The response of Caragana microphylla seedlings to water table changes in Horqin Sandy Land,China
YunHua Ma ,TongHui Zhang ,XinPing Liu ,Wei Mao ,XiangFei Yue
1. Naiman Desertification Research Station,Cold and Arid Regions Environmental and Engineering Research Institute,Chinese Academy of Sciences,Lanzhou,Gansu 730000,China
2. University of Chinese Academy of Sciences,Beijing 100049,China
1 Introduction
In the semi-arid Horqin Sandy Land of Northeast China,Caragana microphylla,a perennial leguminous shrub,is the dominant plant species and is widely planted in this region to control soil erosion and land desertification.Shrub establishment plays an important role in rehabilitating desertified ecosystems in this region(Su and Zhao,2003).Water is a key limiting factor for vegetation restoration in the semi-arid areas of China.Groundwater is an important source of water in sandy land,which influences the plant's physiological and ecological characteristics.The plant's main response to changes in soil moisture content is through the root system.Under dry conditions,the root systems show a certain degree of plasticity in their growth(Readeret al.,1993),and changes in root growth,density,surface area and distribution patterns are more sensitive to change than soil nutrients and other physiological parameters of root systems(Jastrow and Miller,1993;Georgeet al.,1997).However,groundwater is one of the main water sources for natural vegetation in arid areas,and changes in the depth of the groundwater table have a profound effect on the growth and morphological distribution of plants.Plants can adapt to environmental changes by adjusting allotment of resources.Roots are the first organ which changes their growth patterns under the condition of a biotic adversity-stress.Water is the key limiting factor for plant growth(Gregory 1989;Sayeret al.,2006)and also greatly affects fine root distribution(Zhou and Shangguan,2007).Thus,plants can adapt to stressful conditions.
The objective of this study was to monitor the growth response ofC.microphyllato changes of water table controlled artificially in semiarid areas of Horqin Sandy Land.Accurate research on perennialC.microphyllain a natural setting was difficult due to the distribution of a complex root system.Thus,we explored the physiological and ecological characteristics ofC.microphyllaseedlings in an artificially controlled water table.We observed the growth conditions of abovegroundC.microphyllaseedlings over two consecutive years,then removed the root system for further examination.
2 Material and methods
2.1 Site description
This experiment was conducted at the Naiman Desertification Research Station,Chinese Academy of Sciences,located 10 km northeast of Naiman County at Horqin Sandy Land,Inner Mongolia,China(42°55′N,120°42′E;360 m a.s.l.).This region has a semi-arid continental temperate monsoon climate,with windy and dry winter and spring,and warm and comparatively rain-rich summer followed by short and cool autumn.Mean annual precipitation(for 40 years)is 366 mm and mean annual pan evaporation is about 1,935 mm.Rainfall is erratic and shows strong seasonal and annual variability.Annual mean temperature is around 6.4 °C,with the minimum monthly mean temperature of-13.1 °C in January and the maximum of 23.7 °C in July.Annual mean wind velocity ranges 3.2–4.1 m/s,and the directions of prevailing winds are northwest in winter and spring and southwest to south in summer and autumn(Zhu and Chen,1994).The zonal soils are identified as sandy chestnut soils,which are mostly equivalent to the Orthi-Sandic Entisols of sand origin in terms of the FAO-UNESCO system(Suet al.,2006).The degraded sandy grassland is usually covered by weed communities and generally dominated by psammophytes including some grasses(e.g.,Chenopodium acuminatum,Cleistogenes squarrosa(Trin.ex Ledeb.)Keng.,Setaria viridis(L.)P.Beauv.,Phragmites australis(Cav.)Trin.ex Steud.,Digitaria ciliaris(Retz.)Koeler,Leymus chinensis(Trin.)Tzvelev),forbs(e.g.,Mellissitus ruthenicus(L.)C.W.Chang,Salsola collinaPall.,Agriophyllum squrrosum(L.)Moq.,Artemisia scopariaWaldst.Kitma.),shrubs(e.g.,Caragana microphyllaLam.,Lespedeza davurica(Laxm.)Schindl.),and subshrubs(e.g.,Artemisia halodendronTurcz.ex Besser,A.frigidaWilld.).
2.2 Experimental design and sampling
This experiment mainly studied the response ofC.microphyllaseedlings to water supply controlled artificially at different groundwater depths.Considering the actual operability and growth characteristic ofC.microphylla,the groundwater levels were designed for 40,80,120,180 cm and CK(control),and each treatment with three repetitions.Each plant was grown in a 30 cm diameter PVC(Poly Vinyl Chloride)bucket.The PVC buckets of the four treatments contained bottoms,while the PVC buckets of CK contained no bottom.The experiment started in April 2011 and ended in September 2012.First,the buried PVC buckets of the four treatments and CK had heights of 0.6,1.0,0.6,2.0,2.0 m,respectively.Secondly,the treatment buckets were filled with stones(diameter<10 mm)and coarse sand to a height of 20 cm,thus forming a free surface layer in the bottom,then backfilled with undisturbed soil into the buckets,and each bucket had a buried water pipe for the water supply.Thirdly,C.microphyllaseeds were placed into the soil on May 1,2011.An 8 mm of water was applied every other day until germination.Finally,Mariotte bottles were installed to control groundwater depth and record the amount of supplied water.Mariotte bottles were not installed in CK buckets,which accepted only natural precipitation.
The local underground depth is 7 m,thus,CK seedlings ofC.microphyllahas no access to the groundwater.The soil water dynamic content was measured by the portable monitoring soil moisture measuring instrument TRIME-FM-P3(IMKO Micromodultechnik,Ettlingen,Germany)during the period of growth.Measured plant height and canopy of annual seedlings occurred in September 2011,and measured plant height and canopy of two year seedlings,above-ground biomass,root biomass and length occurred in September 2012.The roots in each bucket were excavated every 20 cm with a pointed shovel,trying not to damage the roots.All the roots for each 20 cm sample were removed,then washed with distilled water and divided into two parts,thick roots with a diameter of >2 mm and fine roots with a diameter of<2 mm.The roots were dried at 80 °C for 48 hours,then weighed and data recorded.In this paper the data of all indices are for individual plants.
2.3 Data analysis
Differences in vegetation characteristics and biomass were compared using the multiple comparison and one-way analysis of variance(ANOVA)procedures.The LSD tests were performed to determine the differences between treatment means atP<0.05.The descriptive statistical parameters and significance test were calculated by SPSS(version 17.0).
3 Results and discussion
3.1 Dynamics of soil water content
The monitoring results of soil water content in Figure 1 shows that soil moisture is closely correlated to groundwater depths.The soil volumetric water in the upper 0–20 cm of soil is close to the saturated state when groundwater depth is 40 cm.The soil moisture trend is similar when groundwater depth is 80,120 and 180 cm,which increased at 0–50 cm of soil and then decreased gradually after the peak,and then increased rapidly when close to water sources and finally stabilized in a saturated state.The soil moisture trend of CK is different from other treatments,which increased gradually at 0–50 cm of soil,then decreased at 50–70 cm of soil and finally stabilized around 4% at 70–160 cm.This suggests that the ecological and physiological characteristics ofC.microphyllacan adapt to different soil environments.
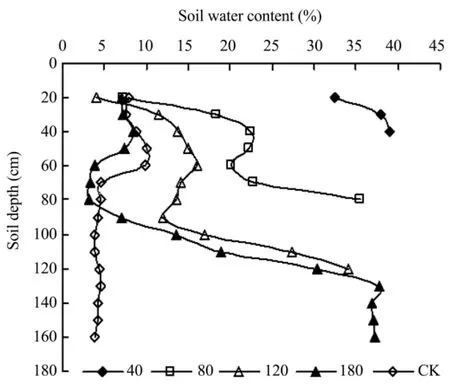
Figure 1 The soil volumetric water content at different water tables
3.2 Ecological characteristics of aboveground parts
As presented in Figure 2a,one-year seedling height in the 40 cm treatment was 5 cm which was the smallest under all treatments,then increased with deeper underground water,and the maximum value of 15 cm appeared in the 120 cm treatment,then decreased to around 10 cm in the 180 cm treatment and CK.The height trend was similar in both years,but two-year seedling height was much higher than that of one-year seedlings.The smallest height was 13.2 cm in the 40 cm treatment,and the maximum value of 75 cm appeared in the 120 cm treatment.The other three treatments are around 60 cm,with no significant difference.
The canopy trend of consecutive years are similar as presented in Figure 2b,where the curves are unimodal according to groundwater depth.The canopy was the smallest for 5 cm2and 232 cm2in 2011 and 2012 separately for the 40 cm treatment,and then increased with groundwater depth.The maximum value was 30 cm2and 1,436 cm2in the 120 cm treatment,and then decrease in the 180 cm treatment and CK.
These results suggest that shallow groundwater at 80 cm restrain the aboveground growth ofC.microphyllaseedlings,and the most suitable water was at 120 cm for plant height and canopy growth ofC.microphyllaseedlings during the first two years.
3.3 Ecological characteristics of underground parts
As presented in Figure 3a,the root biomass of two-year seedlings under the 40 cm treatment was 2.5 g,which is the smallest among all treatments.then the root biomass increased with deeper underground water,and the peak was 52 g under the 120 cm treatment and then declined to 41 g and 26 g under the 180 cm treatment and CK,respectively.The root biomass was 28 g under the 80 cm treatment,with no significant difference in CK.
Root length of two-year seedlings increased gradually from 0.72 to 116 m with increased groundwater depth of 40 to 180 cm in Figure 3b,and all treatments have significant differences.Root length under CK was 24.24 m,54 m under the 80 cm treatment,with significant difference in CK.
These results show that groundwater shallower than 80 cm would restrain the root growth of two-yearC.microphyllaseedlings.The most suitable groundwater was 120 cm for the root biomass and 180 cm for root length during the first two years.
The root biomass was 28 g under the 80 cm treatment,with no significant difference in CK which was 26 g.Root length was 54 m under the 80 cm treatment,with significant differences in CK which was 24.24 m.This suggests that the number of finer roots forC.microphyllaseedlings increased under shallow groundwater at 80 cm than in a natural drought environment.The fine root biomass under the 80 cm treatment and CK was 14.14 g and 2.81 g,respectively,while root length under the 80 cm treatment and CK was 5,195.53 cm and 2,159.50 cm,respectively.These results are presented in Table 1.
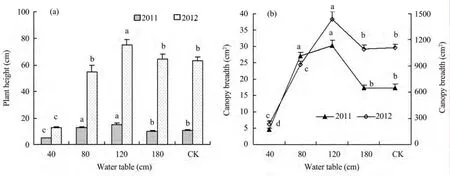
Figure 2 Plant height(a)and canopy breadth(b)of Caragana microphylla in 2011 and 2012 at different water tables.Abscissa 40,80,120,180 cm for manual control of underground water depth,compared to CK.Different letters indicate statistical differences among different groundwater treatments(P<0.05)
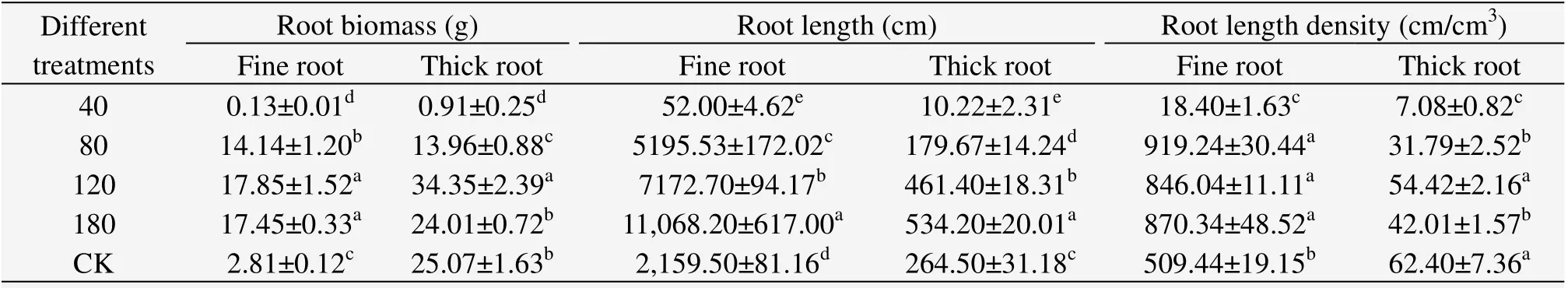
Table 1 Root ecological characteristics of a single Caragana microphyllaplant in 2012
In general,Table 1 shows that thick roots are the determinate factor for root biomass while fine roots are the determinate factor for root length.Table 1 also shows that the minimum value of root length density appeared in the 40 cm treatment,suggesting that shallow groundwater of 40 cm restrains the root length density of two-yearC.microphyllaseedlings.The maximum value of thick root length density appeared in CK,which suggest that thick root growth increases in a natural drought environment.The maximum value of fine root length density appeared in the 80 cm treatment and there was no significant difference in fine root length density between treatments of 80,120 and 180 cm.The fine root length density was much smaller than the other treatments above,which suggests that adding groundwater to the water supply can promote the growth of fine root length density.
3.4 Root-shoot ratio
As presented in Figure 4,the order of root-shoot ratio for different treatments is 80>120>180>CK>40 cm.
The root-shoot ratio under the 40 cm treatment was 0.37,which is the smallest among all treatments;the peak value appeared in the 80 cm treatment,then the root-shoot ratio decreased with deeper underground water depth.Previous studies have found that the root-shoot ratio ofBrassica napusL.(Wanget al.,2013)and winter wheat(Songet al.,2009)increased as the soil became drier.The different trend of the root-shoot ratio between this paper research and previous studies is explained in Figure 5.
Information on root distribution is very important for a better understanding of hydrology and the root system.As presented in Figure 5a,all the root biomass is distributed in the 0–20 cm soil layer under the 40 cm treatment.The root biomass distribution is more than 30% in the 0–20 cm soil layer under the 80,120 and 180 cm treatments,and can reach up to 60% in the 0–20 cm soil layer in CK.The root biomass distribution is 30% in the 20–60 cm soil layer,which becomes smaller layer by layer in the 20–40 and 40–60 cm soil layers under the 80,120,180 cm treatments and CK.There was no root biomass distribution in the 60–80 cm soil layer in CK.The final 30% of root biomass distributed in the soil layer was close to the groundwater under 80,120 and 180 cm treatments.This is more intuitive in Figure 5b where root length distribution is more than 50% under the 80,120 and 180 cm treatments,and the production of fine roots due to groundwater.
Here,we explain why the root-shoot ratio is smaller in the drought soil of CK than in the wet environment of the 80,120 and 180 cm treatments.The soil water content in 60 cm is the maximum in CK(Figure 1).That is because of that the infiltration of rainwater and a lack of underground water promotes horizontal root growth in the 0–60 cm soil.Studies in Horqin Sandy Land(Niuet al.,2013)show that the roots of perennialC.microphyllais mainly distributed in the 0–60 cm soil layer in natural environment.In this paper,roots ofC.microphylla,planted in buckets,could not access water from the surrounding environment,so the horizontal growth of roots was limited in the drought soil of CK.
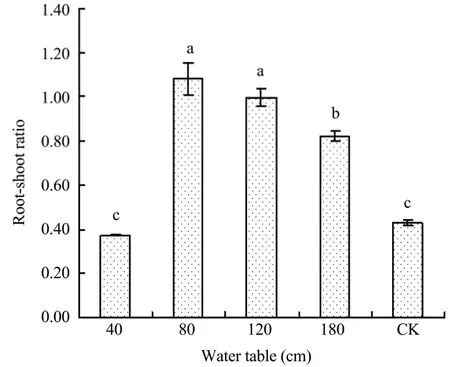
Figure 4 Root-shoot ratio of Caragana microphylla in 2012 at different water tables.Different letters indicate statistical differences among different groundwater treatments(P<0.05)
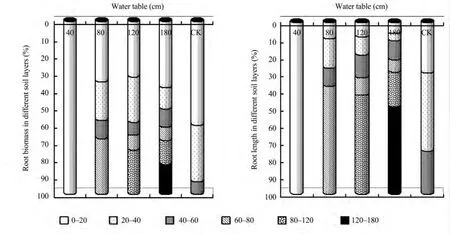
Figure 5 The percentage of root biomass(a)and root length(b)in different soil layers in 2012 at different water tables
4 Conclusion
Caragana microphyllaseedlings adapted to different water table depths in the Horqin Sandy Land of China by changes in the plants ecological characteristics.The shallow groundwater of 40 cm restrained the growth ofC.microphyllaseedlings and the most suitable water was 120 cm for plant height and canopy growth ofC.microphyllaseedlings during the first two years.The growth of vertical roots is positively correlated with groundwater depth;a thick root is the determinate factor for root biomass while the fine root is the determinate factor for root length.The access of groundwater promotes the growth of fine roots and the root-shoot ratio decreases as the underground water deepens from 80 to 180 cm.If there was a need to produce more root of medicinal plant,the method in this paper can provide reference.
We thank all technicians for their help with field investigation.This research was funded by the Chinese National Key Projects for Basic Scientific Research(No.2009CB421303),the Chinese National Support Projects of Science and Technology(No.2011BAC07B02),the Strategic Leading Science and Technology Project of Chinese Academy of Sciences(No.XDA05050201-04-01)and the Chinese National Science Foundation(No.41371053).
George E,Seith B,Schaeffer C,et al.,1997.Responses ofPicea,PinusandPseudotsugaroots to heterogeneous nutrient distribution in soil.Tree Physiology,17(1):39–45.DOI:10.1093/treephys/17.1.39.
Gregory PJ,1989.Water-use efficiency of crops in the semi-arid tropics.In:Soil,Crop and Water Management Systems for Rainfed Agriculture in the Sudano-Sahelian Zone,Niamey,Niger.International Crops Research Institute for the Semi-Arid Tropics(ICRISAT),Patancheru,A.P.502324,India,pp.85–98.
Jastrow JD,Miller RM,1993.Neighbor influences on root morphology and mycorrhizal fungus colonization in tall grass prairie plants.Ecology,74(2):561–569.DOI:org/10.2307/1939316.
Niu CY,Alamusa,Zong Q,et al.,2013.Allocation patterns of above-and below-ground biomass ofCaragana microphyllain Horqin Sandy Land,North China.Chinese Journal of Ecology,32(8):1980–1986.http://www.cje.net.cn/CN/Y2013/V32/I8/1980.
Reader RJ,Jalili A,Grime JP,et al.,1993.A comparative study of plasticity in seedling rooting depth in drying soil.Journal of Ecology,81(3):543–550.http://www.jstor.org/stable/2261532.
Sayer EJ,Tanner EVJ,Cheesman AW,2006.Increased litter fall changes fine root distribution in a moist tropical forest.Plant Soil,281:5–13.DOI:10.1007/s11104-005-6334-x.
Song FP,Hu LY,Zhou GS,et al.,2009.Effects of water table on rapeseed(Brassica napusL.):Growth and yield.Acta Agronomica Sinica,35(8):1508–1515.DOI:10.3724/SP.J.1006.2009.01508.
Su YZ,Li YL,Zhao HL,2006.Soil properties and their spatial pattern in a degraded sandy grassland under post-grazing restoration,Inner Mongolia,northern China.Biogeochemistry,79:297–314.DOI:10.1007/s10533-005-5273-1.
Su YZ,Zhao HL,2003.Soil properties and plant species in an age sequence ofCaragana microphyllaplantations in the Horqin Sandy Land,north China.Ecological Engineering,20:223–235.DOI:10.1016/S0925-8574(03)00042-9.
Wang YZ,Liu XW,Sun HY,et al.,2013.Effects of water and nitrogen on root/shoot ratio and water use efficiency of winter wheat.Chinese Journal of Eco-Agriculture,21(3):282–289.DOI:10.3724/SP.J.1011.2013.00282.
Zhou ZC,Shangguan ZP,2007.Vertical distribution of fine roots in relation to soil factors inPinus tabulaeformisCarr.forest of the Loess Plateau of China.Plant Soil,291:119–129.DOI:10.1007/s11104-006-9179-z.
Zhu ZD,Chen GT,1994.The Sandy Desertification in China.Beijing:Science Press,pp.7–268.(in Chinese)
 Sciences in Cold and Arid Regions2015年1期
Sciences in Cold and Arid Regions2015年1期
- Sciences in Cold and Arid Regions的其它文章
- Seasonal change mediates the shift between resource and pollen limitation in Hedysarum scoparium(Fabaceae)
- Characteristics of high arsenic groundwater in Hetao Basin,Inner Mongolia,northern China
- Effects of sand burial on growth and antioxidant enzyme activities of wheat(Triticum aestivum L.)in northern China
- Photosynthesis of Digitaria ciliarisduring repeated soil drought and rewatering
- Screening of cellulose decomposing fungi in sandy dune soil of Horqin Sandy Land
- Effects of sand burial on survival and growth of Artemisia halodendron and its physiological response
