Robot-assisted laparoscopic radical cystectomy with complete intracorporeal urinary diversion
Jason M.Sandberg,Ashok K.Hemal
Department of Urology,Wake Forest University School of Medicine,Winston-Salem,NC,USA
Robot-assisted laparoscopic radical cystectomy with complete intracorporeal urinary diversion
Jason M.Sandberg,Ashok K.Hemal*
Department of Urology,Wake Forest University School of Medicine,Winston-Salem,NC,USA
Cystectomy;
Robotics;
Urinary bladder
neoplasms;
Urinary diversion
Robot-assisted radical cystectomy with intracorporeal urinary diversion(RARCICUD)has only recently been explored as a viable surgical option for patients with muscle-invasive bladder cancer seeking satisfactory oncologic control while benefiting from minimally invasive surgical techniques.Inspired by earlier open and laparoscopic work, initial descriptions of RARC-ICUD were published in 2003,and have since been followed by multiple larger case series which have suggested promising outcomes for our patients.However,the rate of adoption has remained relatively slow when compared to other robotassisted procedures such as the radical prostatectomy,likely owing to longer operative times,operative complexity,costs,and uncertainty regarding oncologic efficacy.The operative technique for RARC-ICUD has evolved over the past decade and several high-volume centers have shared tips to improve efficiency and make the operation possible for a growing number of urologists.Though there are still questions regarding economic costs,effectiveness,and generalizability of outcomes reported in published data,a growing dataset has brought us ever closer to the answers.Here,we present our current operative technique for RARC-ICUD and discuss the state of the literature so that the urologist may hold an informed discussion with his or her patients.
?2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V. Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons. org/licenses/by-nc-nd/4.0/).
1.Introduction
Open radical cystectomy(ORC)with extended pelvic lymph node dissection and urinary diversion has long been the gold standard for localized muscle invasive urothelial carcinoma of the bladder[1].The growing popularity of laparoscopic surgery in the early 1990s ultimately allowed for the development of the first minimally invasive radical cystectomy (RC)with extracorporeal ileal conduit(IC) reported in 1995[2].Early adoption was stimulated by reports of transitioning from ORC to hand-assisted laparoscopic radical cystectomy (LRC)to pure LRC with extracorporeal diversion[3].Larger series of LRC have since been published demonstrating comparable outcomes to ORC,an important stepping-stone in the journey toward robotic alternatives[4].
The motivation for developing such a technique was that of minimally invasive surgery in all subspecialties:to reduce the morbidity of the operation and improve perioperative outcomes such as blood loss,hospital stay,and patientsatisfaction[5].Five yearslaterin 2000,first LRC with a completely formed intracoporeal ileal conduit(ICIC)was reported[6],which was followed with report of orthotopic neobladder(ONB)in 2002[7].Predictably,the advent and ensuing popularity ofthe da Vinci?SurgicalSystem(Intuitive Surgical Inc.,Sunnyvale,CA,USA)inspired the initial reports of robotic-assisted radical cystectomy(RARC).RARC with extracorporeal neobladder formation was first described in malesin 2003[8],and wassoon followed by a seriesin female patients as well[9].
Throughout the past decade,implementation of RARC with or without intracorporeal urinary diversion(ICUD)has been primarily limited to high volume academic centers. Increased operative time,unfamiliarity with the technology and technique,cost,limited access to quality training initiatives,and uncertainty regarding oncologic and functional outcomes have all been cited as possible barriers to early widespread adoption of the procedure[10-12].More recently,however,data have been published supporting comparable oncologic outcomes to ORC in the setting of decreased blood loss and shorter length of stay.Randomized control trials(RCTs)comparing RARC to ORC have recently been completed with mixed results,but review of the available literature demonstrates increasingly larger prospective series with comparable and sometimes superior perioperative outcomes and complication rates.This review will summarize our surgical technique for complete ICUD as well as the current state of the literature,which suggests a growing number of centers are performing RARCICUD safely and effectively.
2.Operative technique
Following Menon et al.’s original description[8],many different institutions have published various modifications for RARC and urinary diversion,including our own.Over the years,we have continued to make modifications to improve the efficiency of the procedure,and will report our latest technique here,which may also be reviewed with figures and intraoperative images in an upcoming publication[13]. Where applicable,we will also comment on variations reported by other institutions so that the reader may consider the applicability of each of these to his or her own practice.
2.1.Operating room configuration and patient preparation
Prior to induction of general anesthesia,the patient is given 5000 units heparin for deep vein thrombosis prophylaxis. After administering general endotracheal anesthesia,an orogastric tube and Foley catheter are placed.Intravenous antibiotics appropriate for the coverage of enteric organisms are administered.
The patient is moved into the dorsal lithotomy position, ensuring adequate padding of all extremities to avoid potential compartment syndrome and/or neuropraxia.We prefer to tuck the patient’s arms at his or her side.Before prepping and draping,the patient should be placed into the steep Trendelenburg position(30°-45°)to ensure the patent is properly strapped and stable.The use of shoulder pads or Velcro straps has both been described.The Trendelenburg position is applied to slide the bowel out of the pelvis and provide adequate exposure during cystoprostatectomy.During urinary diversion,Trendelenburg should be reduced to 15°or less.For this latter portion of the procedure,the robot may be re-docked between the patient’s legs while in dorsal lithotomy,or side-docked after the patient is moved to the supine position.The patient is prepped and draped in the usual sterile fashion, similar to robot-assisted radical prostatectomy.A preparation time-out is performed,and a sterile field is created by prepping the patient’s penis(vagina in female),perineum and proximal thighs up to the infra-xiphoid abdomen.
2.2.Trocar placement and robotic configuration
We typically perform RARC-ICUD using the da Vinci?Xi Robotic System(Intuitive Surgical Inc.)though it should be noted that this method can be easily adapted for the S and Si systems as well.Pneumoperitoneum is obtained using a Veress needle through a vertical skin incision about 5 cm above the umbilicus.The abdomen is insufflated to 15 mmHg,and the 8-mm camera port is then inserted in the midline.With the camera inserted,the peritoneal cavity is inspected to rule out injury upon entry into the abdomen.
The remaining three robotic ports and two assistant ports are then placed under direct vision 1-2 cm above the level of the umbilicus in a transverse line across the abdomen.The first two robotic ports are placed 10 cm lateral and slightly inferior to the camera port on either side.The additional robotic port is placed another 7-10 cm lateral to the right-sided robotic port and three fingerbreadths superior to the right anterior superior iliac spine (ASIS).This may or may not be in line with the other robotic ports.The 12-mm AirSeal?(SurgiQuest Inc.,Milford,CT, USA)port is then placed on the left side,5-7 cm superior to the ASIS,essentially mirroring the placement of the third robotic port.Placing the third robotic port on the right and Airseal on the left is a departure from a previously described method from our institution in which thesepositions were swapped.We have found this latest configuration facilitates bowel manipulation by avoiding acuteangle stapling.The final assistant port should be placed through the pre-marked stomal site when performing an IC. We have performed this operation with a 5-mm port in this position,though an additional 12-or 15-mm port may be used here to provide additional opportunities for stapling and specimen collection with a laparoscopic bag.When performing ONB,left side placement of the assistant port is advantageous to the assistant,who will be sitting at the patient’s left.The robot is then docked between the patient’s legs.
The camera is placed via the port attached to the second robotic arm.We typically begin with Hot Shears?monopolar curved scissors(Intuitive Surgical Inc.)in the right hand(arm 3),fenestrated bipolar forceps(Intuitive Surgical Inc.)in the left hand(arm 1),and a ProGrasp?forceps(Intuitive Surgical Inc.)in the fourth arm.During the urinary diversion,two needle drivers are also used, which may include a Large SutureCut?(Intuitive Surgical Inc.)depending on surgeon preference.The choice of lens is ultimately dependent on surgeon preference as well.The majority of the operation is performed with the 0°lens, though we do advocate for specialized use of the 30°lens when dissecting deep in the pelvis,for extended lymph node dissection particularly near the aortic bifurcation, and at the time of posterior/retro-apical prostatic dissection.
2.3.Urinary diversion
We have previously described our technique for RARC with or without ePLND and continue to perform it in a similar manner[14,15].After fully dissecting and freely mobilizing the bladder and prostate,attention is turned to the urinary diversion.Several different techniques are available to the urologist,and we will focus our technique for orthotopic ileal neobladder here.ICIC and more recently,intracorporeal continent cutaneous urinary diversion,have also been described.
2.3.1.Completely intracorporeal ileal neobladder
Following RARC,the robot is undocked and the patient moved into shallow Trendelenburg(10°-15°).One advantage of the Xi system is side-docking ability,which allows the diversion to be performed with the patient in the supine position,thereby decreasing the risk of positioning complications[16].Prograsp? (Intuitive Surgical,Inc., Sunnyvale,California,USA)forceps are used in arm 3 on the right and fenestrated bipolar forceps are placed in arm 1 on the left for bowel manipulation.A needle driver may alternatively be used in one of these arms to save on cost. Before isolating bowel,we place two separate 3-0 barbed sutures(V-loc;Covidien,New Haven,CT,USA)at 5-and 7-o’clock in the urethra,and then bring them through the anterior abdominal wall so they can be retrieved later for the neobladder-urethral anastomosis.
We prefer to use 60 cm of distal ileum beginning 15 cm proximal to the ileocecal junction for the formation of the neobladder.Sigmoid colon can alternatively be used if adequate small bowel is lacking.The desired ileal segment is identified and then marked distally with two red vessel loops and proximally with two blue vessel loops(one at 50 cm and one at 60 cm)placed through the mesentery.We prefer this to marking with a suture as it provides better ability to manipulate the bowel with less trauma.The length of the ileal segment may be approximated using a sterile flexible tape measure,premeasured silk suture,or open-ended ureteral catheter.
The ileum is then transected distally with a 60-mm laparoscopic stapler via the left-sided assistant AirSeal?port and a blue stapler load(3.5-mm thickness).We have previously reported on our experience with IV injection of 2 mL of 2.5 mg/mL indocyanine green for identification of mesenteric vessels[17].This may be a useful adjunct for the urologist,particularly when first starting out.We continue dividing the mesentery in this plane using an additional white vascular stapler load(2.5-mm thickness) to allow for proper mobilization of the neobladder.
At this point,we do place a dyed 3-0 Vicryl suture at the distal bowel segment near the red vessel loops.Using an undyed 3-0 Vicryl,the ileal segment is marked at approximately 25 cm and again at 50 cm from the distal end.These sutures represent the apex of the posterior plate and beginning of the afferent limb,respectively.The proximal end of the ileal segment is then divided,again using a blue stapler load.Another purple-dyed 3-0 Vicryl suture is placed to mark the proximally transected ileum.
In restoring bowel continuity,the previously placed vessel loops are very useful for identification,orientation, and manipulation of the ileum.The anastomotic bowel segments are brought adjacent to one another using the vessel loops in order to achieve a side-to-side,functional end-to-end ileoileal anastomosis.We again employ the 60-mm laparoscopic tissue stapler to deploy two loads on the adjacent antimesenteric ileal walls in series.The open ends of ileum are closed with a tissue stapler load deployed transversely,mirroring the open technique.It is imperative to perform the ileoileal anastomosis above the excluded segment so that the neobladder can be formed below the mesentery and easily translocate into the pelvis.
The undyed marking suture at 25 cm(site of urethral anastomosis)is grasped by the fourth robotic arm and retracted into the pelvis.This aligns two 25-cm ileal segments adjacent to each other.The additional 10 cm of ileum is used for the afferent limb.Goh et al.[18]prefers to use 44 cm for the ileal segment and 16 cm for the afferent limb,but ultimately this decision is based on surgeon preference.The 50 cm of ileum is then detubularized. Use of a chest tube or large Foley catheter inserted into the bowel segment is optional,and may prevent injury to the back wall of the bowel.
Several 2-0 absorbable interrupted sutures are placed at 6-8 cm intervals to appose the edges of the posterior plate of the neobladder.A final 6 cm tag is placed to facilitate manipulation during final suturing.A watertight 2-0 barbed suture(V-loc)then runs the entire length of the posterior wall along the previously approximated edges.
With the previously placed 3-0 barbed sutures at the 5 and 7 o’clock positions of the urethra,the urethroneobladder anastomosis is started after rotating theposterior plate counterclockwise 90°with caudal traction. The anastomosis is performed in a running fashion with a barbed suture.Goh and colleagues[18]have described an alternative approach,using double armed 3-0 Monocryl suture on an RB-1 needle starting from the 6 o’clock position.In experimenting with different ways to perform the anastomosis,we have found that the use of barbed sutures provides enhanced technical ability and ensures a watertight connection[19].The posterior portion of the anastomosis is completed over a 22 or 24 Fr Hematuria catheter. The anastomosis is completed anteriorly using interrupted sutures or by continuing to run the previous posterior sutures.
Collins and colleagues[20]perform the urethroneobladder anastomosis at the beginning of the procedure,immediately following identification,but prior to harvest of the ileal segment.This strategy ensures adequate ileal length and mesenteric mobility so that the surgeon may be sure to complete the urethro-neobladder anastomosis under the least tension possible.If there is difficulty reaching the urethra,the surgeon can shift the segment of bowel to be harvested to gain additional length.
Each ureter is then spatulated and separately anastomosed to the afferent limb using the Bricker technique with interrupted or continuous 5-0 monocryl sutures.A Wallace technique may be employed where desired.Each ureter is intubated with a completely internalized 6 Fr x 30 cm JJ ureteral stent prior to completing the ureteral anastomoses [21].We typically place these through the left-sided assistant port,though an alternative technique may be used to introduce 5 Fr stents percutaneously through a 2-mm needle in the abdominal wall.The angle of entry into the ureters using this method creates a more favorable angle for advancing the stents into the renal pelvis.
Neobladder closure is started by cross-folding the posterior plate on itself and fixing the midpoint with a horizontal mattress suture.This aligns the edges for closure and maintains symmetry of the pouch.The anterior wall of the neobladder is closed with running 2-0 barbed V-loc suture. A suprapubic tube(SPT)may be placed into the neobladder prior to final closure if desired.We prefer SPT placement to allow for easier irrigation postoperatively.Next,the neobladder is irrigated via the transurethral Foley catheter to ensure a watertight closure;any leaks can be repaired with interrupted 2-0 Vicryl sutures.Surgical specimens may be extracted vaginally in women,or through extension of the midline camera port incision in men.Vaginal closure and reconstruction should not be overlooked,as this step has important implications for postoperative sexual health and quality of life.A drain is placed in the pelvis through a lateral port site and put to bulb suction.
All 10 mm or greater port sites are re-approximated using 1-0 Vicryl suture at the level of the fascia.The Carter-Thomason method may be used where desired.The fascia and skin are then closed in the standard fashion.
2.3.2.Intracorporeal ileal conduit
Isolating ileum for an ICIC is achieved in a similar fashion to the ONB except a smaller length of ileum is harvested (usually 15 cm).This was first reported by Balaji et al.[22] and later modified to include the Marionette technique by Guru and colleagues[23].The Marionette technique includes placing a long suture into the distal aspect of the isolated bowel segment and then bringing the suture out through one of the assistant ports so that the bowel can be manipulated to provide appropriate exposure for the surgeon.After isolating the bowel segment and restoring bowel continuity with the Endo-GIA stapler through the 15 mm assistant port,the left ureter is delivered under the sigmoid mesocolon to the right side.A small defect may be made in the distal aspect of the conduit and irrigated laparoscopically.Alternatively,irrigation may be performed while maturing the stoma after the robotic is undocked.Ureteroileal anastomosis and intracorporeal stent placement is performed similarly to the ONB.Prior to undocking the robot,full length 3-0 Vicryl suture is then placed in the distal aspect of the conduit and brought extracorporeally through the robotic port closest to the IC site so that it may be readily identified and brought up to the skin during ostomy creation.
3.Choice of urinary diversion
ICUD following RARC may theoretically take many forms. While IC and ONB are the most commonly performed,there have been recent reports of continent cutaneous diversion (Indiana pouch)as well[24].The urologist is encouraged to think broadly about the type of diversion offered to each individual patient.Choice of diversion will ultimately depend not only on patient preference,but also their health and functional status.Many patients may be intrinsically drawn to ONB,owing to superior cosmetics(ostomy is not required)and perceived quality of life advantage. Even here the surgeon has options on the type of neobladder,though a recent 2015 review of RARC with intracorporeal neobadder(ICNB)series reported the Studer pouch as the most commonly performed by far,citing this form of neobladder in over 96%of reviewed ONB patients [25].Indiana pouch is also attractive to patients as it maintains continence,but intracorporeal descriptions are limited and this procedure is not widely performed at this time.Whatever the decision,the urologist must counsel the patient thoroughly and help them to arrive at the best possible choice given their specific comorbidities and functional capabilities.
In order to be considered a candidate for ONB,patients should not have significant stress urinary incontinence, should have intact urethral function,and should not have transitional cell or prostatic carcinoma involving the bladder neck and/or urethra.These authors recommend bladder neck or distal urethral frozen sections at the time of RARC to rule out occult malignancy.ONB also requires the motor and cognitive abilities necessary to perform specified voiding behaviors including self clean intermittent catheterization.Historically,chronic renal insufficiency with serum creatinine>2 mg/dL(or GFR<40 mL/min/ 1.73 m2)is seen as a contraindication to continent diversion as well.If any of these factors are present,the urologist is urged to strongly consider a non-continent diversion such as IC formation.When considering the use of colon in a continent cutaneous diversion,the urologist should also be mindful of concomitant comorbidities of the gastrointestinal system,including inflammatory bowel disorders.
Nazmy et al.[26]recently reviewed the records of 209 patients undergoing consecutive RARC over a 9-year period with median follow up of 35 months and suggested higher complications rates for ONB and cutaneous continent diversion(Indiana pouch)versus IC,despite a less healthy IC cohort.Sixty-eight percent of patients received a continent diversion with a 77%overall and 32%major complication rate,while most complications were gastrointestinal,infectious,of hematologic.In multivariate analyses,ONB was a predictor for 90-day major complication rate compared to IC with OR 4.97(95%CI 1.88-13.2, p=0.001),and both ONB and Indiana pouch had signif icantly higher overall 90-day adverse events.Indiana pouch in particular was a predictor for blood transfusion with OR 3.55(95%CI 1.33-9.44,p=0.01),and the most common contributor to complications in the ONB group was urethral anastomotic leak(24.2%).However,it should be noted that all diversions were performed extracorporeally in this series.
Several large series have been published highlighting single institutional experiences with RARC-ICNB(Table 1)as well as RARC with ICIC(Table 2).Notably,the report by Goh et al.[18]in 2012 detailed perioperative outcomes for both ICNB and ICIC,thus appearing in both accompanying tables here.They performed eight neobladders and seven ICs and reported no difference in perioperative outcomes, including operative time,estimated blood loss(EBL),time to liquid and regular diet,length of hospital stay,and oncologic outcomes.It is generally well accepted that the majority of complications after radical cystectomy are related to the diversion,rather than the extirpative portion of the procedure[27].Thus it stands to reason that a primary goal of the urologist should be to answer the question:which type of diversion,if any,causes the least severe and fewest numbers of complications?For their part,Goh et al.[18]did not find any differences in major or minor complications at both 30-and 90-day follow-up.The overall complication rate was 73%,but high-grade complications comprised only 13% of the total cohort.Readmission rates were also similar,including seven readmissions for minor complications and two for major complications.It should be stated this study included a total of only 15 total patients,and thus even a low number of absolute re-admissions correlates with a relatively large percentage of the cohort.Larger series of patients for both neobladder and IC have since been reported.
Desai et al.[28]reported on 18 patients with ICNB vs.19 patients with ICIC in 2014.All procedures were done completely intracorporeally without any cases converted to open.The ICIC cohort was significantly older(75 vs.62 years)and had higher ASA classification,but did not differ in Charlson Comorbidity Index,tumor histology,grade, stage,or rates of neoadjuvant chemotherapy.There was no significant difference in EBL(250 vs.200 mL),operative time(386 vs.387 min),transfusion rate(32%vs.17%),time to ambulation(3 vs.4 days),time to PO intake(5 vs. 4 days),or length of stay(8 vs.13 days,p=0.078),though this last parameter may be viewed as clinically significant.
This was followed by the largest series of ICNB published to date,including 132 consecutively performed RARC-ICNB at two institutions[29].Mean operative time was 456 min, EBL was 430 mL,and mean hospital stay was 11 days.The overall 30-day complication rate was 47%.An additional 28% complication rate between 30 and 90 days was noted,butthis improved with experience of the surgeon.Major complications were limited to 29%overall.The majority of these were infectious(28.8%)and genitourinary(21.2%).
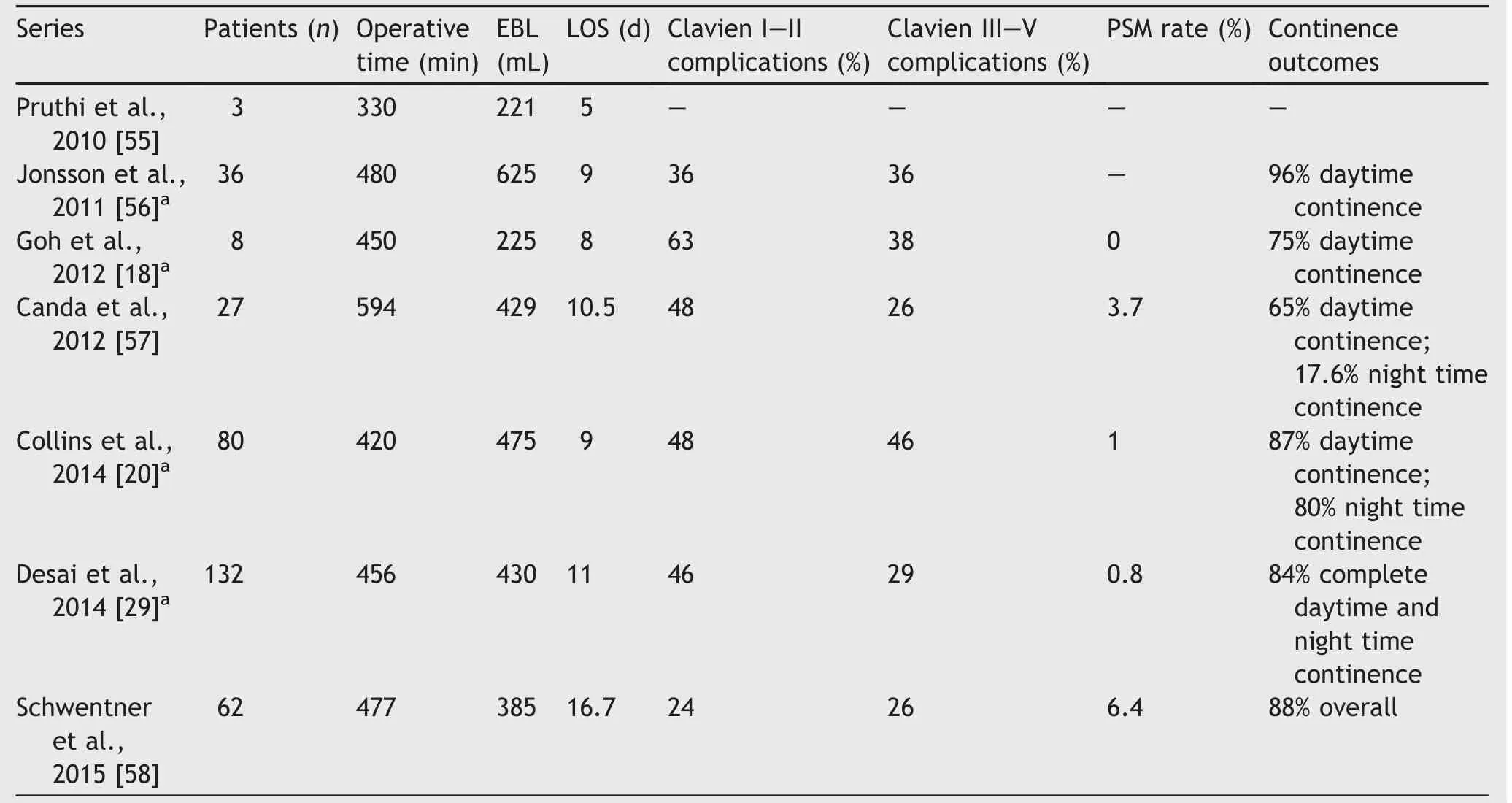
Table 1 Series of RARC with intracoporeal orthotopic ileal neobladder.
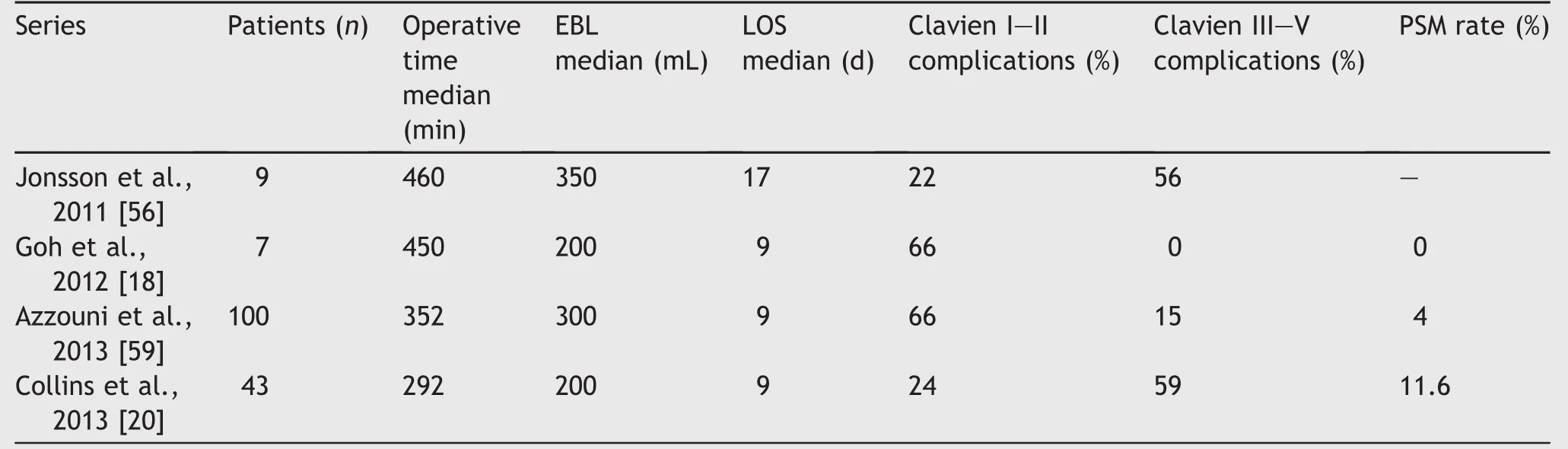
Table 2 Series of RARC in intracorporeal ileal conduit.
In a review of 1000 ONB following RC,Hautmann et al. [27]reported that the majority of complications after RC are related to diversion part of the procedure.It follows then that the majority of complications with a completely intracorporeal approach will also be related to the diversion.Table 3 illustrates diversion-related complications for the two largest published series of ICUD,one with all ICNB and the other with all ICIC.We defined diversion-related complications as those of GU,GI,or infectious etiology. Overall complication rates were comparable in the ICNB vs. ICIC series(75%vs.81%,respectively),but it should be noted that the ICNB series experienced more late complications that the ICIC series.Close inspection of the reported data reveals that the main difference is the timing and incidence of UTI may be responsible for this discrepancy,where ICNB patients seemed to experience higher rates of late UTI,while ICIC patients experienced more UTIs early in their post-operative course.The reason for this is not clear,though may be related to noncompliance with or improper performance self-catheterization after leaving the hospital.If true,this highlights the importance of proper patient selection when considering ONB.
Though most of these listed series are non-randomized and retrospective,data reporting outcomes on ICNB and ICIC in a single publication from single institution hold the distinct advantage of open and direct comparison between the two techniques.It effectively controls for surgeon experience,volume,perioperative care from ancillary staff,and regional idiosyncrasies that are otherwise virtually impossible to capture when comparing separate cohorts in time and space across the literature.Nonetheless,data related to RARC-ICUD remain limited to small institutional series performed by experienced robotic surgeons who also have experienced teams.It is not routinely performed in the community and it is important to consider this reality while interpreting the available literature on the subject.At the same time,experienced surgeons can also perform the described steps of RARCICUD purely laparoscopically,as the steps are by and large the same.
4.Intracorporeal vs.extracorporeal urinary diversion
A completely intracorporeal approach to the urinary diversion is attractive to both the patient and urologist,as it seeks to preserve the minimally invasive bene fits of RARC in the first place.Less intraoperative blood loss,minimal fluid shifts and decreased insensible fluid losses,quicker return of bowel function,and shorter length of stay are all potential advantages[18].In females there is the added opportunity for specimen extraction through the vagina, which may preclude a mini-laparotomy altogether.In such cases,incisional morbidity can be all but eliminated.
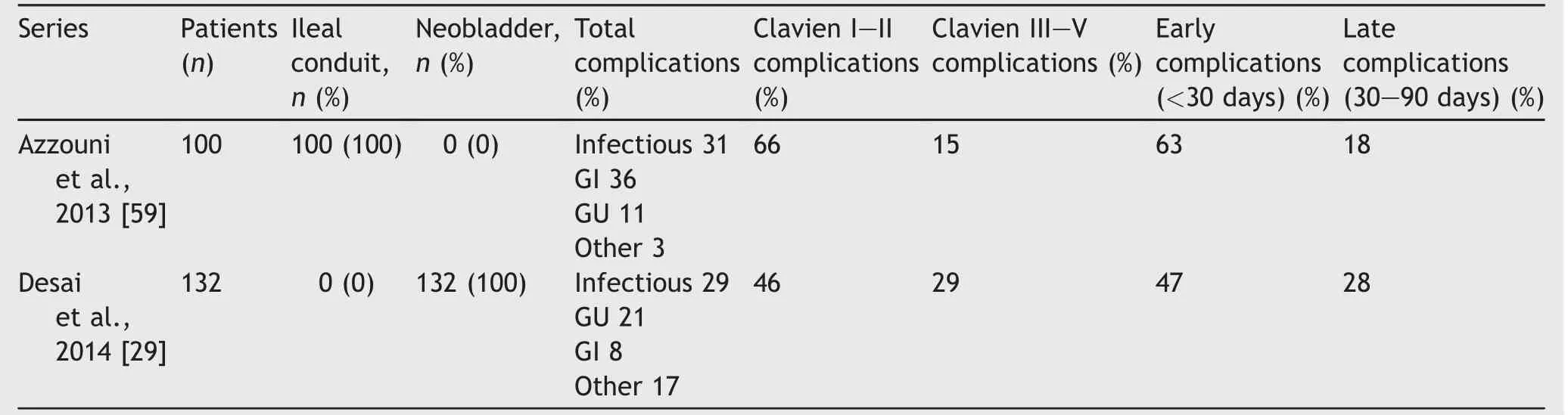
Table 3 Diversion-related complications of RARC-ICUD.
The International Robotic Cystectomy Consortium(IRCC) recently published an extensive analysis of perioperative outcomes in patients undergoing intracorporeal versus extracorporeal urinary diversion(ECUD)after RARC with at least 90-day follow-up[30].The IRCC is a collection of 18 different private and academic international centers that maintains prospective data on what is now nearly 1000 patients treated with RARC for clinically localized bladder cancer.One hundred and sixty-seven patients underwent ICUD(ONB 61;IC 106)while 768 had ECUD(ONB 198;IC 570).Operative times were equivalent(414 min)as were the 30-day reoperation rates.There was no difference in 90-day complication rates,though the results trended in favor of ICUD(41%vs.49%,p=0.05).The ICUD group did have a longer hospital stay by 1 day,which approach statistical significance(9 vs.8 days,p=0.086).This is consistent with another recent review comparing ICUD to ECUD,in which there was not sufficient evidence to conclude one method was superior to the other in terms of length of stay,though ICUD trended toward higher number of hospital days[31].Significantly,however,rates of gastrointestinal complications were lower(p≤0.001)in the ICUD group,and they were far less likely to experience a complication within 90 days(32%reduction)(odds ratio: 0.68;95%confidence interval(CI),0.50-0.94;p=0.02).
The main disadvantage of the IRCC study was its retrospective nature.However,it carries distinct advantages over smaller single institutional series as it suggests generalizability of good outcomes across multiple international centers and surgeons in the performance of ICUD.It is also the largest,most thorough comparison between extracorporeal and intracorporeal diversion to date.
5.RARC vs.ORC
Before deciding whether or not to perform a ICUD the urologist must choose to perform RARC over ORC in the first place.Beyond data on perioperative outcomes,there are many factors that contribute to this decision,including institutional culture,access to necessary equipment and technology,and properly trained ancillary staff.Desire and capability of the urologist are chief amongst these factors, and while the literature suggests an early steep learning curve,there is also evidence to support improvement in perioperative measures with increasing experience.This has direct impact on costs as well,which is seen as a potential barrier to future wide spread adoption of RARC. Indeed,multiple studies have demonstrated a shorter learning curve for RARC when compared to robot-assisted radical prostatectomy,which has been wildly popular amongst urologists[32-34].
Operative time,EBL,lymph node yield,and margin positivity were all evaluated in a 2010 analysis of 496 patients undergoing RARC across 21 surgeons at 14 different institutions[35].The authors of this review suggested a quota of 30 cases to achieve an acceptable level of prof iciency in RARC,and that outcomes were further improved with previous experience with robot-assisted radical prostatectomy.In 2014,Desai et al.[29]reported on significant improvements between multiple parameters for the first 15 cases vs.numbers 76-86 for a single surgeon.Median operative time decreased from 527 to 375 min(p<0.001), median EBL decreased from 550 to 200 mL(p<0.001), median length of stay decreased from 10 to 7 days (p=0.028),and overall 30-90-day complications trended toward significant improvement(60%vs.36%,p=0.057).
Independent of approach,RC has been associated with significant morbidity and post-operative complications. Multiple series for ORC have reported complications rates in the range of 40%-70%,including a 13%-40%rate of highgrade complications[36,37].As previously shown,RARC is associated with a similarly high complication rate,whether the diversion is performed intracorporeally or extracorporeally and regardless of type of diversion performed. However,the vast majority of published series have demonstrated comparable,and in many cases improved complications rates over ORC,a strong conclusion drawn in a recent meta-analysis[38,39].This review of 962 total cases across one RCT,eight prospective studies,and four retrospective studies suggested reduced complications rates for RARC(p=0.04),higher lymph node yield (p=0.009),less EBL(<0.001),lower need for blood transfusion(p<0.001),and shorter length of hospital stay (p< 0.001).Though follow-up was too short across the studies to establish meaningful oncologic and survival data, positive surgical margin(PSM)rates as a surrogate for oncologic control were equivalent between ORC and RARC.
In a more recent meta analysis,there was statistically significant weight mean differences in operative time (83.60 min;95%CI,57.1-110.1 min;p<0.00001 in favor of ORC),blood loss(-521 mL;95%CI,-644 to-399 mL; p<0.00001 in favor of RARC),need for transfusion(OR: 0.16;95%CI,0.1-0.27;p<0.00001 in favor of RARC),and in-hospital stay(WMD:-1.26;95%CI,-2.08 to-0.43; p=0.003 in favor of RARC),whereas rates for intraoperative complications(OR:1.34;95%CI,0.37-4.77; p=0.65)were similar for RARC and ORC.Rates for any grade of complication and for high-grade complications at 90 days favored RARC,whereas all other permutations of complication data were shown to be equivalent between RARC and ORC[40].This represents perhaps some of the strongest data to support the use of RARC over ORC when performed at a high volume center by an experienced surgeon.
Until recently,data have been limited in describing oncologic outcomes,as the previously mentioned series of RARC-ICUD have provided only short-term data,often using surrogates such as PSM and lymph node yield to compare oncologic outcomes.Collins et al.[41]reported on medium-term oncologic data on 113 patients undergoing RARC-ICUD.PSM were reported in 5.3%of patients and a resulting cancer-specific survival of 81%at 3 years and 67% at 5 years,which are favorable when compared to open series.The 2013 meta analysis by Li et al.[39]suggested RARC is associated with higher lymph node yields and equivalent PSM rates when compared to ORC,which have historically ranged from 0 to 6.3%[42,43].
The RARC-ICUD series presented in Tables 1 and 2 demonstrate similar PSM rates to open series,though PSM rates of 11.6%by Collins et al.is indeed an outlier in this respect(Table 2).The higher PSM rate in the IC group was likely due to a higher number of patients with clinical stage T3/T4 disease preoperatively(13.9%compared to 4.3%forthe neobladder group).Indeed,patients with higher clinical T stage translated to higher pathologic T stage,which were also the majority of patients with PSMs.When the PSM rate for the IC and neobladder groups are taken as a whole,it aligns more closely with historically reported figures at 5.3%.
To date,the longest-term data published on oncologic outcomes for minimally invasive RC demonstrate similar results to those reported in ORC[43],though it should be noted that only 17 out of 121 patients in this series underwent RARC while others received either purely laparoscopic or laparoscopic-assisted procedures without robotic assistance.The 10-year cancer-specific survival rate was 63%and overall survival was 35%,which is similar to other series of ORC[44,45].The authors admit the introduction of selection bias in this series as many of the early patients selected for minimally invasive RC were healthier and had lower stage disease as they worked to perfect their surgical technique early on.The largest series of ICNB published by Desai et al.[29]in 2014 reported 5-year overall,cancerspecific,and recurrence free survival was 72%,72%,and 71%,respectively.
Still,the randomized control trial remains the gold standard for comparing two therapies.Three RCTs comparing ORC to RARC have been published to date, though oncologic data are limited.The first,published by Nix et al.[46]in 2010 randomized 21 patients to RARC and 20 patients to ORC while demonstrating non-inferiority of lymph node yield as the primary endpoint.As secondary outcomes,there were no differences in complication rate or hospital stay.The robotic group enjoyed lower mean EBL (258 vs.575 mL,p<0.0001),shorter time to flatus and bowel movement(2.3 vs.3.2 day,p=0.0013;3.2 vs. 4.3 day,p=0.0008),and lower narcotic use(p=0.0044), while the open group had a predictably shorter operative time(4.2 vs.3.52 h,p<0.0001).There was no difference in mean lymph node yield(19 vs.18 LNs,p=0.52)or positive margins(0),while pathologic stage of the specimen was also similar.This was followed by a 2013 RCT evaluating perioperative outcomes and oncologic efficacy in 40 patients[47].Again,there was no significant difference in PSM rates or lymph node yield,and EBL was significantly lower in the robotic group.
The largest and most recent RCT comparing ORC to RARC was published in 2015 with primary endpoints including 90-day Clavien grade II-V complications[48].Secondary outcomes were established as comparison of high-grade complications,EBL,operative time,pathologic outcomes,3-and 6-month patient-reports quality of life measures,and costs.The trial randomized 60 patients to RARC and 58 patients to ORC.All patients regardless of randomization received an open urinary diversion.There were no signif icant differences in 90-day Clavien-complications(62%vs. 66%,p=0.7),and the trial was closed early as these results met interim analysis futility criteria.Not surprisingly, this trial once again demonstrated decreased EBL and longer operative times in the robotic group,and similar rates of PSM and lymph node yields.Mean hospital stay was also similar at 8 days,with similar quality of life measures reported at 3 and 6 months.Importantly,analysis was performed to compare both operating room and total inpatient costs.A robotic vs.open cystectomy and neobladder was shown to be US$3920 more expensive on average(US$19,231 vs.US$15,311,p<0.0001),while the IC was cheaper overall,but still more expensive when performed with the robot(US$18 388 vs.US$16 648, p<0.05).Ultimately,the authors concluded that RARC with either IC or neobladder did not confer any benefit to the patient,while placing increased cost burden on the health care system.
The publication of this most recent RCT has blurred what was,until recently,a picture coming into ever-sharper focus.While the authors should be commended for conducting the largest RCT representing the highest level of evidence published to date,it is important to acknowledge a significant limitation.All urinary diversions in the RARC group were performed extracorporeally,which may have acted to negate the beneficial effects of the minimally invasive approach.Indeed a recent meta-analysis(discussed earlier),reported a 32%reduction in perioperative complications when performing the urinary diversion intracorporeally compared to extracorporeally[30].Nonetheless,this trial did not demonstrate definitive advantage for RARC over ORC.While RARC did appear equivalent to ORC in many respects,it did so at a higher inpatient care cost primarily owing to longer operative times,increased equipment costs,and no benefit in length of stay.
Many studies assessing the feasibility of RARC have been wrought with selection bias,particularly in the earliest series.Patients have historically been excluded from RARC if they are morbidly obese,have other significant comorbidities(e.g.,severe cardiovascular or pulmonary disease), or have large,bulky or extravesical disease,which may lead to better outcomes for RARC cohorts due to more favorable baseline characteristics rather than surgical technique. Indeed early series comparing ORC to RARC demonstrated a selection bias toward less-advanced disease in robotic cohorts[49,50].In addition,patients with a history of pelvic radiation are often excluded from participation in surgical series,and thus published results may not be generalizable to patients with similar history in real-life practice.Novice surgeons tend to select patients by strict criteria.However, as surgeon experience grows,increasingly difficult and complicated cases may be attempted.
Absolute contraindications to RARC include patient inability to tolerate the physiologic stress of surgery to begin with,large body habitus,and multiple previous abdominal surgeries,which may preclude safe laparoscopic access.Those with severe cardiopulmonary disease may be difficult to ventilate while the abdomen is fully insufflated in the steep Trendelenburg position,placing them at increased risk for hypercarbia.Other relative contraindications may include previous extensive abdominal surgery, bleeding diatheses,severe cardiac disease,or Jehovah’s Witnesses.As experience increases,the individual surgeon may feel increasingly comfortable offering RARC to an increasingly comorbid population.
6.Health-related quality of life(HRQoL)and functional outcomes
Table 1 reports the published data regarding continence outcomes in patients following ICNB at times greater than30 days after surgery,many of which extend to 12 months. Daytime continence rates range from 65%to 96%in these series,but this also highlights the need to counsel patients appropriately on the possibility of some level of leakage post operatively.Nighttime continence tends to be lower, likely owning to positional effects of sleeping.Unfortunately,there are very limited HRQoL data directly comparing different types of urinary diversion,including those influenced by the choice between intra-or extracorporeal diversion.
Patients in multiple series universally experience decreased HRQoL score immediately following the operation,but these consistently rebound with time.One study was able to use the Functional Assessment of Cancer Therapy-Bladder(FACT-BL)questionnaire to assess patients’attitudes following RARC-ECUD[51].They reported a return to baseline in HRQoL parameters in about 3 months and in many patients HRQoL actually exceeded precystectomy levels,which is a useful benchmark in preoperative counseling of patients considering RARC.Poch et al. [52]used the Bladder Cancer Index to assess urinary and bowel functional scores,and noted those undergoing ICUD returned to baseline sooner than those in the ECUD group. Overall,both urinary and bowel domain scores returned to baseline by≤6 months,while sexual function did not normalize until 18-24 months.Importantly,multiple series have demonstrated continued improved continence rates at 12 months compared to 6 months post-surgery,thus it is imperative to counsel patients that HRQoL may continue to improve with time.
Tyritzis et al.[53]reported on 70 patients undergoing RARC-ICNB,and reported 81%of patients undergoing nervesparing RARC were potent at 12 months with or without phosphodiesterase type 5 inhibitors,while 67%of women had returned to sexual activity by that time as well.When performing RARC on a woman and extracting the specimen vaginally,it is important to consider proper reconstruction of the vagina,as sexual QoL will be determined by not only the ability to have intercourse,but also preventing dyspareunia.
The authors of the most recent EUA guidelines on muscle-invasive and metastatic bladder cancer noted that in most patient groups studied,the overall HRQoL after cystectomy remains good,irrespective of the type of urinary diversion used.The suggestion that continent diversions are associated with a higher HRQoL has not been sufficiently substantiated.It is also important to realize that QoL will also significantly be determined by the patient’s personality,coping style,and social support[1].
7.Conclusion
The majority of currently published series on RARC report better outcomes with regard to perioperative parameters, functional ability,QoL,and complications when compared to an open operation.Remarkable progress has been made in the technique and performance of this operation,and also there is mounting evidence to also support its use as a safe,reproducible,and oncologically responsible treatment for muscle invasive and other high-risk bladder cancers.While ICUD may increase total operative times and costs,there is also reason to believe that it holds advantages over an extracorporeal approach in perioperative and early post operative domains.
Still,there are no absolute currently available data to suggest the superiority of ICUD over ECUD.Surgeons differ in ability,and thus ICUD should likely be attempted only after the team has mastered the extirpative portion of the operation.The lack of quality long-term randomized control trials continues to make a universal assessment diff icult,and the long-term adoption of RARC will likely depend on economics as much as patient outcomes.Ultimately, engineered bladder substitutes may provide a future solution[54],but until that time urologists must continue to research and define the best options for our patients.
Conflicts of interest
The authors declare no conflict of interest.
[1]Witjes JA,Compe′rat E,Cowan NC,De Santis M,Gakis G, Lebret T,et al.EAU guidelines on muscle-invasive and metastatic bladder cancer:summary of the 2013 guidelines.Eur Urol 2014;65:778-92.
[2]Sanchez de Badajoz E,Gallego Perales JL,Reche Rosado A, Gutierrez de la Cruz JM,Jimenez Garrido A.Laparoscopic cystectomy and ileal conduit:case report.J Endourol 1995;9: 59-62.
[3]Yang S,Huang YH,Ou Yang CM,Huan SK,Chen M,Lin WR, et al.Clinical experience of laparoscopic-assisted radical cystectomy with continent ileal reservoir.Urol Int 2005;74: 240-5.
[4]Hemal AK,Kolla SB,Wadhwa P,Dogra PN,Gupta NP.Laparoscopic radical cystectomy and extracorporeal urinary diversion:a single center experience of 48 cases with three years of follow-up.Urology 2008;71:41-6.
[5]Huang GJ,Stein JP.Open radical cystectomy with lymphadenectomy remains the treatment of choice for invasive bladder cancer.Curr Opin Urol 2007;17:369-75.
[6]Gill IS,Fergany A,Klein EA,Kaouk JH,Sung GT,Meraney AM, et al.Laparoscopic radical cystoprostatectomy with ileal conduit performed completely intracorporeally:the initial 2 cases.Urology 2000;56:26-9.discussion 9-30.
[7]Gill IS,Kaouk JH,Meraney AM,Desai MM,Ulchaker JC, Klein EA,et al.Laparoscopic radical cystectomy and continent orthotopic ileal neobladder performed completely intracorporeally:the initial experience.J Urol 2002;168:13-8.
[8]Menon M,Hemal AK,Tewari A,Shrivastava A,Shoma AM,El-Tabey NA,et al.Nerve-sparing robot-assisted radical cystoprostatectomy and urinary diversion.BJU Int 2003;92: 232-6.
[9]Menon M,Hemal AK,Tewari A,Shrivastava A,Shoma AM,Abol-Ein H,et al.Robot-assisted radical cystectomy and urinary diversion in female patients:technique with preservation of the uterus and vagina.J Am Coll Surg 2004;198:386-93.
[10]Raza SJ,Tawfeeq M,Al-Daghmin A,Guru KA.Robot-assisted intracorporeal urinary diversion:where do we stand in 2014? Urol Clin North Am 2014;41:503-9.
[11]Zehnder P,Gill IS.Cost-effectiveness of open versus laparoscopic versus robotic-assisted laparoscopic cystectomy and urinary diversion.Curr Opin Urol 2011;21:415-9.
[12]Collins JW,Wiklund NP.Totally intracorporeal robot-assisted radical cystectomy:optimizing total outcomes.BJU Int 2014;114:326-33.
[13]Sandberg JM,Manny TB,Hemal AK.Robot-assisted and laparoscopic continent urinary diversion. In: Bishoff JT, Kavoussi LR,editors.Atlas of laparoscopic urologic surgery. 3rd ed.New York:Elsevier;2016[In press].
[14]Richards KA,Hemal AK.Current status and outcomes of robotassisted laparoscopic radical cystectomy and urinary diversion.Curr Urol Rep 2011;12:107-14.
[15]Richards KA,Hemal AK.Anatomic robot-assisted radical cystectomy.J Endourol 2012;26:1586-95.
[16]Manny TB,Gorbachinsky I,Hemal AK.Lower extremity neuropathy after robot assisted laparoscopic radical prostatectomy and radical cystectomy.Can J Urol 2010;17:5390-3.
[17]Manny TB,Hemal AK.Fluorescence-enhanced robotic radical cystectomy using unconjugated indocyanine green for pelvic lymphangiography,tumor marking,and mesenteric angiography:the initial clinical experience.Urology 2014;83:824-9.
[18]Goh AC,Gill IS,Lee DJ,de Castro Abreu AL,Fairey AS, Leslie S,et al.Robotic intracorporeal orthotopic ileal neobladder:replicating open surgical principles.Eur Urol 2012; 62:891-901.
[19]Shah HN,Nayyar R,Rajamahanty S,Hemal AK.Prospective evaluation of unidirectional barbed suture for various indications in surgeon-controlled robotic reconstructive urologic surgery:Wake Forest University experience.Int Urol Nephrol 2012;44:775-85.
[20]Collins JW,Sooriakumaran P,Sanchez-Salas R,Ahonen R, Nyberg T,Wiklund NP,et al.Robot-assisted radical cystectomy with intracorporeal neobladder diversion:the Karolinska experience.Indian J Urol 2014;30:307-13.
[21]Mufarrij PW,Rajamahanty S,Krane LS,Hemal AK.Intracorporeal double-J stent placement during robot-assisted urinary tract reconstruction:technical considerations.J Endourol 2012;26:1121-4.
[22]Balaji KC, Yohannes P, McBride CL, Oleynikov D, Hemstreet 3rd GP.Feasibility of robot-assisted totally intracorporeal laparoscopic ileal conduit urinary diversion:initial resultsofa single institutionalpilotstudy.Urology 2004;63:51-5.
[23]Guru K,Seixas-Mikelus SA,Hussain A,Blumenfeld AJ, Nyquist J,Chandrasekhar R,et al.Robot-assisted intracorporeal ileal conduit:Marionette technique and initial experience at Roswell Park Cancer Institute.Urology 2010;76: 866-71.
[24]Goh AC,Aghazadeh MA,Krasnow RE,Pastuszak AW, Stewart JN,Miles BJ.Robotic intracorporeal continent cutaneous urinary diversion:primary description.J Endourol 2015; 29:1217-20.
[25]Fahmy O,Asri K,Schwentner C,Stenzl A,Gakis G.Current status of robotic assisted radical cystectomy with intracorporeal ileal neobladder for bladder cancer.J Surg Oncol 2015;112:427-9.
[26]Nazmy M,Yuh B,Kawachi M,Lau CS,Linehan J,Ruel NH,et al. Early and late complications of robot-assisted radical cystectomy:a standardized analysis by urinary diversion type.J Urol 2014;191:681-7.
[27]Hautmann RE,de Petriconi RC,Volkmer BG.Lessons learned from 1,000 neobladders:the 90-day complication rate.J Urol 2010;184:990-4.quiz 1235.
[28]Desai MM,de Abreu AL,Goh AC,Fairey A,Berger A,Leslie S, et al.Robotic intracorporeal urinary diversion:technical details to improve time efficiency.J Endourol 2014;28: 1320-7.
[29]Desai MM,Gill IS,de Castro Abreu AL,Hosseini A,Nyberg T, Adding C,et al.Robotic intracorporeal orthotopic neobladder during radical cystectomy in 132 patients.J Urol 2014;192: 1734-40.
[30]Ahmed K,Khan SA,Hayn MH,Agarwal PK,Badani KK, Balbay MD,et al.Analysis of intracorporeal compared with extracorporeal urinary diversion after robot-assisted radical cystectomy:results from the International Robotic Cystectomy Consortium.Eur Urol 2014;65:340-7.
[31]Chan KG,Guru K,Wiklund P,Catto J,Yuh B,Novara G,et al. Robot-assisted radical cystectomy and urinary diversion: technical recommendations from the Pasadena Consensus Panel.Eur Urol 2015;67:423-31.
[32]Richards KA,Kader K,Pettus JA,Smith JJ,Hemal AK.Does initial learning curve compromise outcomes for robot-assisted radical cystectomy?A critical evaluation of the first 60 cases while establishing a robotics program.J Endourol 2011;25: 1553-8.
[33]Sharma NL,Papadopoulos A,Lee D,McLoughlin J,Vowler SL, Baumert H,et al.First 500 cases of robotic-assisted laparoscopic radical prostatectomy from a single UK centre:learning curves of two surgeons.BJU Int 2011;108:739-47.
[34]Collins JW,Wiklund PN,Desai MM,Goh AC,Gill IS.Total intracorporeal robotic cystectomy:are we there yet?Curr Opin Urol 2013;23:135-40.
[35]Hayn MH,Hellenthal NJ,Hussain A,Andrews PE,Carpentier P, Castle E,et al.Does previous robot-assisted radical prostatectomy experience affect outcomes at robot-assisted radical cystectomy?Results from the International Robotic Cystectomy Consortium.Urology 2010;76:1111-6.
[36]Shabsigh A,Korets R,Vora KC,Brooks CM,Cronin AM, Savage C,et al.Defining early morbidity of radical cystectomy for patients with bladder cancer using a standardized reporting methodology.Eur Urol 2009;55:164-74.
[37]Novara G,De Marco V,Aragona M,Boscolo-Berto R, Cavalleri S,Artibani W,et al.Complications and mortality after radical cystectomy for bladder transitional cell cancer.J Urol 2009;182:914-21.
[38]Martin AD,Nunez RN,Castle EP.Robot-assisted radical cystectomy versus open radical cystectomy:a complete cost analysis.Urology 2011;77:621-5.
[39]Li K,Lin T,Fan X,Xu K,Bi L,Duan Y,et al.Systematic review and meta-analysis of comparative studies reporting early outcomes after robot-assisted radical cystectomy versus open radical cystectomy.Cancer Treat Rev 2013;39:551-60.
[40]Novara G,Catto JW,Wilson T,Annerstedt M,Chan K, Murphy DG,et al.Systematic review and cumulative analysis of perioperative outcomes and complications after robotassisted radical cystectomy.Eur Urol 2015;67:376-401.
[41]Collins JW,Tyritzis S,Nyberg T,Schumacher M,Laurin O, Khazaeli D,et al.Robot-assisted radical cystectomy: description of an evolved approach to radical cystectomy.Eur Urol 2013;64:654-63.
[42]Novara G,Svatek RS,Karakiewicz PI,Skinner E,Ficarra V, Fradet Y,et al.Soft tissue surgical margin status is a powerful predictor of outcomes after radical cystectomy:a multicenter study of more than 4,400 patients.J Urol 2010;183:2165-70.
[43]Snow-Lisy DC,Campbell SC,Gill IS,Hernandez AV,Fergany A, Kaouk J,et al.Robotic and laparoscopic radical cystectomy for bladder cancer:long-term oncologic outcomes.Eur Urol 2014;65:193-200.
[44]Madersbacher S,Hochreiter W,Burkhard F,Thalmann GN, Danuser H,Markwalder R,et al.Radical cystectomy for bladder cancer today-a homogeneous series without neoadjuvant therapy.J Clin Oncol 2003;21:690-6.
[45]Stein JP,Lieskovsky G,Cote R,Groshen S,Feng AC,Boyd S, et al.Radical cystectomy in the treatment of invasive bladder cancer:long-term results in 1,054 patients.J Clin Oncol 2001; 19:666-75.
[46]Nix J,Smith A,Kurpad R,Nielsen ME,Wallen EM,Pruthi RS. Prospective randomized controlled trial of robotic versus open radical cystectomy for bladder cancer:perioperative and pathologic results.Eur Urol 2010;57:196-201.
[47]Parekh DJ,Messer J,Fitzgerald J,Ercole B,Svatek R.Perioperative outcomes and oncologic efficacy from a pilotprospective randomized clinical trial of open versus robotic assisted radical cystectomy.J Urol 2013;189:474-9.
[48]Bochner BH,Dalbagni G,Sjoberg DD,Silberstein J,Keren Paz GE,Donat SM,et al.Comparing open radical cystectomy and robot-assisted laparoscopic radical cystectomy:a randomized clinical trial.Eur Urol 2015;67:1042-50.
[49]Wang GJ,Barocas DA,Raman JD,Scherr DS.Robotic vs open radical cystectomy:prospective comparison of perioperative outcomes and pathological measures of early oncological efficacy.BJU Int 2008;101:89-93.
[50]Rhee JJ,Lebeau S,Smolkin M,Theodorescu D.Radicalcystectomy with ileal conduit diversion:early prospective evaluation of the impact of robotic assistance.BJU Int 2006;98:1059-63.
[51]Yuh B,Butt Z,Fazili A,Piacente P,Tan W,Wilding G,et al. Short-term quality-of-life assessed after robot-assisted radical cystectomy:a prospective analysis.BJU Int 2009;103:800-4.
[52]Poch MA,Stegemann AP,Rehman S,Sharif MA,Hussain A, Consiglio JD,et al.Short-term patient reported healthrelated quality of life(HRQL)outcomes after robot-assisted radical cystectomy(RARC).BJU Int 2014;113:260-5.
[53]Tyritzis SI,Hosseini A,Collins J,Nyberg T,Jonsson MN,Laurin O, et al.Oncologic,functional,and complications outcomes of robot-assisted radical cystectomy with totally intracorporeal neobladder diversion.Eur Urol 2013;64:734-41.
[54]Atala A,Bauer SB,Soker S,Yoo JJ,Retik AB.Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet 2006;367:1241-6.
[55]Pruthi RS,Nix J,McRackan D,Hickerson A,Nielsen ME, Raynor M,et al.Robotic-assisted laparoscopic intracorporeal urinary diversion.Eur Urol 2010;57:1013-21.
[56]Jonsson MN,Adding LC,Hosseini A,Schumacher MC,Volz D, Nilsson A,et al.Robot-assisted radical cystectomy with intracorporeal urinary diversion in patients with transitional cell carcinoma of the bladder.Eur Urol 2011;60:1066-73.
[57]Canda AE,Atmaca AF,Altinova S,Akbulut Z,Balbay MD. Robot-assisted nerve-sparing radical cystectomy with bilateral extended pelvic lymph node dissection(PLND)and intracorporeal urinary diversion for bladder cancer:initial experience in 27 cases.BJU Int 2012;110:434-44.
[58]Schwentner C, Sim A, Balbay MD, Todenhofer T, Aufderklamm S,Halalsheh O,et al.Robot-assisted radical cystectomy and intracorporeal neobladder formation:on the way to a standardized procedure.World J Surg Oncol 2015; 13:3.
[59]Azzouni FS,Din R,Rehman S,Khan A,Shi Y,Stegemann A, et al.The first 100 consecutive,robot-assisted,intracorporeal ileal conduits:evolution of technique and 90-day outcomes. Eur Urol 2013;63:637-43.
Received 23 March 2016;received in revised form 3 May 2016;accepted 3 May 2016 Available online 27 May 2016
*Corresponding author.
E-mail address:ahemal@wakehealth.edu(A.K.Hemal).
Peer review under responsibility of Second Military Medical University.
http://dx.doi.org/10.1016/j.ajur.2016.05.004
2214-3882/?2016 Editorial Office of Asian Journal of Urology.Production and hosting by Elsevier B.V.Ltd.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Urology2016年3期
Asian Journal of Urology2016年3期
- Asian Journal of Urology的其它文章
- Current status of laparoscopic and robotassisted nerve-sparing radical cystectomy in male patients
- Stents for malignant ureteral obstruction
- Percutaneous resection of upper tract urothelial cell carcinoma:When,how, and is it safe?
- Techniques to resect the distal ureter in robotic/laparoscopic nephroureterectomy
- Thulium laser treatment for bladder cancer
- Narrow band imaging for bladder cancer
