Complement components of nerve regeneration conditioned fluid influence the microenvironment of nerve regeneration
Guang-shuai Li, Qing-feng Li, Ming-min Dong, Tao Zan Shuang Ding Lin-bo Liu
1 Plastic & Reconstructive Surgery of the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
2 Plastic & Reconstructive Surgery of the Ninth People’s Hospital Affiliated to Shanghai Jiaotong University, Shanghai, China
3 Otolaryngology-Head & Neck Surgery of the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
RESEARCH ARTICLE
Complement components of nerve regeneration conditioned fluid influence the microenvironment of nerve regeneration
Guang-shuai Li1, Qing-feng Li2,*, Ming-min Dong3,*, Tao Zan2, Shuang Ding2, Lin-bo Liu1
1 Plastic & Reconstructive Surgery of the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
2 Plastic & Reconstructive Surgery of the Ninth People’s Hospital Affiliated to Shanghai Jiaotong University, Shanghai, China
3 Otolaryngology-Head & Neck Surgery of the First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province, China
Graphical Abstract
orcid: 0000-0001-5329-4709 (Ming-min Dong)
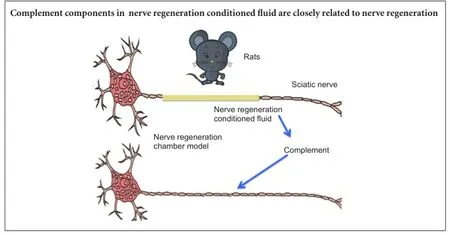
Nerve regeneration conditioned fluid is secreted by nerve stumps inside a nerve regeneration chamber. A better understanding of the proteinogram of nerve regeneration conditioned fluid can provide evidence for studying the role of the microenvironment in peripheral nerve regeneration. In this study, we used cylindrical silicone tubes as the nerve regeneration chamber model for the repair of injured rat sciatic nerve. Isobaric tags for relative and absolute quantitation proteomics technology and western blot analysis confirmed that there were more than 10 complement components (complement factor I, C1q-A, C1q-B, C2, C3, C4, C5, C7, C8β and complement factor D) in the nerve regeneration conditioned fluid and each varied at different time points. These findings suggest that all these complement components have a functional role in nerve regeneration.
nerve regeneration; peripheral nerve injury; nerve regeneration chamber model; sciatic nerve; nerve regeneration conditioned fluid; complement; iTRAQ proteomics technology; neural regeneration
Introduction
Repair of an injured peripheral nerve is a complicated and subtle physiological process. Lundborg and Hansson (1980) successfully repaired rat peripheral nerve transection defect using a cylindrical nerve regeneration chamber, which has become a standard animal model in peripheral nerve injury. Since then, researchers have developed a greater understanding about duct material, luminal contents, soluble regulation elements, functioning cells and other components of the Lundborg’s nerve regeneration chamber model. Nerve regeneration conditioned fluid is secreted by the nerve stump and acts as the functional medium in the nerve regeneration chamber model. The functional components of nerve regeneration conditioned fluid can provide assistance for the regenerated nerve in nerve regeneration chamber model (Li et al., 1999, 2007).
The nerve regeneration conditioned fluid proteinogram shows that the complement parts exist in large numbers and their dynamic changes depend on the different stages of nerve regeneration. The Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis indicates that the complement pathway tops the 10 most significantly enriched pathways (Kyte and Doolittle, 1982; Krogh et al., 2001). Little is currently known about their role in nerve regeneration. From their previous observations (Li et al., 1999, 2007), the authors believe the role of the complement cascade is worth exploring further. Relative and absolute quantification of isobaric Tags for Relative and Absolute Quantitation (iTRAQ) technology is an advanced proteomics technique. It can make a direct comparison of the relative content or absolute content of proteins in 4 or 8 different kinds of samples, making it an effective way to study the dynamic nature of protein expression in nerve regeneration conditioned fluid. A large number of complement components in the nerve regeneration conditioned fluid found through iTRAQ proteomics and its dynamic changes were described, analyzed and summarized. Western blot technique was used to verify some complement components of the nerve regeneration conditioned fluid and possible mechanisms of action for nerve regeneration were discussed.
Materials and Methods
Ethics statement
The animal studies were approved by Animal Ethics Committee of the Shanghai Ninth People’s Hospital of Shanghai JiaoTong University School of Medicine, China and performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Precautions were taken to minimize suffering and the number of animals used in each experiment.
Establishment of nerve regeneration chamber models
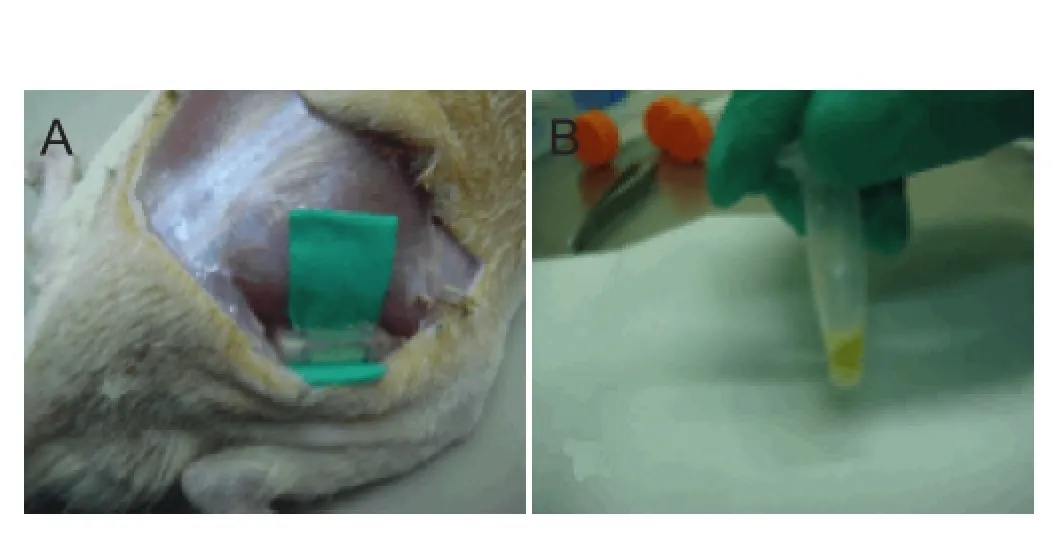
Figure 1 Establishment of nerve regeneration chamber model by anastomosis of the silicone tube with the sciatic nerve broken ends (A) and extraction of nerve regeneration conditioned fluid (B).
Eighty Lewis rats of either sex, aged 6—8 months, weighing 250—300 g, were used for modeling. After anesthesia by an intraperitoneal injection of 10% chloral hydrate solution (0.03 mL/kg), rats were placed in the prone position on a sterile cloth. A 25-mm skin incision was made in the intramuscular space between the superficial muscles of the buttocks, the head of the biceps femoris muscle and the semitendinosus muscle. The subcutaneous tissue was isolated to expose the sciatic nerve. A piece of 2-mm long nerve was cut under the 3-mm piriform aperture. A sterile silicone tube (inner diameter 1.2 mm, outer diameter 2.2 mm, length 14 mm, Shanghai Medical Instrument factory) was inserted for hemostasis. The ends of the silicone tube were attached to the sciatic nerve stumps using a 7/0 suture needle (Lundborg and Hansson, 1980). The two ends of the sciatic nerve were placed 2 mm into the silicone tube; the stump spacing was 10 mm to create the 10-μL nerve regeneration chamber. At 3, 7, 14 and 25 days after the operation, the nerve regeneration chamber was exposed through the original incision and the yellow nerve regeneration conditioned fluid was withdrawn using a microinjector (Figure 1).
Nerve regeneration conditioned fluid sample analyzed by iTRAQ proteomics technology
Each of nerve regeneration conditioned fluid samples, approximately 40 μg, was marked with the iTRAQ Reagent-8 plex Multiplex Kit (AB SCIEX, Framingham, MA, USA). A duplicate of each sample was made but the marking method was the same before and after the experiment. The marked peptide fragments were mixed and classified by strong cation exchange. The details are presented in Table 1.
Each sample was separated by the nano- high-performance liquid chromatography (HPLC), EASY-nLC system, and a Q Exactive mass spectrometer (Thermo Finnigan, Silicon Valley, CA, USA). After capillary HPLC and mass spectrometry the original raw data were documented, identified, and quantitatively analyzed and checked. The raw documents were input into the Mascot server through Proteome DiscovererTM, the databases found were chosen and searched. Proteome Discoverer 1.3 quantified the ion peak intensity, which was reported by the peptides. The result of peptide quantitative analysis was the ratio of the signal intensity of the reference sample label value to the other tag value. The quantitative results for the identification of peptides were expressed as the median value. The last quantitative resultswere normalized by each label median ratio to eliminate sample volume error from human factors in the experiment.
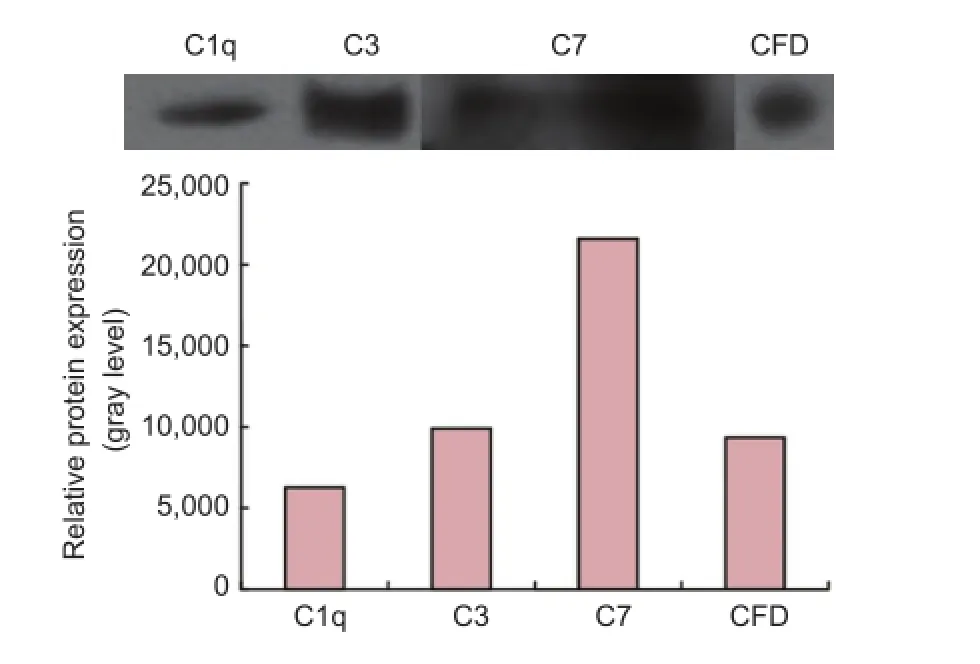
Figure 2 Protein expression of complement components in the nerve regeneratiom conditioned fluid (western blot assay).
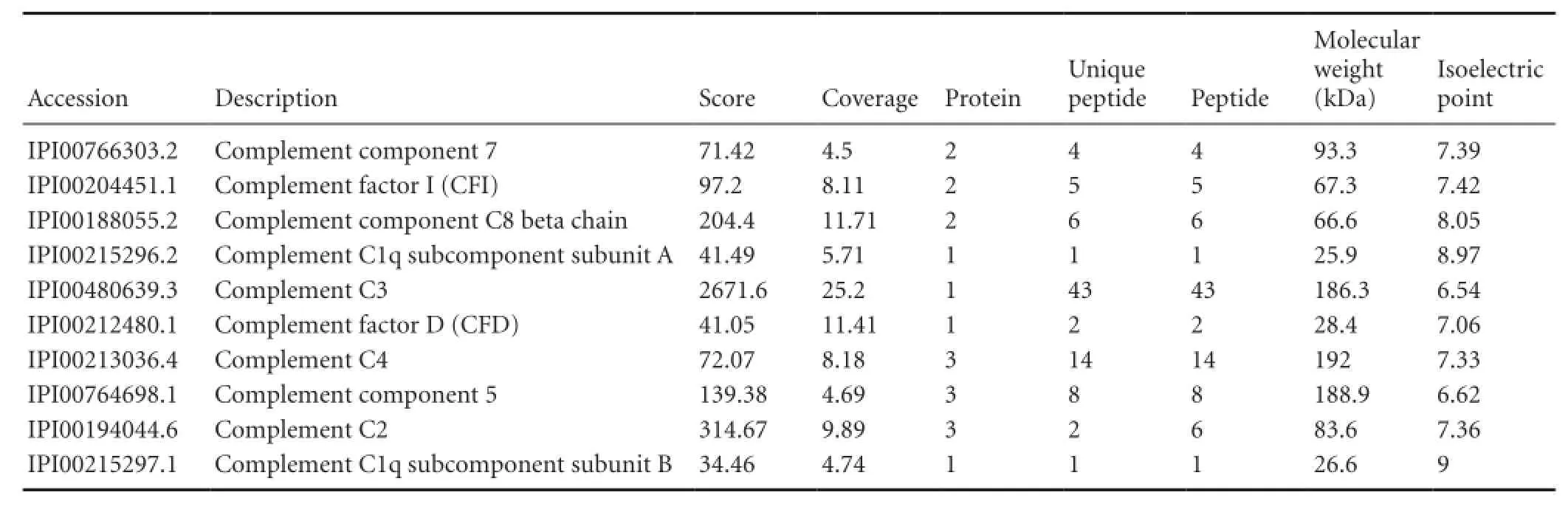
Table 1 The 10 complement components analyzed by nerve regeneration chamber fluid-iTRAQ proteomics
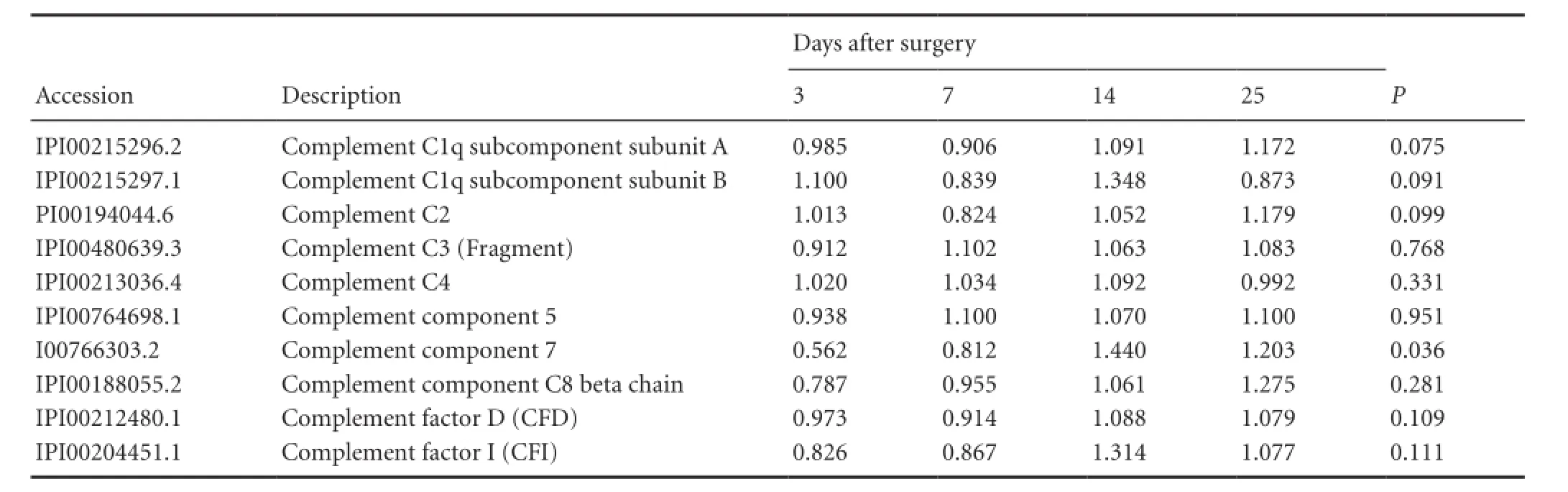
Table 2 The quantitative information of 10 complement constituents in nerve regeneration conditioned fluid after sciatic nerve injury and repair
Western blot analysis
The nerve regeneration conditioned fluid was pretreated for 7 days. Electrophoretic films were soaked in 5% skim milk and shaken gently for 1.5 hours at room temperature. Primary antibodies C1q monoclonal antibody [ab71940, rat, Abcam, AI Bo (Shanghai) Trading Co., Ltd., China], C3 monoclonal antibody [ab11862, rat, Abcam, AI Bo (Shanghai) Trading Co., Ltd.], C7 monoclonal antibody [ab126786, rabbit, Abcam, AI Bo (Shanghai) Trading Co., Ltd.], and Complement factor D (CFD) polyclonal antibody [LSC20320, Goat, Abcam, AI Bo (Shanghai) Trading Co., Ltd.] were diluted with Tris buffered saline with Triton (TBST, pH 7.2, Triton/Tween concentration 0.05%) at a ratio of 1:50, 1:50, 1:100, and 1:200, respectively. The membrane was soaked in 2 mL of the antibodies and incubated for 1.5 hours at room temperature. The primary antibody film was washed three times for 10 minutes each. Secondary antibodies [goat, Abcam, AI Bo (Shanghai) Trading Co., Ltd.] were diluted with TBST at a dilution ratio of 1:10,000, 1:5,000, 1:10,000, and 1:10,000 respectively. The secondary antibody film was placed into 2 mL of the diluted secondary antibody fluid, incubated for 1 hour at room temperature, and washed three times for 10 minutes each. After the addition of enhanced chemiluminescence luminous fluid, the secondary antibody film was transferred to an exposure bag for an X-ray camera (Guangdong Yuehua Medical Instrument Factory Co., Ltd., Shantou, Guangdong Province, China), placed in the dark room, exposed, submerged in the developing solution for 3 minutes and rinsed. The gray scale value of the band was taken as the protein level of the component complements.
Statistical analysis
Data are presented as the mean. One-way analysis of variance was performed by SPSS 21.0 (IBM, Armonk, NY, USA). Each group of data were homogeneous and comparable as confirmed by the homogeneity of variance. A level of P < 0.05 was considered statistically significant.
Results
iTRAQ proteomics of nerve regeneration conditioned fluid indicated some qualitative information on complements such as Complement factor I (CFI), C1q , as described in Table 1. Some qualitative information on a large number of complement components like CFI, C1q, C2, C3, C4, C5, C7, C8β and CFD existed in iTRAQ proteomics of nerve regeneration conditioned fluid. We used curve graph to exhibit how its dynamic change trended on 3, 7, 14 and 25 days (Table 2).
iTRAQ proteomics of nerve regeneration conditionedfluid at 7 days after sciatic nerve injury were confirmed by western blot analysis that complement components such as C1q, C3, C7 and CFD occurred in the nerve regeneration conditioned fluid (Figure 2).
Discussion
The cascading synthesis and regulation of the complement system is confirmed in the peripheral nervous system (de Jonge et al., 2004), but their role in maintaining normal peripheral nervous system function is controversial. The complements which are produced in situ and their regulatory factors can protect against infection and other damage from the nerves, but also attack target tissue. The complements clean myelin debris to promote the regeneration of damaged axons and aggravate damage to the tissue and prevent undamaged nerve from growing/sprouting.
In our tests, peripheral nerve regeneration was analyzed at four important times (3, 7, 14, and 25 days after dividing the peripheral nerve) with iTRAQ technology. At 3 days, the nerve regeneration chamber model was full of nerve regeneration conditioned fluid and represented the early environment of peripheral nerve regeneration. At 7 days, there were longitudinal arrangements of fibrin fibers and translucent cord structures connecting the two cut ends of the sciatic nerve in the nerve regeneration chamber model, referred to as the “nerve bridge” (Williams and Varon, 1985; Kotani et al., 1996). Basement membrane synthesis, which is an important structure to promote axon stretching, appeared at 14 days. The latter period of the peripheral nerve regeneration occurs around 25 days when the myelin axons stopped extending from the proximal to distal ends. At 35 days, the myelinogenesis completed (Yannas, 2001), but the nerve regeneration chamber model was left out of this study because there was not enough nerve regeneration conditioned fluid to be extracted.
The proteomics results showed the qualitative and quantitative information of more than 10 complements (C1q-A, C1q-B, C2, C3, C4, C5, C7, C8β, CFI, and CFD), however, each exhibited a different variation at different time points. So far we have only tested for C1q, C3, C7, and CFD in nerve regeneration conditioned fluid by western blot assay and the results proved that the complements of nerve regeneration conditioned fluid existed. Bioinformatics analysis (gene ontology analysis, KEGG access analysis) indicated that the complements might participate in cellular communication, defensive reaction, response to irritants, and the regulation of biological activity and function. The complement pathway is at the top of 10 significantly enriched pathways.
To prove whether nerve regeneration conditioned fluid exists or participates in peripheral nerve regeneration, our research group has conducted a series of studies starting in early 1990. The results were as follows: (1) The protein bands of nerve regeneration conditioned fluid in which molecular weight was within 232—440 kDa had neurotrophic and neutrophil chemotaxic properties. (2) Using gel electrophoresis, gunshot proteomics, and mass spectrometry technology, we identified 54 kinds of proteins from the protein bands within 232—440 kDa. most had an isoelectric point between 5.5 and 8.0 and their relative molecular weights were in the range 10—40 × 103. However, the functions of 17% of the proteins were unknown (Li et al., 2007). (3) The complement C3 α-chain, identified from the 54 proteins, might become a C3-like factor and has the same nontranslated regions subassembly as netrins (nerve guiding proteins). At the particular concentration measured, C3 could increase the length of neurites, so that C3 of nerve regeneration conditioned fluid might promote nerve regeneration (Li et al., 1999, 2007). (4) We chose different concentrations of exogenous C3 protein, C3a receptor antagonist SB290157, and the nerve growth factor, put them into the cortical neurons of 16-day-old mouse embryos (primary culture), and analyzed the effects of different concentrations of C3 and SB290157 on neuron growth. At a lower concentration, C3 had an obvious promoting effect on the growth of axons and dendrites which might be mediated by active fragment C3 receptor (Li et al., 2008).
The proteomics results in this study indicated that the C3 was expressed at each of the four important nerve regeneration points and there were no significant changes at the different stages of quantitative analysis. Our tests also gathered quantitative and qualitative information on C1q-A, C1q-B, C2, C4, C5, C7, C8β, CFI, and CFD of nerve regeneration conditioned fluid, however, only C7 had a significant change at different stages of the quantitative analysis.
In the central nervous system, the complements come mainly from the astrocytes, microglial cells, and neurons (Thomas et al., 2000). The nerve regeneration chamber model is a closed system and the complements are secreted from the end of the nerve rather than the immune system. The endogenous complement synthesis and a serial analysis of gene expression in the healthy human sciatic nerve have been confirmed and more than 10 complements (C1q, C3, C4, C5, MAC, CFI, CFD, CFH, CD59 and so on) were found, which are consistent with our results. The Schwann cells are considered the main source of the complement mRNA rather than fibroblasts and macrophagocytes. The classical complement pathway (C1q, C1r, C1s, and C4), alternative pathway (CFD), and their communal channel (C3) and the relevant regulatory factor (CLU, MCP, DAF, and CD59) all function and differentially express in different structures of peripheral nerves, such as nerve membrane, epineurium, Schwann cells, axons, and myelin. Although the complement regulators, including CR1, MCP, DAF, and CD59, are expressed in the myelin sheath, Schwann cell plasma membrane expresses only C5b-9s complex regulator CD59, without C3 invertase and other regulators. The situation mentioned above can protect the Schwann cells from the regulation and phagocytosis of macrophagocytes (de Jonge et al., 2004; Ramaglia et al., 2008) and are mediated by C3b and iC3b knocked off.
Not only does the activation of the complement system aggravates the non-specific damage to the local nerve tissue, but also terminates the inflammatory processes. It also guides the macrophages to remove the collapsed axon andmyelin debris to promote nerve regeneration.
The results of a previous study (de Jonge et al., 2004) indicated that C3, C4, C5, C9, and C1q were widespread in the nervous system and acted as important factors in nerve regeneration. The complements cascade is activated in an injured area soon after a transverse or crush injury to the peripheral nerve (de Jonge et al., 2004). Then the epitope of the axon and myelin sheath will be exposed after mechanical injury and activated by the corresponding pathway. The activated complement system creates an opsonin and enables the membrane attack complex. The larger splitting products of C3 and C5 could influence the directional migration of phagocytes and determine the membrane attack complex anchor point. The smaller splitting products have the potential for chemotaxis. The clearance of the myelin sheath is realized by mediation of the cAMP cascade of CR3 and scavenger-receptor (SR) AI/II (Reichert and Rotshenker, 2003). In the Lewis mice C3 knock-out model the recruitment of macrophages was slower and the clearance of the myelin was significantly reduced compared with normal mice (Dailey et al., 1998). The mice with a congenital reduction of C5 also exhibited a delay in recruitment of macrophages about 1—21 days after injury that could influence the degradation of the axon and myelin (Liu et al., 1999). The axon and myelin sheath epitope exposure after a trauma promotes the creation of C1q and activates the classic pathway, leading to hole formation in the axolemma on the membrane attack complex and inflow of calcium ions, eventually resulting in acute nerve injuries. The clearance of the nerve fragment from the membrane attack complex is mediated by a positive feedback pathway, led by C1q, that promotes ongoing regeneration.
In our test, C1q, C3, C5, C2, C4, C8, C7, and other complements were expressed at 3 days and the concentrations maintained at high levels until 25 days. This suggests that the complements have a relationship with peripheral nerve regeneration before nerve myelinogenesis. The changes of expression of C3, C4, C5, C8, and other related complements at different time points were not obvious, suggesting that the function of the activated complement system may be stable and continuous before myelinogenesis.
The research into the relationship between the complement and nerve regeneration has been sporadic and still lacks a systematic strategy. The study of complements focused on C3, C4, C5, C6 and C1q, short of in-depth discussion on other complements, such as C7, which have significant changes at different periods of nerve regeneration. In the standard and controlled nerve regeneration chamber model, our tests covered broad observations of complements in the nerve regeneration conditioned fluid, which have given new evidence and insights. These will be the basis of further studies to examine the mechanisms of action of complements in healing peripheral nerve injuries.
Author contributions: GSL, QFL and MMD designed the study, performed experiments, collected the data, and wrote the paper. MMD, TZ, SD and LBL performed statistical analysis and participated in design of the study. TZ and SD helped to wrote the paper. All authors approved the final version of this paper.
Conflicts of interest: None declared.
Plagiarism check: This paper was screened twice using Cross-Check to verify originality before publication.
Peer review: This paper was double-blinded and stringently reviewed by international expert reviewers.
Dailey AT, Avellino AM, Benthem L, Silver J, Kliot M (1998) Complement depletion reduces macrophage infiltration and activation during Wallerian degeneration and axonal regeneration. J Neurosci 18:6713-6722.
de Jonge RR, van Schaik IN, Vreijling JP, Troost D, Baas F (2004) Expression of complement components in the peripheral nervous system. Hum Mol Genet 13:295-302.
Dougherty KD, Dreyfus CF, Black IB (2000) Brain-derived neurotrophic factor in astrocytes, oligodendrocytes, and microglia/macrophages after spinal cord injury. Neurobiol Dis 7:574-585.
Gok B, Okutan O, Beskonakli E, Palaoglu S, Erdamar H, Sargon MF (2007) Effect of immunomodulation with human interferon-beta on early functional recovery from experimental spinal cord injury. Spine 32:873-880.
Kotani Y, Matsuda S, Sakanaka M, Kondoh K, Ueno S, Sano A (1996) Prosaposin facilitates sciatic nerve regeneration in vivo. J Neurochem 66:2019-2025.
Krogh A, Larsson B, von Heijne G, Sonnhammer ELL (2001) Predicting transmembrane protein topology with a hidden markov model: application to complete genomes1. J Mol Biol 305:567-580.
Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157:105-132.
Li QF, Xu LP, Jing NH (1999) The separation and detection of the bioactive proteins in nerve regeneration conditioned fluids. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 13:199-201.
Li YP, Meng FJ, Lu PH, Li QF (2008) Effects of complement C3 exposure on survival and neurite growth in primary rat cortical neuron cultures. Shanghai Jiaotong Daxue Xuebao: Yixue Ban 28:953-956.
Li YP, Jiang H, Li QF, Wang HY, Tang LJ, Zheng SW, Liu XQ, Zan T, Xie Y, Chen Y, Zheng DN (2007) Analysis of protein band in peripheral nerve regeneration conditioned fluid by shotgun technique. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 21:596.
Liu L, Lioudyno M, Tao R, Eriksson P, Svensson M, Aldskogius H (1999) Hereditary absence of complement C5 in adult mice influences Wallerian degeneration, but not retrograde responses, following injury to peripheral nerve. J Peripher Nerv Syst 4:123-133.
Lundborg G, Hansson HA (1980) Nerve regeneration through preformed pseudosynovial tubes. A preliminary report of a new experimental model for studying the regeneration and reorganization capacity of peripheral nerve tissue. J Hand Surg Am 5:35-38.
Ramaglia V, Daha MR, Baas F (2008) The complement system in the peripheral nerve: friend or foe? Mol Immunol 45:3865-3877.
Reichert F, Rotshenker S (2003) Complement-receptor-3 and scavenger-receptor-AI/II mediated myelin phagocytosis in microglia and macrophages. Neurobiol Dis 12:65-72.
Tabakman R, Lecht S, Sephanova S, Arien-Zakay H, Lazarovici P (2004) Interactions between the cells of the immune and nervous system: neurotrophins as neuroprotection mediators in CNS injury. Prog Brain Res 146:387-401.
Thomas A, Gasque P, Vaudry D, Gonzalez B, Fontaine M (2000) Expression of a complete and functional complement system by human neuronal cells in vitro. Int Immunol 12:1015-1023.
van Beek J, Elward K, Gasque P (2003) Activation of complement in the central nervous system: roles in neurodegeneration and neuroprotection. Ann N Y Acad Sci 992:56-71.
Williams LR, Varon S (1985) Modification of fibrin matrix formation in situ enhances nerve regeneration in silicone chambers. J Comp Neurol 231:209-220.
Yannas IV (2001) Tissue and Organ Regeneration in Adults. New York: Springer.
Copyedited by Dawes EA, Raye W, YJ, Li CH, Song LP, Zhao M
10.4103/1673-5374.180758 http://www.nrronline.org/
How to cite this article: Li GS, Li QF, Dong MM, Zan T, Ding S, Liu LB (2016) Complement components of nerve regeneration conditioned fluid influence the microenvironment of nerve regeneration. Neural Regen Res 11(4):682-686.
Funding: This study was supported by grants from the National Natural Science Foundation of China, No. 30925034, 81101437.
Accepted: 2015-10-23
*Correspondence to: Qing-feng Li, M.D., or Ming-min Dong, M.D., dr.liqingfeng@shsmu.edu.cn or chnwills@hotmail.com.
 中國(guó)神經(jīng)再生研究(英文版)2016年4期
中國(guó)神經(jīng)再生研究(英文版)2016年4期
- 中國(guó)神經(jīng)再生研究(英文版)的其它文章
- Gait deterioration due to neural degeneration of the corticoreticular pathway: a case report
- Electrical stimulation of dog pudendal nerve regulates the excitatory pudendal-to-bladder reflex
- Supplementary motor area deactivation impacts the recovery of hand function from severe peripheral nerve injury
- Combined use of Y-tube conduits with human umbilical cord stem cells for repairing nerve bifurcation defects
- Senegenin inhibits neuronal apoptosis after spinal cord contusion injury
- Human umbilical cord blood-derived stem cells and brain-derived neurotrophic factor protect injured optic nerve: viscoelasticity characterization
