Effects of sand burial on growth and antioxidant enzyme activities of wheat(Triticum aestivum L.)in northern China
Hao Qu ,HaLin Zhao ,RuiLian Zhou ,Jin Li ,ChengChen Pan
1.Cold and Arid Regions Environmental and Engineering Research Institute,Chinese Academy of Sciences,Lanzhou,Gansu 730000,China
2.School of Life Science,Ludong University,Yantai,Shandong 264025,China
3.University of Chinese Academy of Sciences,Beijing 100049,China
1 Introduction
Sand burial,a common and recurrent event in dune ecosystems,influences growth of native plants and crops(Brown,1997;Yu and Tang,2004;Quet al.,2012b).Research on survival and growth of plants in dune ecosystems indicate positive responses to moderate burial,e.g.,an increase in leaf area to make up for the reduced photosynthetic rate(Martínez and Moreno-Casasola,1996),elongation of stem and leaf petioles to promote vertical growth(Maun,1996),increased number of seeds and flowers per plant to benefit reproduction(Maun,1994)and production of more adventitious roots for nutrient uptake(Dech and Maun,2006).However,burial is fatal above the tolerance limit,since the physical barrier caused by deep burial will hamper vertical growth,minimize photosynthetic area,and reduce the flux of oxygen needed for root respiration(Harris and Davy,1988;Maun,1994).The tolerance limit varies considerably between species.For example,no seedlings ofCirsium pitcherisurvived from 100% of seedling height burial(Maun,1996).In contrast,Agriophyllum squarrosumseedlings can endure more serious sand burial stress.When buried 10 cm below seedling height,9% ofA.squarrosumseedlings survived for at least five months(Quet al.,2012b).In general,plant species from habitats with high risk of burial show adaptations leading to higher survival rates than those for species from other biotopes(Liuet al.,2008;Zardiet al.,2008).Research on antioxidant enzyme response to environmental stress has focused on freezing(Yoshidaet al.,1997),drought(Zhanget al.,2004),or high salt concentrations(Panet al.,2006).The results show that antioxidant enzyme activities to repair or resist damage caused by reactive oxygen species(ROS)increased by environmental stress.
However,compared to dune plants,little is known on how sand burial influences survival,growth and yield of common agricultural crops grown in desertified regions with strong sand activity(Quet al.,2012a,c),and wheat is a major crop in the dry(but irrigated)farmland of these regions(Liuet al.,2013;Tariqet al.,2013).In particular,little is known about the response of antioxidant enzyme activity of crops to sand burial stress.Therefore,wheat grown in irrigated fields in Horqin Sandy Land was selected to study sand burial effects on survival,growth and physiological responses.As one of the most seriously desertified areas in northern China,Horqin Sandy Land has intensive sand activity,and burial of plants and crops by sand is a common phenomenon in this region(Liuet al.,1992;Zhaoet al.,2007a).
The aim of this project are:(1)to investigate the tolerance limit of wheat to sand burial by measuring the survival rate;(2)to quantify the sand burial effects on wheat growth and production by measuring seedling height,biomass and crop yield;(3)to measure the dynamic antioxidant enzyme response of wheat under sand burial;(4)to increase the base for rational advice for efficient agriculture in the sandy land.
Two hypotheses were set up,according to previous studies:(1)shallow burial is beneficial to the survival and growth of wheat,whereas deeper burial is harmful to growth and yield;(2)antioxidant enzyme activity of wheat will be stimulated by shallow burial,but deep burial beyond the tolerance limit will result in an obvious decrease of antioxidant enzyme activity.
2 Materials and methods
2.1 Experimental site
The experimental site is located in the southwestern part of Horqin Sandy Land(Naiman Desertification Research Station,Chinese Academy of Sciences),Inner Mongolia(42°55′N,120°42′E),which is one of the most seriously desertified areas of China due to pressures caused by grazing,cultivation and the collection of fuel wood(Liuet al.,1992;Andrénet al.,1994;Zuoet al.,2008).The climate is temperate,semi-arid continental and monsoonal;the average annual temperature is 6.4 °C,average annual precipitation is 360 mm and average annual pan evaporation is about 1,900 mm.The mean wind speed in spring is 4.3 m/s,and flying sand events occur between 20 and 30 days a year.Most of the cropland is in lowland areas,and maize is the dominant crop.However,wheat areas have increased in recent years,and since wheat is a primary choice for newly reclaimed sandy wastelands,it is often affected by sand burial(Zhanget al.,1999;Zhaoet al.,2006;Luoet al.,2010).
2.2 Experimental design
The experiment was from the end of April to mid-September(a full growing season)in 2011.At the end of April,seeds of wheat(Triticum aestivumL.cv.Linyou2069)were sown in 15 2m×2m×2m-deep concrete-lined plots filled with sandy farmland soil.Thinning was performed after the seedlings emerged.According to local plant density standards,200 wheat seedlings with similar growth were kept and marked in each plot.Twenty days after thinning,sand was added to the burial treatments(about 40 days after sowing,when the average seedling height was 6.6±1.0 cm),and the seedlings were kept vertical while being buried.The sand was taken from local mobile dunes.There was one control(C,no burial)and four burial treatments:B25(burial to 25% of seedling height),B50(burial to 50% of seedling height),B75(burial to 75%of seedling height),and B100(burial to 100% of seedling height).A completely randomized design was used,and every treatment had three replicates,i.e.,15 plots in total.Leaf wilting and deaths were recorded;in B100,sand was carefully removed and replaced after observation.Flag leaves from five living seedlings of each treatment were randomly sampled six and 12 days after sand burial,to measure the malondialdehyde(MDA)concentration,and the activity of superoxide dismutase(SOD),peroxidase(POD)and catalase(CAT).The survival rate,seedling height,crop yield,above-and belowground biomass of wheat were measured in mid-September.Irrigation and fertilizer were applied according to the local farming practice during the entire experimental period(total water 6,200 m3/ha,total N 750 kg/ha).Any unmarked seedlings emerging during the experiment were removed.
2.3 Analytical methods
2.3.1 Survival,growth and yield
At harvest time,the survival rate of wheat was determined as the fraction of surviving wheat plants of the initial 200.Plant height was measured by a ruler and all living wheat were excavated and dried to constant weight to determine above-and belowground biomass.The crop yield was calculated as seed weight per square meter.
2.3.2 Physiological indices
Only seedlings that survived from the burial treatment were selected.SOD activity was measured using the method devised by Beauchamp and Fridovich(1971),and POD activity by the method of Srivastava and van Huystee(1973).CAT activity was measured according to Patraet al.(1978),and MDA concentration was determined according to Hernandez and Almansa(2002).
2.4 Statistical analysis
SPSS version 18.0(SPSS Inc.,Chicago,Illinois,USA)was used for two-way analysis of variance with the factors:Treatment and time.Means were compared using Tukey's test.All statistics were evaluated at the 5% significance level.
3 Results
3.1 Effects of sand burial on survival and growth of wheat
3.1.1 Leaf performance
No wheat leaf died or wilted under C(no burial)during the whole experiment.However,in B25 leaf wilting appeared after 17 days,and wilted leaves appeared earlier with increased burial depth;after 14 days in B50,7 days in B75,and 4 days in B100.
No wheat seedling died in any treatment except in B100,where no seedling survived for more than four days after burial(Table 1).
Leaf survival rates of wheat in C and B25 were significantly higher(P<0.05)than those in B50 and B75;all higher than B100.The same grouping was found for the seedling heights,with a gradual decrease with burial depth(Table 2).

Table 1 Leaf performance of wheat under different burial depths
3.1.2 Survival rate and seedling height
Leaf survival rates of wheat in C and B25 were significantly higher(P<0.05)than those in B50 and B75;and they are all higher than that in B100.The same grouping was found for the seedling heights,with a gradual decrease with burial depth(Table 2).
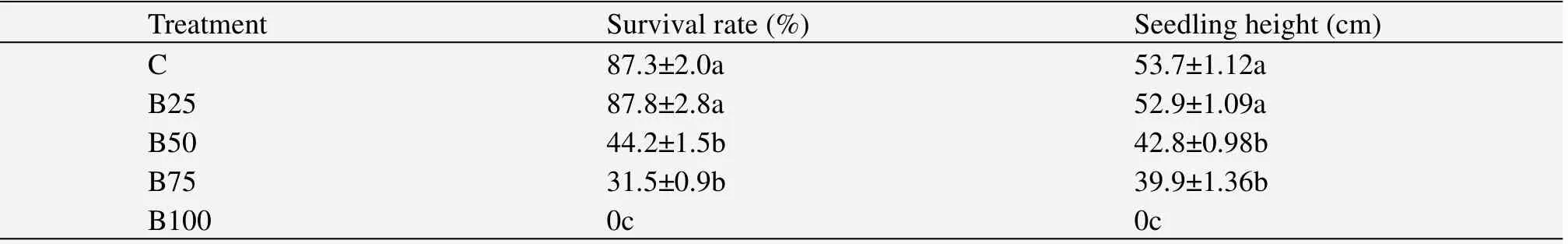
Table 2 Leaf survival rate and seedling height of wheat under different burial depths
3.1.3 Biomass and yield
The aboveground biomass of wheat decreased with increased burial depth,and all buried treatments showed lower values than those of C.Belowground biomass was not reduced under B25 and B50,but significantly reduced under the deeper burials.Root/shoot ratios were significantly higher than those in C,but similar among all buried treatments.Grain yield decreased with increased burial depth,and B75 was significantly lower than B25 and B50(Table 3).
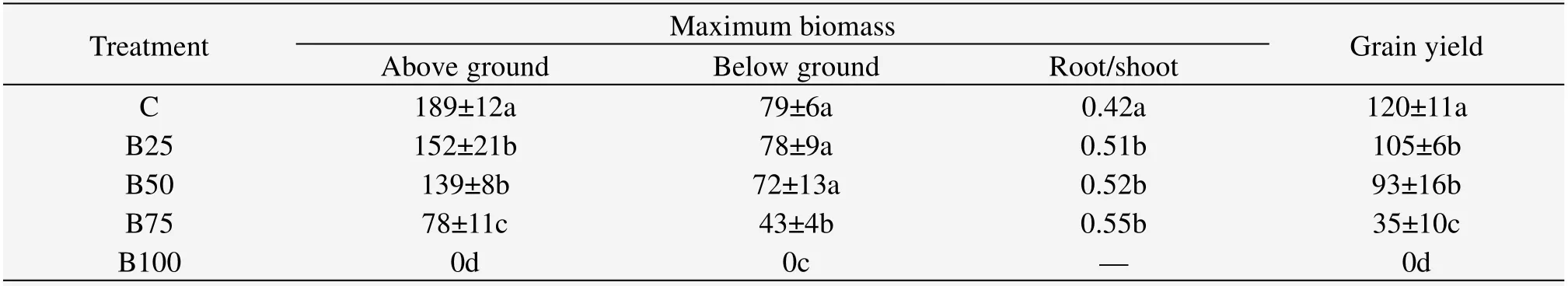
Table 3 Maximum biomass and grain yield(g/m2)of wheat under different burial depths
3.2 Response of antioxidant enzymes activities of wheat to sand burial
3.2.1 SOD activity
The aboveground biomass of wheat decreased with increased burial depth,and all buried treatments showed lower values than those of C.Belowground biomass was not reduced under B25 and B50,but significantly reduced under the deeper burials.Root/shoot ratios were significantly higher than those in C,but similar among all buried treatments.Grain yield decreased with increased burial depth,and B75 was significantly lower than B25 and B50.
Six days after burial,SOD activity of wheat increased with increased burial depth,and all buried treatments showed higher values than those of C except in B100.Similar results were found 12 days after burial(Figure 1a).
3.2.2 POD activity
Six days after burial,POD activity showed no significant increase with increased burial depth except in B100.Twelve days after sand burial,POD activity in all burial treatments were significantly higher than that in C,and B25 was significantly higher than B50 and B100.We also found that POD activity increased with burial time,in all burial treatments,and the value under 12 days after burial was significant higher than before burial and 6 days after burial(Figure 1b).
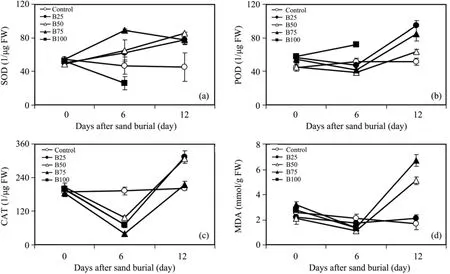
Figure 1 The antioxidant enzyme SOD,superoxide dismutase(a);POD,peroxidase(b);CAT,catalase activities(c)and MDA,malondialdehyde content(d)of wheat to sand burial stress after 6 and 12 days,respectively.Mean ± SE.Control:no burial;B25,B50,B75,B100,respectively:burial to 25%,50%,75%,or 100% of seedling height.Note that all plants died in B100;thus no values(zero activity)after 12 days
3.2.3 CAT activity
The difference of CAT activity among all treatments and C were not significant both before sand burial and 6 days after burial.Twelve days after burial,CAT activity under B25 and B50 were significantly higher than C.With increased burial time,CAT activity of wheat decreased firstly and then increased significantly in all burial treatments.Therefore,CAT activity after 6 days after burial was significantly lower than C,while CAT activity after 12 days after burial was significantly higher than C(Figure 1c).
3.2.4 MDA content
The MDA content in C and all treatments were maintained at a lower level both before burial and 6 days after burial,their difference were not significant.Twelve days after burial,MDA content increased significantly with increased burial depth,the value under B50 and B75 were significantly higher than C and B25.With increased burial time,MDA content under C and B25 did not show an obvious change.The MDA content under B50 and B75 increased significantly(Figure 1d).
4 Discussion
4.1 Effects of sand burial on the survival and growth of wheat
Several studies show that shallow burial can promote growth and survival of plants,possibly because sand burial can decrease soil temperature and maintain soil moisture in the root zone(Brown,1997;Shiet al.,2004;Liuet al.,2008).These factors have been shown to limit plant growth(Niuet al.,2003).Shallow burial can increase the growth rate of plants as a strategy for avoiding full burial(Olson,1958;van Der Puttenet al.,1993).In our study,shallow sand burial did not increase survival and growth of wheat,but had no negative effects.The overall effects on seedling height and survival rate were similar,and decreased significantly when sand burial depth was 50% or deeper.The first wilting date appeared earlier with increased burial depth and all the seedlings died on the sixth day after sand burial in B100.Most of the plants previously studied are psammophytes,adapted to habitats where burial events are common(Martínez and Maun,1999;Liuet al.,2008;Zardiet al.,2008).
Wheat is not a psammophyte and is not adapted to such environments,and is susceptible to direct mechanical obstruction and photosynthetic area reduction caused by sand burial(Harris and Davy,1988;Maun,1994).Numerous reports have indicated that dune plants can adapt to sand burial by changing biomass allocation;for instance,woody plants from central Canadian coastal dunes re-allocated their available energy to aboveground parts after sand burial(Dech and Maun,2006),and the species more tolerant to burial stress among the seven woody species produced more adventitious roots,indicating that these are adaptive features(Dech and Maun,2006).Artemisia ordosicareduced the number of new branches but invested higher biomass in shoots after sand burial(Liet al.,2010a,b).This was explained as a strategy response to burial stress:fewer large branches are more efficient for the emergence ofA.ordosicaafter burial.Shiet al.(2004)also found that the biomass and its allocation ofUlmus pumilaseedlings were significantly influenced by burial depth;complete burial reduced the biomass,but partial burial enhanced it.Zhaoet al.(2007b)suggested that shoot sprouting and growth at an individual scale ofSophora moorcroftianawas promoted by sand burial and further stimulated the overall population development,because the greater leaf mass will increase the photosynthetic area.In our study,the aboveground biomass and yield decreased by increased burial depth,while the belowground biomass did not show obvious change until the burial depth was 75% of seedling height.As a consequence,sand burial caused the root/shoot ratio to increase significantly.However,this cannot be regarded as a positive strategy for survival and growth of wheat;the increased root/shoot ratio is just a consequence of the massive reduction in seedling growth after sand burial(Table 3).
4.2 Response of antioxidant enzyme activities to sand burial
When stressed,ROS(reactive oxygen species)will accumulate in the plant,resulting in cell membrane lipid peroxidation and metabolic disorders which lead to oxidative stress(Sheokandet al.,2008).As the first product of membrane lipid peroxidation,MDA concentration can indicate the degree of injury of plant cells(Demiral and Türkan,2005;Chenet al.,2007).In our experiment,MDA content 6 days after sand burial was lower compared with those before burial,indicating that wheat was not damaged after sand burial.However,after 12 days,the MDA content of wheat increased significantly,and the deeper the burial,the higher the MDA content.This suggests that prolonged burial caused injury to wheat,increasing with burial depth,in agreement with the results on the survival rate of wheat.
There is a wide range of defensive mechanism systems in higher plants,involving the antioxidant protective enzyme systems to help them adapt to various environmental stresses(Chaveset al.,2003;Yu and Tang,2004).As the first defense against membrane lipid peroxidation caused by ROS,SOD can repair injured cells by catalyzing the dismutation of O2-to H2O2and O2,therefore,high SOD activity suggests low membrane lipid peroxidation(Monket al.,1989;Zhanget al.,2004).POD can then catalyze H2O2and ROOH into H2O and ROH to avoid cell damage(Liuet al.,2010).CAT can repair plant injuries by inhibiting H2O2,because when plants are stressed,H2O2can be converted into H2O and result in lipid peroxidation(Quartacciet al.,1995;Navari-Izzoet al.,1996),destruction of the electron transport chain in mitochondria and chloroplasts(Jiménezet al.,1997;Meneguzzoet al.,1998),DNA(Conteet al.,1996)and protein denaturation(Di Baccioet al.,2004).Therefore,the ability to adjust antioxidant enzyme systems of plants is associated with their ability to withstand sand burial(Quet al.,2012a,c).In our study,SOD activity in deep burial treatments on the sixth and twelfth day after burial were higher than those before burial.This indicates that after sand burial,SOD acted to decrease cell damage.Naturally,the sharp decrease of SOD activity under B100(burial to 100% of seedling height)showed that the stress was too extreme and the system crashed,resulting in plant death.The POD and CAT activity were at a lower level after six days of sand burial,but increased significantly after a prolonged burial time,showing that POD and CAT activity in wheat needed a certain lag period before responding to environmental stress.This lag period in POD and CAT activity may partly explain why wheat is susceptible to sand burial.
5 Conclusions
In summary,wheat is not a burial resistant species;the tolerance limit of wheat to sand burial was equal to its height.Hypotheses 1 and 2(see Introduction)were not disproved.Shallow burial(B25)had no positive effects on survival and growth of wheat,on the other hand no clear negative effects were found.Deep burial had obvious negative effects on survival,seedling height and crop yield,and the decreased root/shoot ratio should not be regarded as a positive strategy to sand burial,but caused by reduced seedling biomass.Shallow burial stimulated SOD activity,protecting plants from injury,whereas deep burial resulted in a sharp decrease of SOD activity and increase of MDA content.Contrary to SOD,the response of POD and CAT was very slow.The lack of a rapid response mechanism increasing POD and CAT activity as well as a limited ability to adjust SOD activity may be the causes for the death of the wheat plants.
Arable crops grown in Horqin Sandy Land will suffer from sand burial due to strong wind and sand movement.Our results show that sand burial has no positive effects on wheat;and deep burial is very harmful.Therefore,the establishment of sand barriers(e.g.,buried wheat straw in a checkerboard pattern and fences)is necessary to avoid sand burial of wheat.In the long run,it may be possible to develop burial-tolerant wheat or similar crops for securing crop yields and the income of farmers.
This research was funded by Foundation for Excellent Youth Scholars of CAREERI,CAS(Y451081001)and National Natural Science Foundation of China(41401620,41201249).The Chinese Academy of Sciences has kindly granted Prof.O.Andrén a 'Professorship for Senior International Scientists'(Grant No.Y229D91001)which made it possible for him to review and edit late versions of the manuscript.
Andrén O,Zhao X,Liu X,1994.Climate and litter decomposition in Naiman,Inner Mongolia,China.Ambio,23(3):222–224.
Beauchamp C,Fridovich I,1971.Superoxide dismutase:Improved assays and an assay applicable to acrylamide gel.Analytical Biochemistry,44(1):276–287.DOI:10.1016/0003-2697(71)90370-8.
Brown JF,1997.Effects of experimental burial on survival,growth and resource allocation of three species of dune plants.Journal of Ecology,85:151–158.
Chaves MM,Maroco JP,Pereira JS,2003.Understanding plant responses to drought-from genes to the whole plant.Functional Plant Biology,30(3):239–264.DOI:10.1071/FP02076.
Chen J,Zhu C,Lin D,et al.,2007.The effects of Cd on lipid peroxidation,hydrogen peroxide content and antioxidant enzyme activities in Cd-sensitive mutant rice seedlings.Canadian Journal of Plant Science,87(1):49–57.DOI:10.4141/P06-048.
Conte D,Narindrasorasak S,Sarkar B,1996.In vivo and in vitro iron-replaced zinc finger generates free radicals and causes DNA damages.Journal of Biological Chemistry,271(9):5125–5130.
Dech JP,Maun MA,2006.Adventitious root production and plastic resource allocation to biomass determine burial tolerance in woody plants from central Canadian coastal dunes.Annals of Botany,98(5):1095–1105.DOI:10.1093/aob/mcl196.
Demiral T,Türkan I,2005.Comparative lipid peroxidation,antioxidant defense systems and proline content in roots of two rice cultivars differing in salt tolerance.Environmental and Experimental Botany,53(3):247–257.DOI:10.1016/j.env expbot.2004.03.017.
Di Baccio D,Navari-Izzo F,Izzo R,2004.Seawater irrigation:antioxidant defense responses in leaves and roots of a sunflower(Helianthus annuusL.)ecotype.Journal of Plant Physiology,161(12):1359–1366.
Harris D,Davy AJ,1988.Carbon and nutrient allocation inElymus farctusseedlings after burial with sand.Annals of Botany,61(2):147–157.
Hernandez JA,Almansa MS,2002.Short-term effects of salt stress on antioxidant systems and leaf water relations of pea leaves.Acta Physiologiae Plantarum,115(2):251–257.DOI:10.1034/j.1399-3054.2002.1150211.x.
Jiménez A,Hernández JA,Del Ryo LA,et al.,1997.Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea(Pisum sativumL.)leaves.Plant Physiology,114(1):275–284.
Li SL,Zuidema PA,Yu FH,et al.,2010b.Effects of denudation and burial on growth and reproduction ofArtemisia ordosicain Mu Us sandland.Ecological Research,25(3):655–661.DOI:10.1007/s11284-010-0699-x.
Li SL,Werger MJA,Zuidema PA,et al.,2010a.Seedlings of the semi-shrubArtemisia ordosicaare resistant to moderate wind denudation and sand burial in Mu Us sandland,China.Trees-Structure and Function,24(3):515–521.DOI:10.1007/s00468-010-0422-0.
Liu B,Liu ZM,Guan DX,2008.Seedling growth variation in response to sand burial in fourArtemisiaspecies from different habitats in the semi-arid dune field.Trees-Structure and Function,22(1):41–47.DOI:10.1007/s00468-007-0167-6.
Liu JG,Zhang XL,Sun YH,et al.,2010.Antioxidative capacity and enzyme activity inHaematococcus pluvialiscells exposed to superoxide free radicals.Chinese Journal of Oceanology and Limnology,28:1–9.DOI:10.1007/s00343-010-9244-6.
Liu XM,Zhao HL,Xu B,1992.Destruction causes of Korqin Sandy Land and approaches to its restoration.Chinese Journal of Ecology,11(5):38–41.
Liu Y,Yang L,Gu DD,et al.,2013.Influence of tillage practice on soil CO2emission rate and soil characteristics in a dryland wheat field.International Journal of Agriculture and Biology,15(4):680–686.
Luo YY,Zhao XY,Zuo XA,et al.,2010.Leaf nitrogen resorption pattern along habitats of semi-arid sandy land with different nitrogen status.Polish Journal of Ecology,58(4):707–716.
Martínez ML,Maun MA,1999.Responses of dune mosses to experimental burial by sand under natural and greenhouse conditions.Plant Ecology,145(2):209–219.DOI:10.1023/A:1009850304137.
Martínez ML,Moreno-Casasola P,1996.Effects of burial by sand on seedling growth and survival in six tropical sand dune species from the Gulf of Mexico.Journal of Coastal Research,12(2):406–419.
Maun MA,1994.Adaptations enhancing survival and establishment of seedlings on coastal dune systems.Vegetatio,111(1):59–70.DOI:10.1007/BF00045577.
Maun MA,1996.The effects of burial by sand on survival and growth ofCalamovilfa longifolia.Ecoscience,3:93–100.
Meneguzzo S,Sgherri CLM,Navari-Izzo F,et al.,1998.Stromal and thylakoid-bound ascorbate peroxidases in NaCl treated wheat.Physiologia Plantarum,104(4):735–740.DOI:10.1034/j.1399-3054.1998.1040431.x.
Monk LS,Fagerstedt KV,Crawford RMM,1989.Oxygen toxicity and superoxide dismutase as an antioxidant in physiological stress.Physiologia Plantarum,76(3):456–459.DOI:10.1111/j.1399-3054.1989.tb06219.x.
Navari-Izzo F,Quartacci MF,Sgherri CML,1996.Superoxide generation in relation to dehydration and rehydration.Biochemical Society Transactions,24(2):447–451.
Niu SL,Jiang GM,Li YG,et al.,2003.Comparison of photosynthetic traits between two typical shrubs:legume and non-legume in Hunshandak Sandland.Photosynthetica,41(1):111–116.DOI:10.1023/A:1025824916389.
Olson JS,1958.Rates of succession and soil changes on southern Lake Michigan dunes.Botanical Gazette,119(3):125–170.DOI:10.1086/335973.
Pan Y,Wu LJ,Yu ZL,2006.Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice(Glycyrrhiza uralensisFisch).Plant Growth Regulation,49(2–3):157–165.DOI:10.1007/s10725-006-9101-y.
Patra HL,Kar M,Mishra D,1978.Catalase activity in leaves and cotyledons during plant development and senescence.Journal of Plant Physiology,172(4):385–390.
Qu H,Zhao HL,Zhao XY,et al.,2012a.Effects of sand burial on survival and yield of mung bean.Journal of Food Agriculture and Environment,10(2):687–689.
Qu H,Zhao HL,Zhou RL,et al.,2012b.Effects of sand burial on the survival and physiology of three annuals of Northern China.African Journal of Biotechnology,11:4518–4529.
Qu H,Zhao HL,Zhou RL,et al.,2012c.Effects of sand burial stress on maize(Zea maysL.)growth and physiological responses.Australian Journal of Crop Science,6(5):869–876.
Quartacci MF,Pinzino C,Sgherri CLM,et al.,1995.Lipid composition and protein dynamics in thylakoids of two wheat cultivars differently sensitive to drought.Plant Physiology,108:191–197.DOI:http://dx.doi.org/10.1104/pp.108.1.191.
Sheokand S,Kumari A,Sawhney V,2008.Effect of nitric oxide and putrescine on antioxidative responses under NaCl stress in chickpea plants.Plant Molecular Biology,14(4):355–362.DOI:10.1007/s12298-008-0034-y.
Shi L,Zhang ZJ,Zhang CY,et al.,2004.Effects of sand burial on survival,growth,gas exchange and biomass allocation ofUlmus pumilaseedlings in the Hunshandak Sandland,China.Annals of Botany,94(4):553–560.DOI:http://dx.doi.org/10.1093/aob/mch174.
Srivastava OP,van Huystee PB,1973.Evidence for close association of POD Polyphenol oxidase and IAA oxidase isoenzyme of peanut suspension culture medium.Canadian Journal of Botany,51:2207–2214.
Tariq M,Mahmood A,Mian MA,et al.,2013.Dharabi-11:A new high yielding drought and disease tolerant wheat variety.International Journal of Agriculture and Biology,15(4):701–706.
van Der Putten WH,van Dijk C,Peters BAM,1993.Plant-specific soil-borne diseases contribute to succession in foredune vegetation.Nature,362:53–56.DOI:10.1038/362053a0.
Yoshida M,Abe J,Moriyama M,et al.,1997.Seasonal changes in the physical state of crown water associated with freezing tolerance in winter wheat.Physiologia Plantarum,99(3):363–370.DOI:10.1111/j.1399-3054.1997.tb00548.x.
Yu SW,Tang KX,2004.MAP kinase cascades responding to environmental stress in plants.Acta Botanica Sinica,46(2):127–136.
Zardi GI,Nicastro KR,McQuaid CD,et al.,2008.Sand and wave induced mortality in invasive(Mytilus galloprovincialis)and indigenous(Perna perna)mussels.Marine Biology,153(5):853–858.DOI:10.1007/s00227-007-0857-z.
Zhang F,Guo JK,Yang YL,et al.,2004.Changes in the pattern of antioxidant enzymes in wheat exposed to water deficit and rewatering.Acta Physiologiae Plantarum,26(3):345–352.DOI:10.1007/s11738-004-0024-9.
Zhang TH,Zhao HL,Zhao XY,et al.,1999.Study on water consumption of spring maize in Horqin Sandy Land.Journal of Desert Research,19:137–139.
Zhao HL,Zhou RL,Drake S,2007a.Effects of aeolian deposition on soil properties and crop growth in sandy soils of northern China.Geoderma,142(3–4):342–348.DOI:10.1016/j.geoderma.2007.09.005.
Zhao HL,Zhou RL,Zhang TH,et al.,2006.Effects of desertification on soil and crop growth properties in Horqin sandy cropland of Inner Mongolia,north China.Soil and Tillage Research,87(2):175–185.DOI:10.1016/j.still.2005.03.009.
Zhao WZ,Zhang ZH,Li QY,2007b.Growth and reproduction ofSophora moorcroftianaresponding to altitude and sand burial in the middle Tibet.Environmental Geology,53(1):11–17.DOI:10.1007/s00254-006-0613-6.
Zuo XA,Zhao HL,Zhao XY,et al.,2008.Spatial pattern and heterogeneity of soil properties in sand dunes under grazing and restoration in Horqin Sandy Land,Northern China.Soil Tillage Research,99(2):202–212.DOI:10.1016/j.still.2008.02.008.
 Sciences in Cold and Arid Regions2015年1期
Sciences in Cold and Arid Regions2015年1期
- Sciences in Cold and Arid Regions的其它文章
- Seasonal change mediates the shift between resource and pollen limitation in Hedysarum scoparium(Fabaceae)
- Characteristics of high arsenic groundwater in Hetao Basin,Inner Mongolia,northern China
- The response of Caragana microphylla seedlings to water table changes in Horqin Sandy Land,China
- Photosynthesis of Digitaria ciliarisduring repeated soil drought and rewatering
- Screening of cellulose decomposing fungi in sandy dune soil of Horqin Sandy Land
- Effects of sand burial on survival and growth of Artemisia halodendron and its physiological response
