Photosynthesis of Digitaria ciliarisduring repeated soil drought and rewatering
YaYong Luo ,XueYong Zhao,JingHui Zhang,YuLin Li,XiaoAn Zuo,DianChao Sun
Naiman Desertification Research Station,Cold and Arid Regions Environmental and Engineering Research Institute,Chinese Academy of Sciences,Lanzhou,Gansu 730000,China
1 Introduction
The water availability in most arid and semi-arid ecosystems usually does not meet the demands of indigenous vegetation(Chenet al.,2005).Furthermore,the water availability is spatially and temporally heterogeneous(Huxmanet al.,2004).As a result,plants are repeatedly exposed to drought during their life cycles due to continuous changes in climatic factors in these regions(Miyashitaet al.,2005).However,arid ecosystems tend to rapidly show the effects of variation in precipitation(Xuet al.,2007).The responses of crops(Amlin and Rood,2003;Souzaet al.,2004;Zhanget al.,2004;Miyashitaet al.,2005)and trees(Ortunoet al.,2005;Gallé and Feller,2007;Galléet al.,2007;Perez-Perezet al.,2007)exposed to drought and rewatering cycles have been well documented,but little is known about the adaptive strategies of psammophytes during frequent water shortage and rewatering cycles.Most past studies about the effects of drought and rewatering assessed only one drought and rewatering cycle(Sionitet al.,1980;Lianget al.,2002),but it is difficult to understand plant responses to drought and rewatering processes with only one drought and rewatering cycle(Lianget al.,2002;Luoet al.,2011).
The Horqin Sandy Land in northern China is one of the most seriously desertification-threatened areas in China(Andrénet al.,1994).Plants in this area often survive during longer soil drought periods and recover upon precipitations,even though some soil water is used by other plants or is rapidly lost due to evaporation or percolation,but little information exists on the physiological mechanisms involved.Digitaria ciliarisis one of the dominant annuals in this sandy land(Liet al.,2005)and plays an important role in vegetation restoration.However,the mechanisms of its capacity to recover from drought have not yet been elucidated.The capacity of plants to recover from drought stress is crucial in terms of growth and survival in a rapidly changing climate.Accordingly,we examined the responses of leaf RWC(relative water content),gas exchange characteristics,and chlorophyll fluorescence to repeated soil drought and rewatering processes.Our objectives were to(1)determine the response patterns of photosynthesis to repeated soil drying and watering;and(2)determine whether the drought and rewatering alternation improved the photosynthesis in subsequent drought processes.
2 Materials and methods
2.1 Experimental site,plant material,and experimental design
Our study was conducted in Naiman County(42°55′N,120°44′E;360 m a.s.l.)located in the southwestern part of the Horqin Sandy Land,representing the most desertification-threatened area in North China(Andrénet al.,1994).This area has a temperate,semi-arid,and continental monsoonal climate,receiving 360 mm annual mean rainfall,with 75% of it occurring between June and September.The annual mean latent evaporation is 1,935 mm(Liet al.,2005).A number of psammophilous species are dominant in the area,such asD.ciliaris,Aristida adscensionis,Cleistogenes squarrosa,andChloris virgata(Liet al.,2005).
In our study,specimens ofD.ciliariswere uprooted from sandy dunes.They were of uniform size(height of 20 cm)and were transferred to plastic pots(diameter 22 cm,height 19 cm)containing sandy soil near the Naiman Desertification Research Station.Thirty plants(15 plants per treatment)were nurtured to recover from transplanted injury under natural conditions with well irrigation until the onset of the experiments for each species.Excess water was allowed to drain through holes in bottoms of the pots.Experimental treatment was put into practice on August 13,when leaf had been completely developed and matured.All pots were placed into a mobile rain shelter,which was drawn back to avoid rainfall,and undrawn to ensure the natural conditions after rain.Stressed plants were kept without water until net photosynthesis approached zero during the late morning.
The control group consisted of well-watered plants(irrigated daily in the evening)and kept constant pot weight.For drought stressed plants,three drought spells were from August 14 to 19,from August 23 to 29,and from September 2 to 9 in 2008.In the evening of the fifth day without water,the plants were rewatered for four days.In each drought spell,parameter measurements were taken on every other day(that is,the first,third,and fifth days after watering,excluding the overcast and rainy days)on sunny and windless days.The soil moisture was determined according to the changes of whole pot weight.
2.2 Soil water status and climatic data
Changes in pot weights of the drought-stressed plants and control plants were monitored by the method of Galléet al.(2007)to reflect soil water status.Photosynthetic active radiation(PAR),air temperature,and the air relative humidity were recorded with a LI-6400 portable photosynthesis system(LI-COR Inc.,Lincoln,NE,USA)during gas exchange measurements throughout the experiment.
2.3 Determination of RWC
On each sampling day,six leaves were taken from each treatment during the late morning(09:30–10:30).After determining the fresh weight(FW),the leaves were rehydrated by immersing the petiole in distilled water in a petri dish capped with nylon mesh.Full rehydration was achieved in 3 h;then the leaves were blotted dry and weighed for turgid weight(TW).Subsequently,the dry weight(DW)was determined after oven-drying the leaf samples at 70 °C for 24 h(Izanlooet al.,2008).Five replicates per treatment were taken.
The relative water content(RWC)of the leaves was calculated according to the following equation:
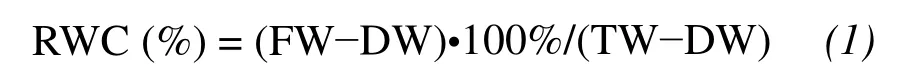
2.4 Gas exchange measurements
At the same time,photosynthetic traits were measuredin situon fully expanded and sun-exposed leaves of five different plants per treatment(one leaf per plant).The net photosynthetic rate(Pn),transpiration rate(Tr),stomatal conductance(gs)and intercellular CO2concentration(Ci)of leaves were measured using the LI-6400 photosynthesis system.The measurements were conducted in the condition of ambient CO2concentration,a block temperature of 30 °C,and a saturating irradiance(1,500 μmol/(m2·s))provided by a built-in red LED light source.The ratio ofPntoTrwas calculated to determine instantaneous water use efficiency(WUE)(Chenet al.,2005).
2.5 Chlorophyll fluorescence measurements
Measurements of chlorophyll fluorescence and gas exchange were carried out on the same leaf.Chlorophyll fluorescence was measured using a Handy-PEA portable plant efficiency analyzer(Hansatech,King's Lynn,UK).With no dark adaptation,the measurements were conducted with the excitation light of 3,000 μmol/(m2·s)for 1 s.
2.6 Analysis of the chlorophyll fluorescence transients:JIP-test
Partial JIP test parameters(Table 1)are shown mainly to explain the distribution of the absorbance.The energy fluxes contain trapping and dissipation.The Fv/Fmratio(φP0),φE0,and ψ0represent the trapping probability(TR0/ABS),the electron transport probability,and the probability that the trapped excitation moves an electron into the electron transport chain beyond electron transport chain beyond,respectively(Strasser,1978,1981;Forceet al.,2003).Cross-section parameters were calculated to determine the distribution of energy flux for trapping,dissipation,and electron transport.
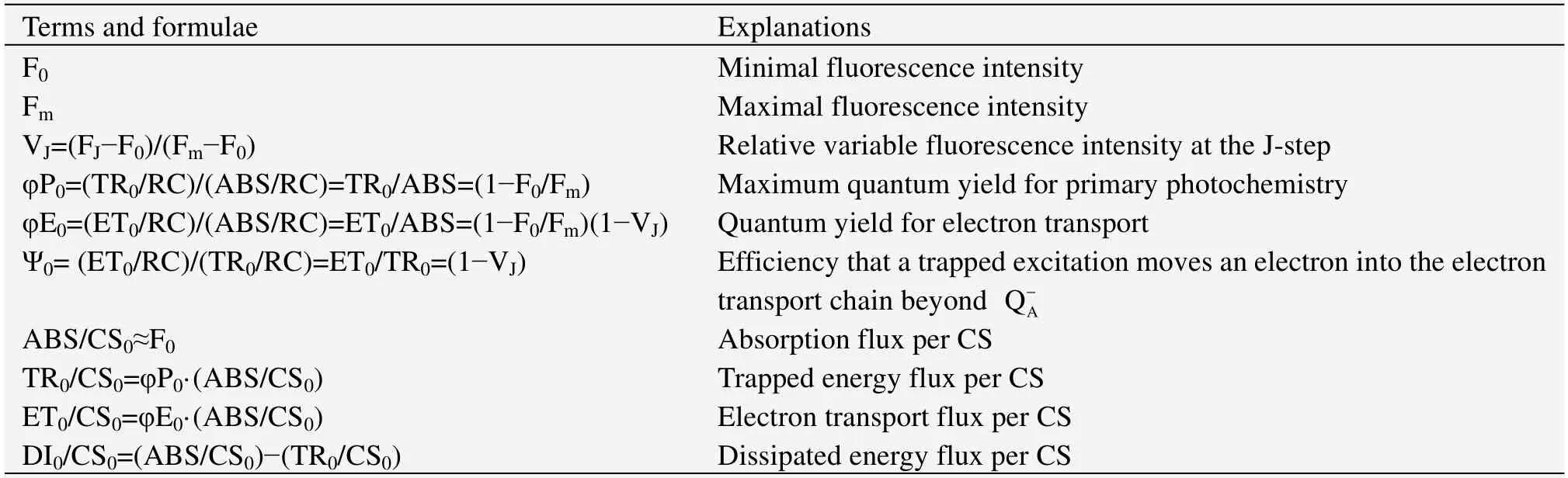
Table 1 JIP test parameters with explanations and formulae calculated using data extracted from the fast fluorescence transient
3 Results
3.1 Environmental conditions and water status
The climatic conditions during the experimental periods were typical of summer in the Horqin Sandy Land.Photosynthetic active radiation(PAR),the relative humidity,and air temperature on the sampling days throughout the experiment ranged between 1,103 and 1,604 μmol/(m2·s),26.6%,and 37.2%(Figure 1),and 29.3 °C and 30.4 °C,respectively(Figures 2a,b).Ambient CO2concentration ranged between 358 and 380 μmol(CO2)/mol(data not shown)throughout the whole experimental period.
In the control group,the soil moisture was kept at 80% during the entire experimental period(Figure 2a).In each treatment,the soil moisture decreased progressively with drought,and recovered upon rewatering.The changes of soil moisture in three repeated drought and watering cycles were the same(Figure 2a).At the end of the three drought treatment periods,the soil moisture reached minimum values of 45.20%,43.91%,and 46.31%,respectively.
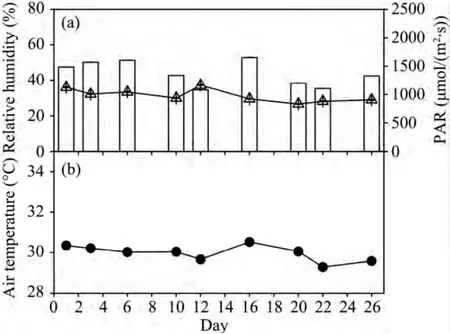
Figure 1 Changes in photosynthetic active radiation(PAR)(columns),relative humidity of air(▲ line),and air temperature for each sampling day through experiment.Drought-stressed plants were rewatered on days 6–9 and 16–19(hatched areas).Means and SE of five replicates are shown
The RWC of the leaves indicates the extent of dehydration and is used to assess cellular damage(Baiet al.,2006).In the control,the RWC of the leaves was relatively constant throughout the experiment(Figure 2b).However,the leaf RWC in the drought treatments changed sharply depending on the soil moisture(Figure 2b).It declined from the onset of the drought period,but upon rewatering it recovered and reached levels similar to the control.The pattern of leaf RWC had a tendency similar to that of the soil moisture.At the ends of the three drought periods,the RWC of the leaves declined to 37%,41%,and 44%,respectively,indicating that the decreasing level of leaf RWC diminished with the increasing number of drought cycles.Leaf RWC was restored to the control level after rewatering four days later(Figures 2b).The control plants showed slight changes in the leaf RWC throughout the experimental period,ranging between 75.37% and 89.53%(Figure 2b).
3.2 Gas exchange characteristics
Compared to the control plants,soil drought led to a rapid suppression ofPnduring soil drought periods(Figure 3a).Pndecreased by 92%,95%,and 63% at the ends of the three drought periods,respectively,while the reverse tendency was observed in the changes ofCi(Figure 3b)compared with thePnunder drought.TheCiincreased by 0.60,0.08,and 0.07 times on the third day after watering ceased during the three drought cycles,respectively,while it increased by 1.08,0.88,and 0.45 times at the ends of the drought.
The changes of WUE had a tendency similar to that ofPn(Figure 3c).The WUE decreased by 67%,54%,and 48% at the ends of the three drought periods,respectively.It was always lower than in the control.The average WUE was 4.83,3.30,and 2.07,respectively,on the first,third,and fifth days after watering ceased,while it was 5.21,5.20,and 5.11 in the control.This suggests that the plants used water to grow as quickly as possible after drought stress,even if at a reduced WUE.
3.3 Chlorophyll fluorescence characteristics
Fv′/Fm′(Figure 4a)and ET0′/CS0′(Figure 4c)were lower in drought-stressed plants than in the control.The Fv′/Fm′ decreased by 55%,51%,and 9% at the end of three drought cycles,respectively,while the ET0′/CS0′decreased by 63%,42%,and 18%.This suggests that the trapping probability of PS II and the electron transport flux were inhibited under drought.However,the reverse tendency was observed in F0′ and DI0′/CS0′under drought,reflecting photoinhibition(Forceet al.,2003).The F0′ increased by 15%,12%,and 9% in the three drought cycles,respectively,while the DI0′/CS0′increased by 98%,28%,and 22%(Figure 4b).
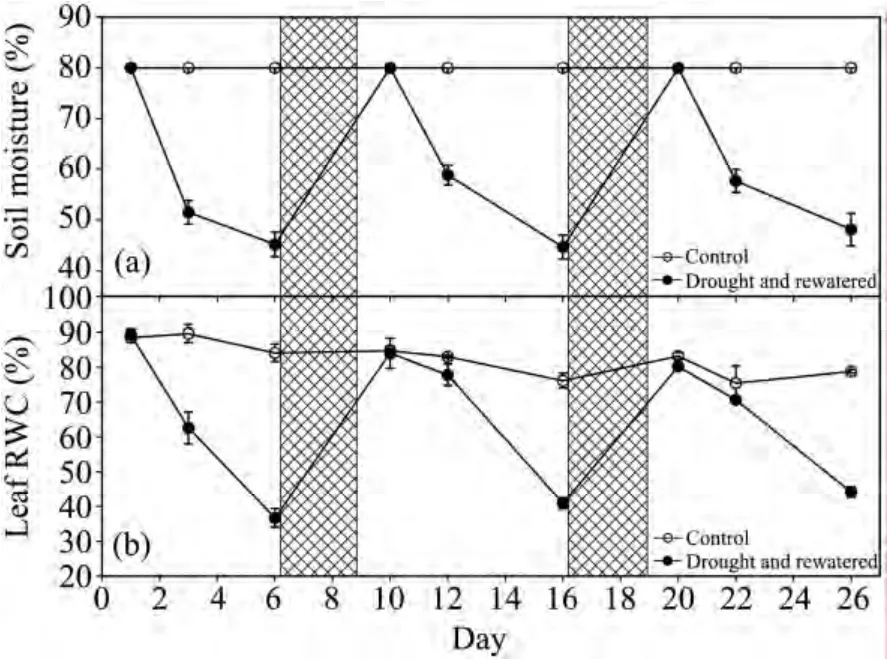
Figure 2 Changes of soil moisture content and leaf RWC in D.ciliarisduring repeated soil drought and rewatering.Drought-stressed plants were rewatered on days 6–9 and 16–19(hatched areas).Soil moisture(n = 15 pots)and leaf RWC(n = 5)are shown
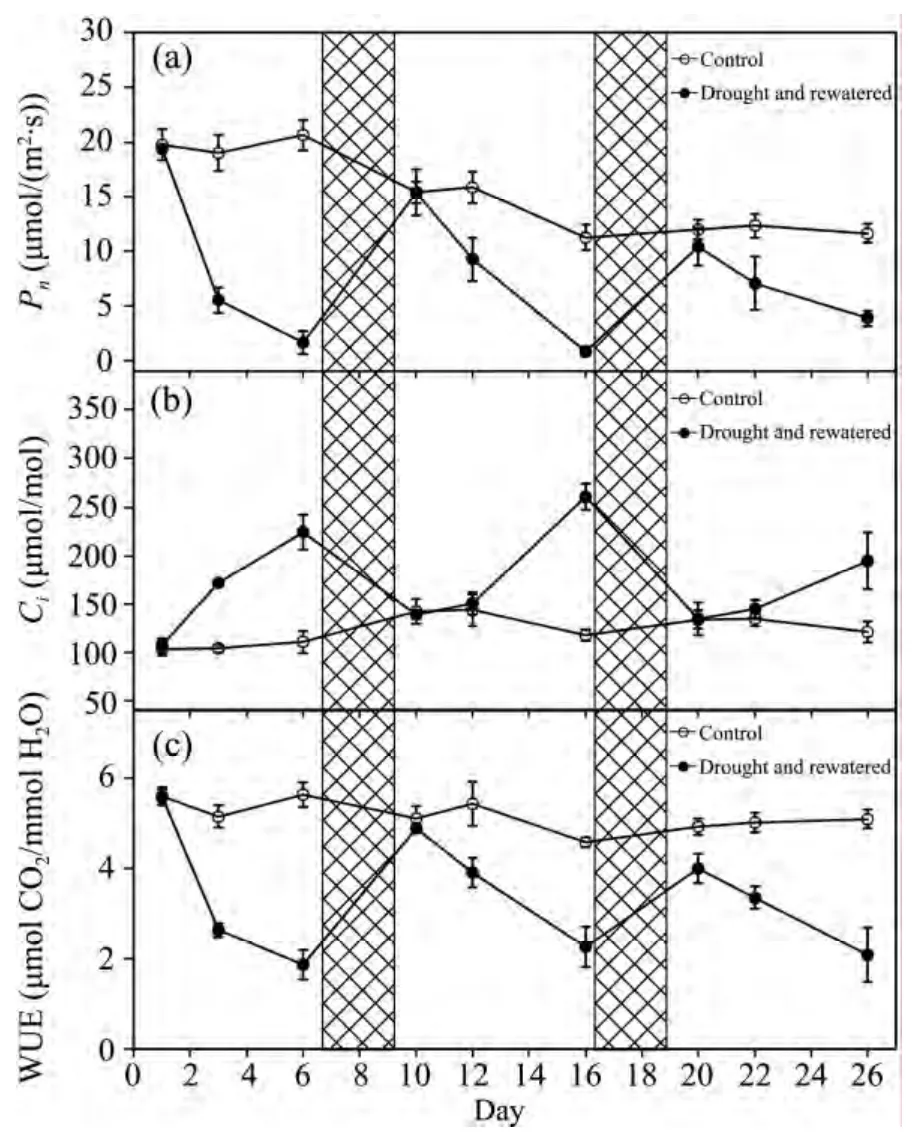
Figure 3 Changes in D.ciliaris Pn,Ci and WUE during repeated soil drought and rewatering.Values are means ±SE(n = 5)
4 Discussions
4.1 Effect of drought on gas exchange and chlorophyll fluorescence characteristics
Compared to the control plants,soil drought led to a rapid suppression ofPn(Figure 3)during soil drought periods,consistent with previous studies(Souzaet al.,2004;Gallé and Feller,2007).Ciincreased with the soil water shortage,especially on the last days of the drought periods(Figure 3).An overestimatedCicould result from heterogeneous(or "patch")stomatal closure and cuticular conductance,which are the two main problems invalidatingCicalculation under drought;decreasing mesophyll conductance can cause the CO2concentration in chloroplasts of stressed leaves to be considerably lower than theCi(Flexas and Medrano,2002;Flexaset al.,2004b).Decreases inPnmay indicate changes in the mesophyll capacity forPn,but may also be caused by heterogeneous stomatal closure(Mott and Parkhurst,1991).Furthermore,the dramatic decreases inPnand sharp increases inCithat we found(Figure 3)suggest the predominance of non-stomatal limitations to photosynthesis on the last days of drought periods(Flexas and Medrano,2002;Flexaset al.,2004a),which in turn indicates that the photosynthetic apparatus was damaged,presumably due to decreases in photochemistry and Rubisco activity(Flexaset al.,2006).Malfunction of the photosynthetic apparatus may reduce the efficiency of electron transport for photosynthetic reaction(Figure 4).
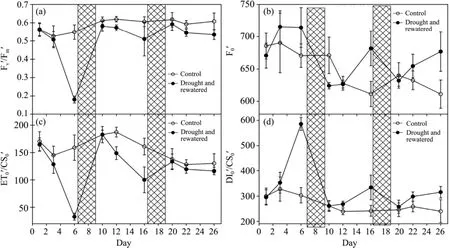
Figure 4 Changes in D.ciliarisFv′/Fm′,ET0′/CS0′,F0′ and DI0'/CS0' during repeated soil drought and rewatering.Values are means ±SE(n = 5)
Potential higher WUE is due to stomatal closure causing decreases in transpiration more thanPn(Hetherington and Woodward,2003;Donovanet al.,2007).However,higher WUE comes at the cost of lowerPnand productivity(Chaveset al.,2002;Donovanet al.,2007).In our study,changes in WUE had a tendency similar to leaf RWC(Figures 2,3);thus,photosynthesis may have been more restricted by the chloroplast's capacity to fix CO2(metabolic limitations)than by the increased diffusive resistance(Flexaset al.,2004a),especially on the last days of the drought periods.Additionally,WUE in the drought-stressed plants was lower than in the control plants(Figure 3).As a result,D.ciliarisphotosynthesized quickly and had high WUE on the first days of the drought periods,while suppression ofPnand low WUE were observed during later drought periods(Figure 3).This "drought escape" strategy may therefore have been adopted by the psammophytes to cope with decreases of water availability(Sherrard and Maherali,2006),allowing them to increase their growth rate and accelerate development.Chaveset al.(2002)reported that annuals often primarily rely on rapid growth to escape drought stress,as well as on fast photosynthetic and C metabolism machinery responses in semi-arid regions.This"fast growing" strategy allows psammophytes to acclimatize themselves and compete with other plants for available water in semi-arid sandy land regions where there is a lack of precipitation and water is easily lost due to evaporation and percolation(Donovanet al.,2007).
Chlorophyll fluorescence is widely accepted as a means of understanding the structure and function of the photosynthetic apparatus(Forceet al.,2003).Although measurement with no dark adaptation could increase ABS/CS0and DI0/CS0,and could decrease TR0/CS0and ET0/CS0,the JIP-test could also be used to analyze the energy fluxes in this case(Forceet al.,2003).TR0/ABS with no dark adaptation denotes the trapping probability,namely Fv′/Fm′,reflecting the effective quantum yield of PS II under light.The F0′value can represent ABS/CS0,which shows the total number of photons absorbed by the antenna molecules of active and inactive PS II reaction center over the sample cross-section(Forceet al.,2003).As a result,the absorbed flux at the level of a PS II cross-section increases under drought.However,in our study the increased level was much lower in ABS/CS0than the DI0′/CS0′,which shows total dissipation measured over the cross-section of the sample that contained active and inactive reaction center.The results indicated that the excess photons absorbed were dissipated effectively,so the trapping probability and electron transport flux were reduced under drought.Increased levels of energy dissipation,which decrease Fv′/Fm′,may help to protect PS II from overexcitation and photodamage(Schindler and Lichtenthaler,1994),so it is an important mechanism under conditions of water deficit(Chaveset al.,2002).The reduction in electron transport flux was higher than the trapping probability of PS II under light.These results were consistent with the proposal of Forceet al.(2003)that photoinhibition can be assessed more sensitively and accurately from changes in the dissipation and electron transport probability,than by the trapping probability(Fv′/Fm′)with no dark adaptation.
4.2 Recovery of gas exchange and chlorophyll fluorescence characteristics
Upon rewatering,our drought-stressed plants reached levels ofPn,Ci,and WUE that were similar to those in the control group.Studies by Souzaet al.(2004)and Gallé and Feller(2007)also reported that the plants reached the levels ofPnthat were similar to those in the control groups.In contrast,Miyashitaet al.(2005)reported that the fractional recovery inPnwas higher thanTrand stomatal conductance.Another study(Gallé and Feller,2007)showed that thePnrecovered completely within four weeks in stressed beech saplings,while gsremained permanently lower in stressed plants than in control plants.The reason for this difference in the recovery of gas exchange characteristics might be species-specific or stress-specific,and requires further investigations(Galléet al.,2007).
In parallel with thePnand WUE,our plants reached levels of Fv′/Fm′,ET0′/CS0′,F0′,and DI0′/CS0′that were similar to those in the control upon rewatering.Some studies reported that,upon rewatering,fluorescence parameters under severe drought were recovered gradually and completely(Souzaet al.,2004;Miyashitaet al.,2005;Gallé and Feller,2007;Galléet al.,2007).In parallel with thePn,our plants that had no water for five days reached the levels of Fv′/Fm′,ET0′/CS0′,F0′,and DI0′/CS0′ that were similar to those in the control group upon rewatering.
Similar to the changes ofPnand WUE,the Fv′/Fm′and ET0′/CS0′ of leaves decreased or slowed down during subsequent progressions of drought,especially in the third drought spell.This indicated that,after drought hardening,the photosynthetic apparatus itself may have enhanced the plants' future capacity to tolerate soil drought(Yordanovet al.,2003).Therefore,it might be speculated that photosynthetic adjustments at the leaf level enhance the resistance and adaptive evolution to drought in order to prevent further drought stress(Galléet al.,2007).
Because we investigated only the data pertaining soil drought,the data pertaining to rewatering were insufficient,and the delayed recovery ofPnof stressedD.ciliarisduring rewatering remains unexplained.The patterns of recovery forD.ciliarisrequire further investigations in order to clarify its capability to withstand and survive extreme drought events.In this experiment,after four days of rewatering,the plants reached the levels of photosynthetic parameters that were similar to those in the control group.This capability to recover quickly from drought may indicate thatD.ciliarisuses water to grow as quickly as possible after drought stress.This strategy can enableD.ciliaristo succeed in the competition with other species for water and to flourish in semi-arid sandy lands.
The authors thank all the staff at the Naiman Desertification Research Station,Chinese Academy of Sciences(CAS).This paper was financially supported by the National Natural Science Foundation of China(No.41201249),the Open Fund of the Key Laboratory of Land Surface Process and Climate Change in Cold and Arid Regions(No.SKLFSE201203),the National Science and Technology Support Program(No.2011BAC07B02),the Knowledge Innovation Program of the Chinese Academy of Sciences(No.KZCX2-EW-QN313),and the National Basic Research Program of China(No.2009CB421303).
Amlin NM,Rood SB,2003.Drought stress and recovery of riparian cottonwoods due to water table alteration along Willow Creek,Alberta.Trees-Structure and Function,17:351–358.DOI:10.1007/s00468-003-0245-3.
Andrén O,Zhao X,Liu X,1994.Climate and litter decomposition in Naiman,Inner Mongolia,China.AMBIO,23:222–224.
Bai LP,Sui FG,Ge TD,et al.,2006.Effect of soil drought stress on leaf water status,membrane permeability and enzymatic antioxidant system of maize.Pedosphere,16:326–332.DOI:10.1016/S1002-0160(06)60059-3.
Chaves MM,Pereira JS,Maroco J,et al.,2002.How plants cope with water stress in the field:Photosynthesis and growth.Annals of Botany,89:907–916.DOI:10.1093/Aob/Mcf105.
Chen SP,Bai YF,Zhang LX,et al.,2005.Comparing physiological responses of two dominant grass species to nitrogen addition in Xilin River Basin of China.Environmental and Experimental Botany,53:65–75.DOI:10.1016/j.envexpbot.2004.03.002.
Donovan LA,Dudley SA,Rosenthal DM,et al.,2007.Phenotypic selection on leaf water use efficiency and related ecophysiological traits for natural populations of desert sunflowers.Oecologia,152(1):13–25.DOI:10.1007/s00442-006-0627-5.
Flexas J,Medrano H,2002.Drought-inhibition of photosynthesis in C3plants:Stomatal and non-stomatal limitations revisited.Annals of Botany,89(2):183–189.
Flexas J,Bota J,Cifre J,et al.,2004a.Understanding down-regulation of photosynthesis under water stress:Future prospects and searching for physiological tools for irrigation management.Annals of Applied Biology,144:273–283.DOI:10.1111/j.1744-7348.2004.tb00343.x.
Flexas J,Bota J,Loreto F,et al.,2004b.Diffusive and metabolic limitations to photosynthesis under drought and salinity in C3plants.Plant Biology(Stuttgart),6:269–279.
Flexas J,Bota J,Galmés J,et al.,2006.Keeping a positive carbon balance under adverse conditions:Responses of photosynthesis and respiration to water stress.Physiologia Plantarum,127(3):343–352.DOI:10.1111/j.1399-3054.2006.00621.x.
Force L,Critchley C,van Rensen JJS,2003.New fluorescence parameters for monitoring photosynthesis in plants.Photosynthesis Research,78(1):17–33.DOI:10.1023/A:102601 2116709.
Gallé A,Feller U,2007.Changes of photosynthetic traits in beech saplings(Fagus sylvatica)under severe drought stress and during recovery.Physiologia Plantarum,131:412–421.DOI:10.1111/j.1399-3054.2007.00972.x.
Gallé A,Haldimann P,Feller U,2007.Photosynthetic performance and water relations in young pubescent oak(Quercus pubescens)trees during drought stress and recovery.New Phytologist,174(4):799–810.DOI:10.1111/j.1469-8137.2007.02047.x.
Hetherington AM,Woodward FI,2003.The role of stomata in sensing and driving environmental change.Nature,424:901–908.DOI:10.1038/nature01843.
Huxman TE,Snyder KA,Tissue D,et al.,2004.Precipitation pulses and carbon fluxes in semiarid and arid ecosystems.Oecologia,141(2):254–268.
Izanloo A,Condon AG,Langridge P,et al.,2008.Different mechanisms of adaptation to cyclic water stress in two South Australian bread wheat cultivars.Journal of Experimental Botany,59(12):3327–3346.DOI:10.1093/jxb/ern199.
Li FR,Zhang AS,Duan SS,et al.,2005.Patterns of reproductive allocation inArtemisia halodendroninhabiting two contrasting habitats.Acta Oecologica,28(1):57–64.DOI:10.1016/j.actao.2005.02.005.
Liang ZS,Zhang FS,Shao MG,et al.,2002.The relations of stomatal conductance,water consumption,growth rate to leaf water potential during soil drying and rewatering cycle of wheat(Triticum aestivum).Botanical Bulletin of Academia Sinica,43:187–192.
Luo YY,Zhao XY,Zhou RL,et al.,2011.Physiological acclimation of two psammophytes to repeated soil drought and rewatering.Acta Physiologiae Plantarum,33:79–91.DOI:10.1007/s11738-010-0519-5.
Miyashita K,Tanakamaru S,Maitani T,et al.,2005.Recovery responses of photosynthesis,transpiration,and stomatal conductance in kidney bean following drought stress.Environmental and Experimental Botany,53:205–214.DOI:10.1016/j.envexpbot.2004.03.015.
Mott KA,Parkhurst DF,1991.Stomatal responses to humidity in air and helox.Plant,Cell and Environment,14(5):509–515.DOI:10.1111/j.1365-3040.1991.tb01521.x.
Ortuno MF,Alarcon JJ,Nicolas E,et al.,2005.Sap flow and trunk diameter fluctuations of young lemon trees under water stress and rewatering.Environmental and Experimental Botany,54:155–162.DOI:10.1016/j.envexpbot.2004.06.009.
Perez-Perez JG,Syvertsen JP,Botia P,et al.,2007.Leaf water relations and net gas exchange responses of salinizedCarrizo citrangeseedlings during drought stress and recovery.Annals of Botany,100:335–345.DOI:10.1093/Aob/Mcm113.
Schindler C,Lichtenthaler HK,1994.Is there a correlation between light-induced zeaxanthin accumulation and quenching of variable chlorophyll a fluorescence? Plant Physiology and Biochemistry(Paris),32:813–823.
Sherrard ME,Maherali H,2006.The adaptive significance of drought escape inAvenabarbata,an annual grass.Evolution,60(12):2478–2489.
Sionit N,Teare ID,Kramer PJ,1980.Effects of repeated application of water-stress on water status and growth of wheat.Physiologia Plantarum,50:11–15.
Souza RP,Machado EC,Silva JAB,et al.,2004.Photosynthetic gas exchange,chlorophyll fluorescence and some associated metabolic changes in cowpea(Vigna unguiculata)during water stress and recovery.Environmental and Experimental Botany,51:45–56.DOI:10.1016/S0098-8472(03)00059-5.
Strasser RJ,1978.The grouping model of plant photosynthesis.In:Akoyunoglou G(ed.).Chloroplast Development.North Holland:Elsevier,pp.513–524.
Strasser RJ,1981.The grouping model of plant photosynthesis:heterogeneity of photosynthetic units in thylakoids.In:Akoyunoglou G(ed.).Photosynthesis III.Structure and Molecular Organisation of the Photosynthetic Apparatus.Philadelphia:Balaban International Science Services,pp.727–737.
Xu H,Li Y,Xu GT,et al.,2007.Ecophysiological response and morphological adjustment of two central Asian desert shrubs towards variation in summer precipitation.Plant,Cell and Environment,30(4):399–409.
Yordanov I,Velikova V,Tsonev T,2003.Plant responses to drought and stress tolerance.Bulgarian Journal of Plant Physiology,17(l):187–206.DOI:10.1023/A:1007201411474.
Zhang F,Guo JK,Yang YL,et al.,2004.Changes in the pattern of antioxidant enzymes in wheat exposed to water deficit and rewatering.Acta Physiologiae Plantarum,26(3):345–352.DOI:10.1007/s11738-004-0024-9.
 Sciences in Cold and Arid Regions2015年1期
Sciences in Cold and Arid Regions2015年1期
- Sciences in Cold and Arid Regions的其它文章
- Seasonal change mediates the shift between resource and pollen limitation in Hedysarum scoparium(Fabaceae)
- Characteristics of high arsenic groundwater in Hetao Basin,Inner Mongolia,northern China
- The response of Caragana microphylla seedlings to water table changes in Horqin Sandy Land,China
- Effects of sand burial on growth and antioxidant enzyme activities of wheat(Triticum aestivum L.)in northern China
- Screening of cellulose decomposing fungi in sandy dune soil of Horqin Sandy Land
- Effects of sand burial on survival and growth of Artemisia halodendron and its physiological response
