Human umbilical cord blood-derived stem cells and brain-derived neurotrophic factor protect injured optic nerve: viscoelasticity characterization
Xue-man Lv, Yan Liu, Fei Wu, Yi Yuan,, Min Luo
1 Department of Ophthalmology, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
2 Department of Hand Surgery, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
3 Department of Gynaecology and Obstetrics, Second Hospital of Jilin University, Changchun, Jilin Province, China
4 Department of Pain Management, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
RESEARCH ARTICLE
Human umbilical cord blood-derived stem cells and brain-derived neurotrophic factor protect injured optic nerve: viscoelasticity characterization
Xue-man Lv1, Yan Liu2, Fei Wu3, Yi Yuan1,*, Min Luo4
1 Department of Ophthalmology, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
2 Department of Hand Surgery, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
3 Department of Gynaecology and Obstetrics, Second Hospital of Jilin University, Changchun, Jilin Province, China
4 Department of Pain Management, China-Japan Union Hospital of Jilin University, Changchun, Jilin Province, China
Graphical Abstract
orcid: 0000-0002-7007-2857 (Yi Yuan)
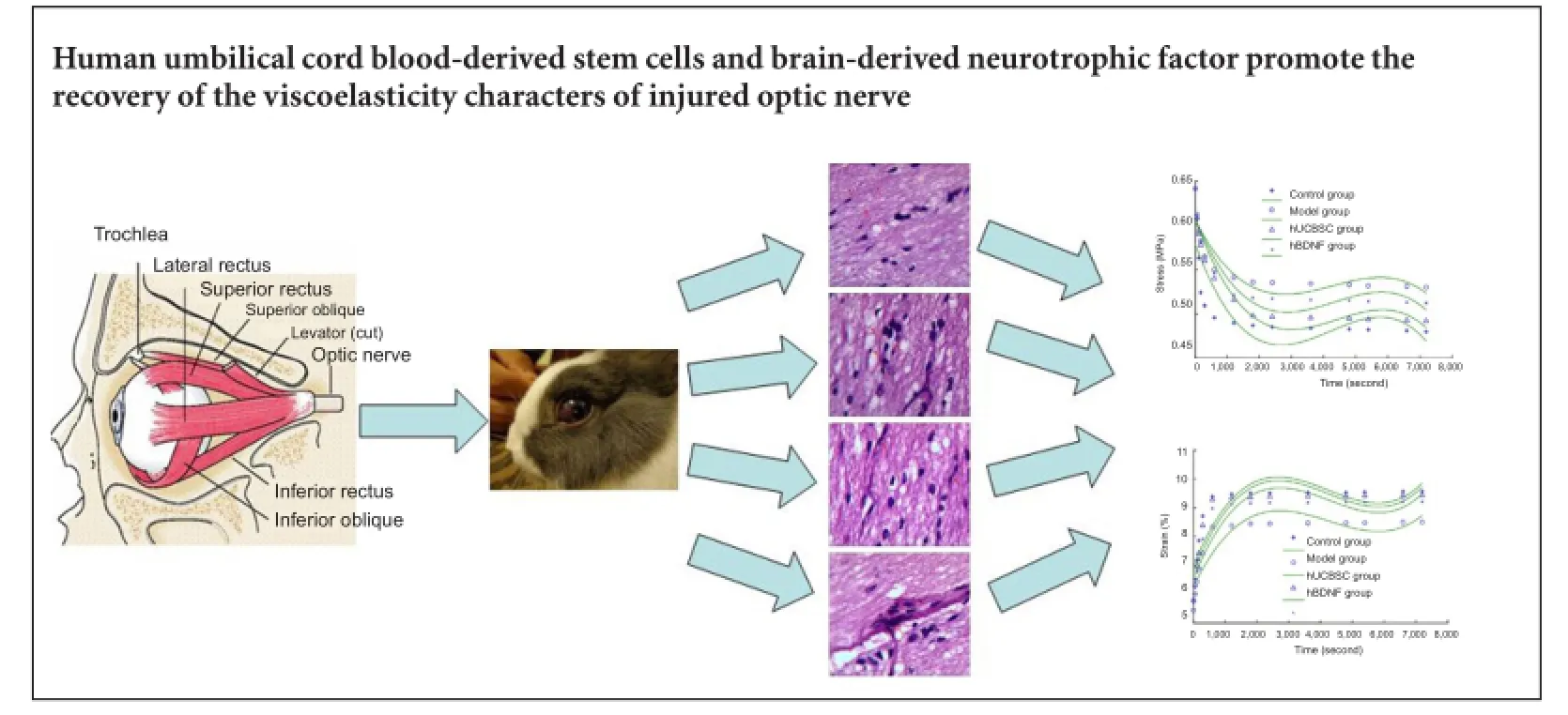
The optic nerve is a viscoelastic solid-like biomaterial. Its normal stress relaxation and creep properties enable the nerve to resist constant strain and protect it from injury. We hypothesized that stress relaxation and creep properties of the optic nerve change after injury. Moreover, human brain-derived neurotrophic factor or umbilical cord blood-derived stem cells may restore these changes to normal. To validate this hypothesis, a rabbit model of optic nerve injury was established using a clamp approach. At 7 days after injury, the vitreous body received a one-time injection of 50 μg human brain-derived neurotrophic factor or 1 × 106human umbilical cord blood-derived stem cells. At 30 days after injury, stress relaxation and creep properties of the optic nerve that received treatment had recovered greatly, with pathological changes in the injured optic nerve also noticeably improved. These results suggest that human brain-derived neurotrophic factor or umbilical cord blood-derived stem cell intervention promotes viscoelasticity recovery of injured optic nerves, and thereby contributes to nerve recovery.
nerve regeneration; optic nerve injury; human umbilical cord blood-derived stem cells; brain-derived neurotrophic factors; creep; histomorphology; stress relaxation; viscoelasticity; neural regeneration
Introduction
Currently used medication and gene therapy for the treatment of optic nerve injury have no ideal therapeutic effects (Schwartz, 2004; Dahlmann-Noor et al., 2010; Zrenner et al., 2011; Dagnelie, 2012; Hollander et al., 2012; Munemasa et al., 2012; Bodanapally et al., 2013; Chiu et al., 2013; Hunsberger et al., 2013; Lv et al., 2013; Yi et al., 2013; Mar et al., 2014). Stem cells exhibit neuroprotective effects in various models of central nervous system injury (Bi et al., 2009; Chakraborty et al., 2014). There is evidence that intravitreous injection of human umbilical cord blood-derived stem cells (hUCBSCs) can reduce progressive retinal ganglion cell (RGC) death after optic nerve injury, and accordingly, reduce loss of visual function (Gluckman et al., 1989; Zhao et al., 2011). Li et al. (2015) confirmed that intravitreous injection of hUCBSCs in rats with optic nerve injury reduces RGC loss by protectingRGCs. In addition, Liu et al. (2009) found increased number of surviving RGCs after transplantation of human brain-derived neurotrophic factor (hBDNF)-green fluorescent protein (GFP) gene-transfected neural stem cells into the injured rat retina. Moreover, Hou et al. (2004) showed that intravitreous injection of recombinant adenovirus-containing BDNF gene in rats with incomplete optic nerve injury led to flash visual-evoked potentials (F-VEP) with increased P1 wave amplitudes and shortened latencies, while rat neurological function recovered to a certain degree.
The optic nerve is a viscoelastic solid-like biomaterial. Its normal stress relaxation and creep properties enable the nerve to resist constant strain and protect it from injury. In this study, we hypothesized that BDNF or hUCBSC treatment may, to a certain extent, recover the altered stress relaxation and creep properties of the injured optic nerve. Using a rabbit model of optic nerve injury, we treated the animals with BDNF or hUCBSCs and then examined the therapeutic effect of each intervention from a viscoelastic and histomorphological perspective. Our intention was to provide information regarding viscoelasticity for the clinical treatment of optic nerve injury.
Materials and Methods
Ethics statement
Rabbits were allowed free access to water in their cages. The experimental protocol was approved by the Animal Ethics Committee, China-Japan Union Hospital of Jilin University, China. This study was performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Precautions were taken to minimize suffering and the number of animals used in each experiment.
Animals
Sixty male healthy Japanese rabbits, aged 5 months, weighing 2.5—2.8 kg, were provided by Changchun Gaoxin Medical Experimental Animal Center, China (license No. SCXK (Ji) 2003-0004). They were fed with whole grain and raised in an environment with air circulation (23—24°C), relative humidity (55—70%), and natural illumination.
Prior to experimentation, all rabbits were anesthetized by intraperitoneal injection of 10% chloral hydrate (35 mg/ kg). Rabbits with no lesions in the outer eyes or ocular fundus were included in the experiments. Sixty rabbits were randomly divided into model (optic nerve injury), optic nerve injury + hUCBSC (hUCBSC) and optic nerve injury + hBDNF (hBDNF) groups. Normal eyes (right eyes) of 20 randomly selected rabbits from these 60 rabbits were included as controls (control group).
Preparation of rabbit models of optic nerve injury
Precisely, following anesthesia by intravenous injection of 0.1% urethane (5 mg/kg) via the ear edge vein, rabbits were fixed on the pre-sterilized operating table in a prone position. The supraorbital skin of the left eye was incised to expose the supraorbital margin incisure and orbital wall. The part bone lamella of the orbital wall was removed, thereby passing through the supraorbital margin incisures (7—8 mm in depth and 6 mm in width). To expose the optic nerve, the posterior aponeurosis and orbicularis oculi muscles were cut and then bluntly separated along the superior rectus muscle towards the back of the eye ball. An approximately 5 mm length of optic nerve was dissociated, and the nerve (approximately 3 mm away from the posterior pole) was clamped for 5 seconds using a vascular clamp (W40160, specification: 3.7 cm; Shanghai Medical Apparatus and Instruments Factory, Shanghai, China) at 98 g constant pressure. After two gentamicin washes, the injured optic nerve was sutured layer by layer. After surgery, erythromycin was administered to the conjunctival sac and the eye examined using an ophthalmoscope (YZ6F; Liuliu Science Co., Ltd., Suzhou, Jiangsu Province, China). Successful rabbit models of optic nerve injury presenting no retinal vascular hemorrhage or infarction were included for further experiments.
BDNF and hUCBSC interventions
Seven days after optic nerve injury, rabbits in the hBDNF and hUCBSC groups received intravitreous injection of 50 ng BDNF (Beike Biological Science and Technology Co., Ltd., Shenzhen, Guangdong Province, China) or 1 × 106hUCBSCs (Beike Biological Science and Technology Co., Ltd.) using a microsyringe (YZ6F; Shanghai Meilian Biological Science and Technology Co., Ltd., Shanghai, China).
Sample harvesting
Thirty days after injury, 20 rabbits selected from each group were sacrificed by intravenous gas injection via the ear edge vein. The optic nerve was exposed. Under the ophthalmoscope, a segment of orbital optic nerve was harvested and stored in a container with physical saline at 4°C.
Hematoxylin-eosin staining
Optic nerve samples were fixed in 10% neutral formalin for 24 hours, degraded through an ethanol series, cleared with xylene, embedded with paraffin, successively transversely sliced into 3 μm thick sections, and enveloped with wax on albumen- and glycerol-coated slides. Sections were then routinely dewaxed, stained with hematoxylin for 3 minutes, washed with running water, treated with hydrochloric acid, washed with running water, treated with mild ammonia water, washed with running water, stained with eosin for 2 minutes, dehydrated through an ethanol series, cleared with xylene, and mounted with neutral gum. Injured optic nerve morphology was observed under an optical microscope (BX51; Olympus, Tokyo, Japan).
Stress relaxation testing of injured optic nerve
The length and diameter of injured optic nerve samples were measured using a CCs-3 reading microscope (Changchun Third Optical Instrument Co., Ltd., Changchun, Jilin Province, China). The length of each optic nerve sample was 10 ± 0.1 mm. Each optic nerve sample was loaded and unloaded 10 times. At 36.5 ± 1.0°C, each optic nerve sample was fixed in an electronic universal testing machine and the strain increased at a speed of 50%/min. When the strain reached3.98%, 3.85%, 3.78%, and 3.92% in the control, BDNF, model, and hUCBSC groups, respectively, and stress reached 0.61 MPa in each group, the strain was maintained constant in each group. Experimental sessions lasted 7,200 seconds. Once the experimental session was complete, stress-relaxation data and curves were automatically outputted.
Creep testing
Sample fixation, experimental temperature, data collection, and preliminary adjustment of the creep testing were identical to those used for stress-relaxation testing. The stress added to the optic nerve sample increased at a speed of 0.5 GPa/min. When the strain reached 4.00%, 3.77%, 3.86%, and 3.85% in the control, model, hUCBSC, and hBDNF groups, respectively, the stress was maintained at 0.61 MPa. Creep data and curves and time-dependent strain changes were determined.
Statistical analysis
All data are expressed as the mean ± SD and statistically processed using SPSS16.0 software (SPSS, Chicago, IL, USA). One-way analysis of variance and Scheffé’s method were used for comparisons between groups. A level of P < 0.05 was considered statistically significant. The normalized stress relaxation function and normalized creep function equation of the optic nerve sample in each group were established.
Results
BDNF and hUCBSC improved injured optic nerve pathology
Hematoxylin-eosin staining of transverse sections revealed that in the control group, fibers with an ordered structure were densely packed and arranged in parallel. In addition, glial cells were even in size and arrangement. In the model group, optic nerve fiber structure disappeared, nerve fibers and glial cells degenerated, and necrotic and deliquescent nuclei were occasionally observed. In the hUCBSC group, optic nerve fiber structure was absent with a few optic nerve fibers irregularly arranged, yet obviously thinned optic nerves were not observed. In the BDNF group, some optic nerve fibers were irregularly arranged but optic nerve fiber structure was visible. Additionally, an increased number of glial cell nuclei were observed, with only a small number dissolved (Figure 1).
Effects of hBDNF and hUCBSC on stress-relaxation curves in rabbits with optic nerve injury
In the hUCBSC and hBDNF groups, stress at 7,200 seconds was greatly decreased compared with the model group (P< 0.05). Stress at 7,200 seconds in the hUCBSC group was significantly decreased compared with the hBDNF group (P< 0.05; Figure 2). Functional expression of stress-relaxation curves from optic nerves in each group changed in a logarithmic manner.
The optic nerve stress-relaxation function was significantly greater at 7,200 seconds in the hUCBSC and hBDNF groups than in the model group (P < 0.05). Additionally, the stress-relaxation function was significantly greater in the hUCBSC group compared with the hBDNF group (P < 0.05; Figure 3).
We found that stress-relaxation curves changed in a logarithmic manner (Figure 3). Therefore, they could be designated according to a method by Ma et al. (2004) as follows:

Where c and d are undetermined constants,
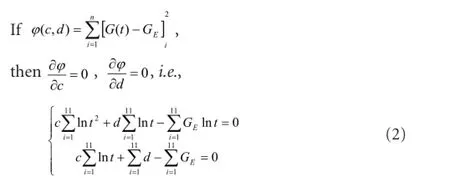
The data were introduced into equation (2) to obtain c and d values in each group, which were then introduced into equation (1) to obtain a normalized stress relaxation functional equation as follows:
Model group:

Effects of hBDNF and hUCBSC on creep curves of injured rabbit optic nerves
Our creep testing results demonstrated significantly increased strain in the injured optic nerve at 7,200 seconds in the hUCBSC and hBDNF groups compared with the model group (P < 0.05). Moreover, strain was also significantly increased in the hUCBSC group compared with the hBDNF group (P < 0.05; Figure 4). Creep curves of optic nerve samples in each group changed in a logarithmic manner.
Our normalized creep function results show that the normalized creep function value of the optic nerve at 7,200 seconds in the hUCBSC and hBDNF groups was significantly greater than in the model group (P < 0.05). Similarly, the normalized creep function value in the hUCBSC group was significantly greater than in the hBDNF group (P < 0.05; Figure 5).
Normalized creep function curves of optic nerves in each group changed in a logarithmic manner. According to Ma et al. (2004), we hypothesized that:
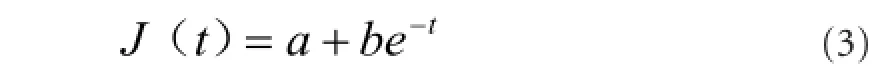
Optic nerve creep data in each group were introduced into equation (4) to obtain a and b values in each group, which were then introduced into equation (3) to obtain the normalized creep functional equation as follows: Control group:
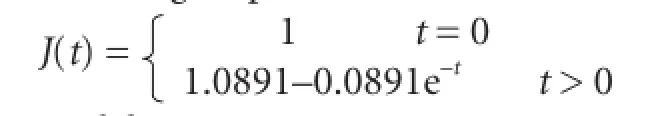
Model group:

hBDNF group:
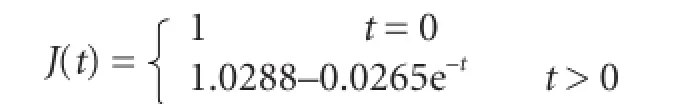
hUCBSC group:
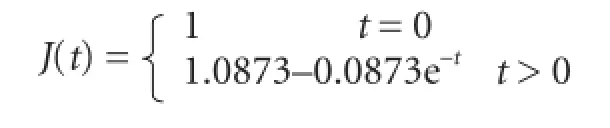
Discussion
Only optic nerves with complete nerve fiber structure exhibit the capacity to counteract stress and strain. Our results show that after repair by hUCBSC transplantation, optic nerve fiber structure is present in rabbits after optic nerve injury, with the majority of optic nerve fibers well arranged, and obviously thinning optic nerves not observed. However, after hBDNF treatment, some optic nerve fibers were poorly arranged, although optic nerve fiber structure was present, and glial cell nuclei were increased in number with only a small number dissolved. These findings suggest that hUCBSC and hBDNF can recover injured optic nerve morphology in animal models. We also found that after optic nerve injury, the nerve structure was disordered and the fibers poorly arranged, which led to changes in stress relaxation and creep properties. However, after hUCBSC or hBDNF intervention, stress relaxation and creep properties recovered to a certain degree in our rabbit model of optic nerve injury. These results suggest that hUCBSC and hBDNF intervention promote recovery of viscoelastic properties of injured optic nerves, and as expected, contribute to recovery from optic nerve injury.
The therapeutic effects of animal models of optic nerve injury have mostly been examined from the biological and histomorphological perspective. Ma et al. (2000) investigated the viscoelasticity of optic nerves harvested from a male cadaver using the normalized stress relaxation function at 6,600 seconds, and concluded that optic nerves exhibit striking viscoelasticity. In this study, we also obtained stress relaxation and creep curves of injured optic nerves after hUCBSC or hBDNF intervention.
The number of optic nerve samples in our study is limited and the optic nerves are different among individuals, which may result in bias. Additionally, we performed stress relaxation and creep experiments under only one stress and strain state because of our limited sample size. Nevertheless, our present results are still valuable for clinical treatment of optic nerve injury.
Author contributions: XML and YY conceived and designed this study. YL, FW and ML integrated experimental data. XML wrote the paper. YY guided the study and authorized the paper. ML performed statistical analysis, was responsible for fundraising, and provided assistance in technique or material application. All authors performed experiments and approved the final version of this paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Bi YY, Feng DF, Pan DC (2009) Stem/progenitor cells: a potential source of retina-specific cells for retinal repair. Neurosci Res 65:215-221.
Bodanapally UK, Kathirkamanathan S, Geraymovych E, Mirvis SE, Choi AY, McMillan AB, Zhuo J, Shin RK (2013) Diagnosis of traumatic optic neuropathy: application of diffusion tensor magnetic resonance imaging. J Neuroophthalmol 33:128-133.
Chakraborty SK, Banu LA, Rahman MF, Paul S (2014) Cord blood stem cells - a dream for future medicine. Mymensingh Med J 23:614-620.
Chiu CT, Wang Z, Hunsberger JG, Chuang DM (2013) Therapeutic potential of mood stabilizers lithium and valproic acid: beyond bipolar disorder. Pharmacol Rev 65:105-142.
Dagnelie G (2012) Retinal implants: emergence of a multidisciplinary field. Curr Opin Neurol 25:67-75.
Dahlmann-Noor A, Vijay S, Jayaram H, Limb A, Khaw PT (2010) Current approaches and future prospects for stem cell rescue and regeneration of the retina and optic nerve. Can J Ophthalmol 45:333-341.
Gluckman E, Broxmeyer HE, Auerbach AD, Friedman HS, Douglas GW, Devergie A, Esperou H, Thierry D, Socie G, Lehn P, Cooper S, English D, Kurtzberg J, Bard J, Boyse EA (1989) Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. New Engl J Med 321:1174-1178.
Hollander A, D’Onofrio PM, Magharious MM, Lysko MD, Koeberle PD (2012) Quantitative retinal protein analysis after optic nerve transection reveals a neuroprotective role for hepatoma-derived growth factor on injured retinal ganglion cells. Invest Ophthalmol Vis Sci 53:3973-3989.
Hou X, Hu D, Hui YN, Guo SY, Zhang ZM (2004) Adenovirally delivered BDNF to murine ocular tissues after optic nerve injury improves electrophysiology of vision. Yanke Xinjinzhan 24:247-249.
Hunsberger JG, Fessler EB, Chibane FL, Leng Y, Maric D, Elkahloun AG, Chuang DM (2013) Mood stabilizer-regulated miRNAs in neuropsychiatric and neurodegenerative diseases: identifying associations and functions. Am J Transl Res 5:450-464.
Li YQ, Zou Y, Hu ZL, Xiao LB, Wu M, Liu M, Chen YY, Zhang Y (2015) Protective effects of human umbilical cord blood stem cells vitreous transplantation on function restoration after traumatic optic nerve injury. Yanke Xinjinzhan 35:125-131.
Liu Y, Chen ET, Feng DF, Pan DC, Wang Y (2009) Treatment of optic nerve injury by transplanting hBDNF-GFP gene transfected neural stem cells. Zhonghua Chuangshang Zazhi 25:456-460.
Lv Q, Zhao FG, Zhang WQ, Zhang YQ, Shi J (2013) Single intravitreal injection of ciliary neurotrophic factor (CNTF) promotes optic nerve re-growth in golden hamsters. Shenjing Sunshang yu Gongneng Chongjian 8:414-417.
Ma HS, Dong XH, Liu E (2000) Normalized stress relaxation function of optic nerve. Yiyong Shengwu Lixue 15:125.
Ma HS, Zhang ZJ, Li XH (2004) Experimental study on viscoelasticity of the upper branch of fetal brachial plexus. Zhongguo Shengwu Yixue Gongcheng Xuebao 23:274-277.
Mar FM, Bonni A, Sousa MM (2014) Cell intrinsic control of axon regeneration. Embo Rep 15:254-263.
Munemasa Y, Chang CS, Kwong JM, Kyung H, Kitaoka Y, Caprioli J, Piri N (2012) The neuronal EGF-related gene Nell2 interacts with Macf1 and supports survival of retinal ganglion cells after optic nerve injury. PLoS One 7:e34810.
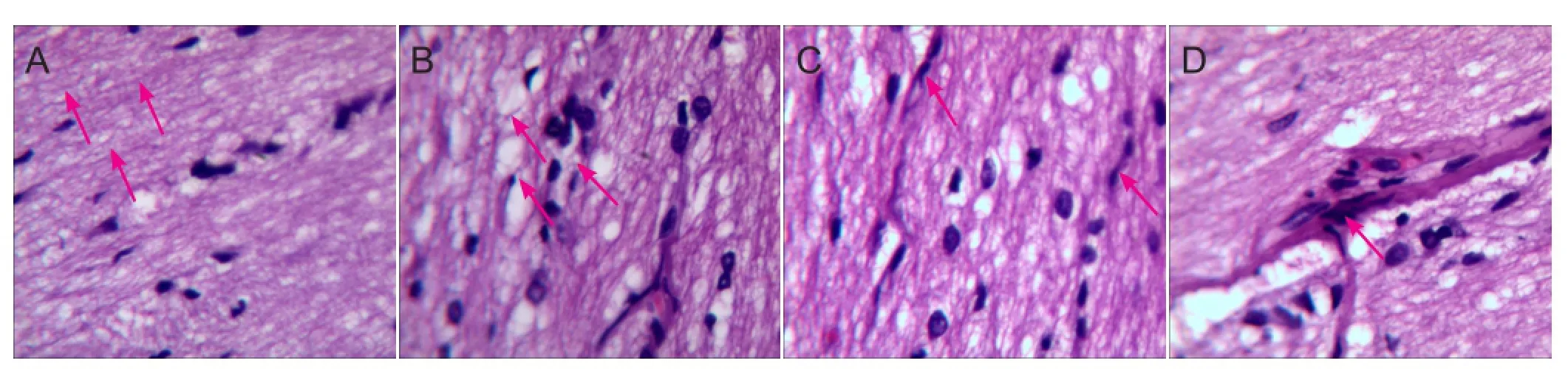
Figure 1 Effects of human brain-derived neurotrophic factor (hBDNF) and umbilical cord blood-derived stem cells (hUCBSC) on pathological morphology of injured optic nerve (optical microscope, hematoxylin-eosin staining, × 400).
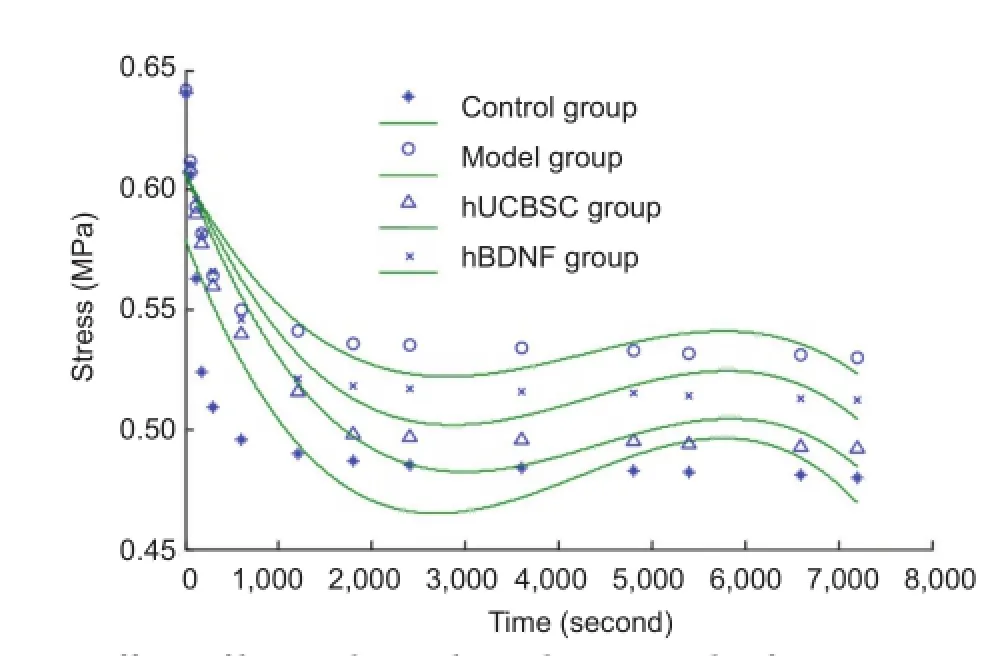
Figure 2 Effects of human brain-derived neurotrophic factor (hBDNF) and umbilical cord blood-derived stem cells (hUCBSC) on stress-relaxation curves in rabbits with optic nerve injury.
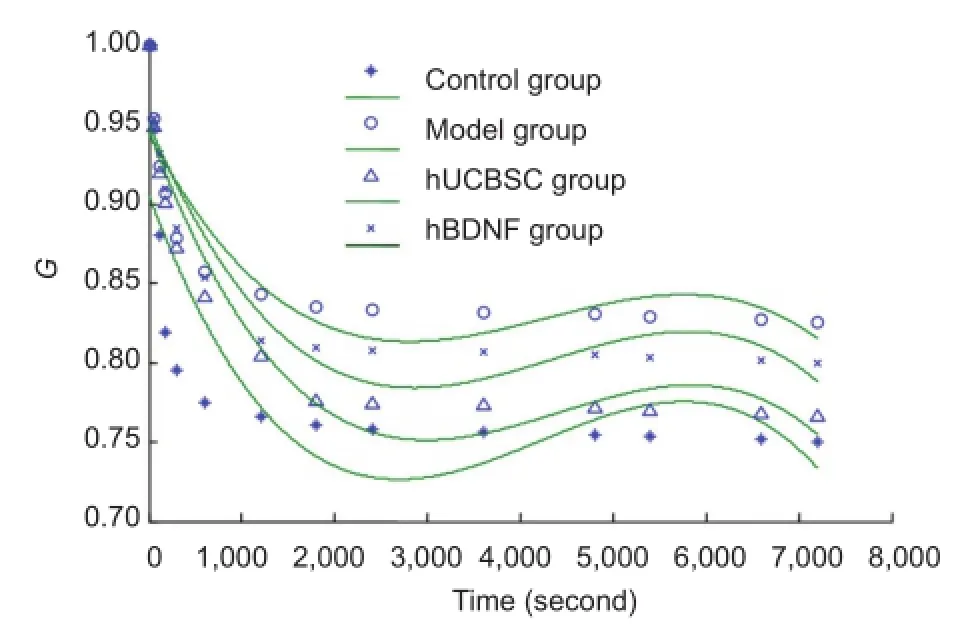
Figure 3 Effects of human brain-derived neurotrophic factor (hBDNF) and umbilical cord blood-derived stem cells (hUCBSC) on stress-relaxation curves (G) of injured rabbit optic nerves.
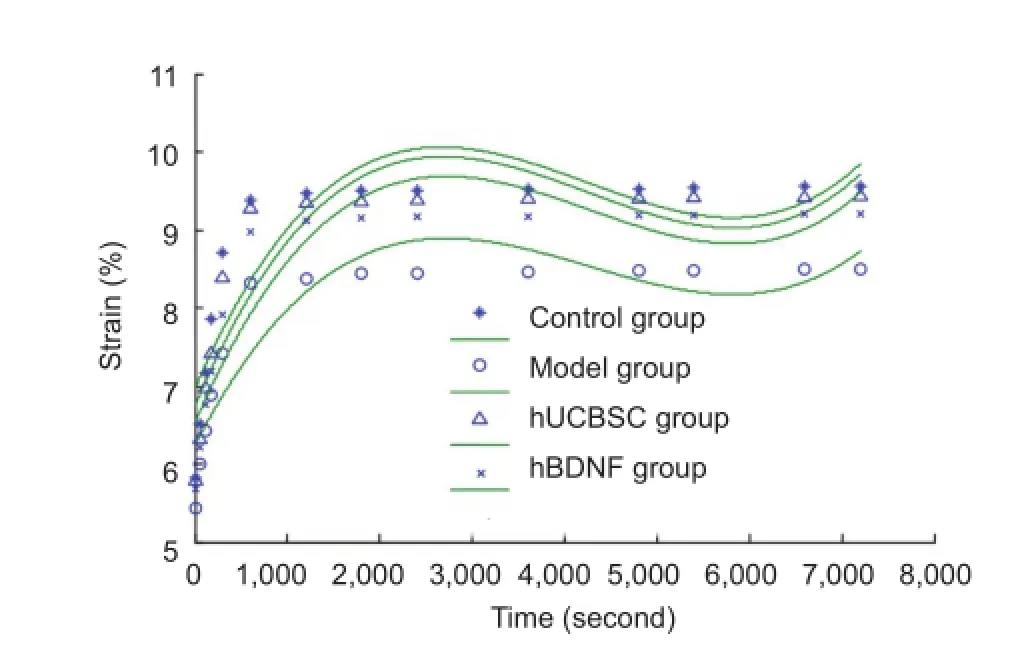
Figure 4 Effects of human brain-derived neurotrophic factor (hBDNF) and umbilical cord blood-derived stem cells (hUCBSC) on creep curves of injured rabbit optic nerve.
Schwartz M (2004) Optic nerve crush: protection and regeneration. Brain Res Bull 62:467-471.
Yi J, Zhang L, Tang B, Han W, Zhou Y, Chen Z, Jia D, Jiang H (2013) Sodium valproate alleviates neurodegeneration in SCA3/MJD via suppressing apoptosis and rescuing the hypoacetylation levels of histone H3 and H4. PLoS One 8:e54792.
Zhao T, Li Y, Tang L, Li Y, Fan F, Jiang B (2011) Protective effects of human umbilical cord blood stem cell intravitreal transplantation against optic nerve injury in rats. Graefes Arch Clin Exp Ophthalmol 249:1021-1028.
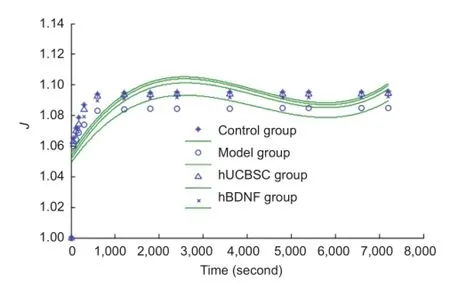
Figure 5 Effects of human brain-derived neurotrophic factor (hBDNF) and umbilical cord blood-derived stem cells (hUCBSC) on normalized creep function curves (J) of injured rabbit optic nerve.
Zrenner E, Bartz-Schmidt KU, Benav H, Besch D, Bruckmann A, Gabel VP, Gekeler F, Greppmaier U, Harscher A, Kibbel S, Koch J, Kusnyerik A, Peters T, Stingl K, Sachs H, Stett A, Szurman P, Wilhelm B, Wilke R (2011) Subretinal electronic chips allow blind patients to read letters and combine them to words. Proc Biol Sci 278:1489-1497.
Copyedited by James R, Waye W, Yu J, Li CH, Song LP, Zhao M
10.4103/1673-5374.180753 http://www.nrronline.org/
How to cite this article: Lv XM, Liu Y, Wu F, Yuan Y, Luo M (2016) Human umbilical cord blood-derived stem cells and brain-derived neurotrophic factor protect injured optic nerve: viscoelasticity characterization. Neural Regen Res 11(4):652-656.
Funding: This study was supported by a grant from High-Tech Research and Development Program of Jilin Province of China, No. 20110492.
Accepted: 2015-08-07
*Correspondence to: Yi Yuan, M.D., yuany@jlu.edu.cn.
- 中國神經(jīng)再生研究(英文版)的其它文章
- Gait deterioration due to neural degeneration of the corticoreticular pathway: a case report
- Complement components of nerve regeneration conditioned fluid influence the microenvironment of nerve regeneration
- Electrical stimulation of dog pudendal nerve regulates the excitatory pudendal-to-bladder reflex
- Supplementary motor area deactivation impacts the recovery of hand function from severe peripheral nerve injury
- Combined use of Y-tube conduits with human umbilical cord stem cells for repairing nerve bifurcation defects
- Senegenin inhibits neuronal apoptosis after spinal cord contusion injury

