Chu, XL & Chen YR (eds), The Fishes of Yunnan, China. Part I. Beijing: Science Press, 229-286.
Cui GH, Li ZY. 1984. Description of a new cyprinid fish of the subfamily Barbinae from China.Acta Zootaxonomica Sinica, 9(1): 110-112. (in Chinese)
Kottelat M. 2001. Fishes of Laos. Colombo, Sri Lanka: Wildlife Heritage Trust Publications.
Li MH, Zhou W, Yuan J, Qi M, Li QS. 2016. Comparison study on species status ofPlacocheilus(Teleostei: Cypriniformes) from Red River.Journal of Guangxi Normal University (Natural Science Edition). (in Chinese) (Accepted)
Li X, Li FL, Liu K, Zhou W. 2008. A study on morphologic differentiation and taxonomic status ofPseudecheneis(Siluriformes: Sisoridae) from Irrawaddy and Salween drainages, China.Zoological Research,29(1): 83-88.(in Chinese)
Min R, Ye L, Chen XY, Yang JX. 2009. Morphometrics analysis ofSinocyclocheilus grahami(Cypriniformes: Cyprinidae).Zoological Research,30(6): 707-712. (in Chinese)
Wang WY. 2012. Systematrics, Phylogenetics and Biogeography of Several Chinese Labeononiae Subgroups andGarra. A dissertation for doctoral degree. Graduate University of Chinese Academy of Sciences (Kunming Institute of Zoology, Chinese Academy of Sciences), 1-144.
Wu XW. 1977. Cyprinidae Fishes of China. Part II. Shanghai: Science &Technology Press.
Xie ZG, Xie CX, Zhang E 2003. Morphological variations among the Chinese species ofSinibrama(Pisces: Teleostei: Cyprinidae), with comments on their species validities.Zoological Research, 24(5): 321-330. (in Chinese)
Yang Q, Zhou W, Shu SS. 2011. Morphological varations and differentiation ofDiscogobio yunnanensisfrom different population.Acta Zootaxonomica Sinica, 36(1): 117-124. (in Chinese)
Yang XP, Zhang MY, Liu HZ. 2003. Studies on morphometrics of the genusSaurogobio.Acta Hydrobilogica Sinica, 26(3): 281-285. (in Chinese)
Yang X, Zhou W, Li X, Li QS. 2013. Morphological differentiation ofGarra orientalis(Cyprinidae) among different geographical populations.Zoological Research, 34(5): 471-474. (in Chinese)
Zhang E, He SP, Chen YY. 2002. Revision of cyprinid genusPlacocheilusWu, 1977 in China, with description of a new species form Yunnan.Hydrobiologia, 487 (1): 207-217.
- Zoological Research的其它文章
- Patterns of reptile and amphibian species richness along elevational gradients in Mt. Kenya
- Stress-relevant social behaviors of middle-class male cynomolgus monkeys (Macaca fascicularis)
- Morphometric variability of Arctodiaptomus salinus(Copepoda) in the Mediterranean-Black Sea region
- Accelerated evolution of constraint elements for hematophagic adaptation in mosquitoes
- Physiological approaches to understanding molecular actions on dorsolateral prefrontal cortical neurons underlying higher cognitive processing
- Editor’s comments

