An intravenous clarithromycin lipid emulsion with a high drug loading,H-bonding and a hydrogen-bonded ion pair complex exhibiting excellent antibacterial activity
Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China
An intravenous clarithromycin lipid emulsion with a high drug loading,H-bonding and a hydrogen-bonded ion pair complex exhibiting excellent antibacterial activity
Haoyu Gong,Sicong Geng,Qi Zheng,Puxiu Wang,Lifeng Luo, Xiuzhi Wang,Yan Zhang,Yu Zhang,Haibing He,Xing Tang*
Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China
A R T I C L EI N F O
Article history:
Received 24 February 2016
Received in revised form 13 April 2016
Accepted 22 April 2016
Available online 10 May 2016
Clarithromycin
Cholesteryl hemisuccinate
H-bonding and hydrogen-bonded ion pair complex
Antibacterial activity
Thin-flm dispersed
homogenization
The aim of this study was to develop an intravenous clarithromycin lipid emulsion(CLE) with good stability and excellent antibacterial activity.The CLE was prepared by the thinflm dispersed homogenization method.The interaction between clarithromycin(CLA)and cholesteryl hemisuccinate(CHEMS)was confrmed by DSC,FT-IR and1H NMR analysis.The interfacial drug loading,thermal sterilization,freeze–thaw stability,andin vitroandin vivoantibacterial activity were investigated systematically.DSC,FT-IR and1H NMR spectra showed that CHEMS(CLA:CHEMS,M ratio 1:2)could interact with CLA through H-bonding and a hydrogen-bonded ion pair.The CHEMS was found necessary to maintain the stability of CLE. Ultracentrifugation showed that almost 88%CLA could be loaded into the interfacial layer. The optimized CLE formulation could withstand autoclaving at 121°C for 10 min and remain stable after three freeze–thaw cycles.Thein vitrosusceptibility test revealed that the CLA–CHEMS ion-pair and CLE have similar activity to the parent drug against many different bacterial strains.Thein vivoantibacterial activity showed that the ED50of intravenous CLE was markedly lower than that of CLA solution administrated orally.CLE exhibited pronounced antibacterial activity and might be a candidate for a new nanocarrier for CLA with potential advantages over the current commercial formulation.
?2016 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/ licenses/by-nc-nd/4.0/).
1.Introduction
Clarithromycin(CLA),an erythromycin analogue with a 14-membered ring structure,exhibits important new properties following substitution of a methoxy group(-OCH3)for the C6hydroxy group(-OH)of erythromycin[1].These structural modifcations allow CLA to perform better than erythromycin in terms of acid stability,pharmacokinetics, gastrointestinal adverse effects and antibacterial spectrum. Clarithromycin is widely used in the treatment of bacterial infections,and it has been proved to be effective inMycobacterium aviumcomplex(MAC)infections bothin vitroandin vivo[2–4].Currently,clarithromycin lyophilized powder for injection contains ingredients like lactobionic acid and sodium hydroxide,which causes serious irritation at the injection site.Therefore,the development of an alternative formulation for intravenous injection with less irritation is necessary [5].
To obtain a less painful and more stable CLA treatment, various drug delivery systems for CLA have been studied,such as micelles[5],liposomes[6,7],emulsions[8–10],and nanoparticles[11].However,there are still many problems limiting the clinical applications of these formulations,such as low entrapment effciency,intolerable pain,a complex manufacturing process,poor physiochemical stability during longterm storage and the very high cost.
Lipid emulsions have been proven to be ideal carriers for parenteral drug delivery,due to their unique properties such as being biodegradable,biocompatible,physically stable,with low toxicity,and being easy to prepare even on a large scale. In addition,it had been shown that the oil-in-water emulsion system can signifcantly lower the incidence and the intensity of pain on injection because direct contact of the drug with body fuids and tissues is prevented by incorporating the drug into the oil phase or the interfacial layer[12,13]. So,the possibility of reducing irritation by preparing CLA lipid emulsions has been demonstrated and a number of related research studies have been launched.Lovell et al.developed a less painful clarithromycin emulsion with the aid of lipophilic counterions,like hexanoic acid and oleic acid,which improved the drug solubility in the oil phase[8].However, the fnal emulsion was unable to withstand thermal sterilization which limited its industrial application.Yan et al.and Jie et al.reported clarithromycin emulsions containing vitamin E,a phospholipid complex,and tocopherol succinate(TS)respectively[9,10].The fnal emulsions remained stable after undergoing sterilization at 100°C in a rotating water bath for 30 min.However,too many excipients were used in the formulation,which limited the safety and tolerability of the preparation.Currently,many studies are being conducted on ion-pair,which is a suitable chemical approach to increase the lipophilicity of drugs,making it easier to incorporate drugs into the lipid matrix[14–16].According to Br?nsted–Lowry acid–base theory,strong acids and bases tend to form hydrogenbonded ion pair(HIP)by proton transfer,especially in solvents with a low dielectric constant[17].
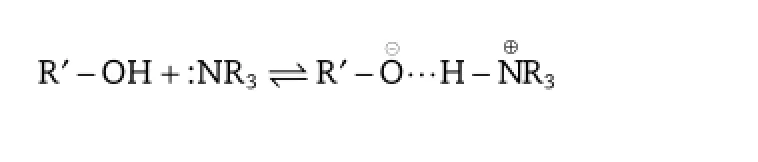
In the case of CLA,it is expected that the dimethylamino group will probably form strong hydrogen bonds like OH...N followed by proton transfer:OH...N?O?...HN+,with the excipient carrying the hydroxyl group.Therefore,it is essential to fnd an appropriate counter-ion to interact with CLA.In addition,to effectively localize the drug in the interfacial lecithinrich layer,a thin-flm dispersed homogenization method was developed.The resulting lipid emulsion exhibited good physical stability.
Based on the above factors,a novel parenteral clarithromycin lipid emulsion(CLE)with cholesteryl hemisuccinate(CHEMS) was developed and investigated.Considering that CLA has poor solubility in both oil and water media,efforts were made to localize the drug in the interfacial flm by H-bonding and the formation of a hydrogen-bonded ion-pair between CLA and CHEMS.Also,characterization by DSC and FT-IR analysis was performed to examine a possible interaction between CLA and CHEMS.Furthermore,a stability test was carried out after sterilization and freeze–thawing,which proved that the clarithromycin lipid emulsion was of high quality.Finally,the antibacterial activity of the drug was assessed bothin vitroandin vivo.The experimental results obtained showed that CLE exhibited good physical stability,thermal stability,and excellent antibacterial activity,suggesting that it had great prospects for clinical applications and its production could also be scaled up without great expense.
2.Materials and methods
2.1.Materials
The following materials were purchased from or provided by the sources in parentheses:CLA(Zhejiang Huayi Pharma Ltd. Co.,Zhejiang,China),cholesterol(CHO),CHEMS,and PL-100M (Shanghai Advanced Vehicle Technology Ltd.Co.,Shanghai, China),Lipoid E80,Lipoid S100,medium chain triglyceride(MCT) and long chain triglyceride(LCT)(Lipoid KG,Ludwigshafen, Germany),PC-98T(Q.P.Corporation,Tokyo,Japan),potassium bromide(FTIR grade)and DMSO-d6(Sigma Aldrich),Poloxamer 188(Pluronic F68)(BASF AG,Ludwigshafen,Germany),and glycerol(ZheJiang Suichang Glycerol Plant,Zhejiang,China).Doubledistilled water(DDW)was used throughout the study.All other chemicals and reagents were of analytical or chromatographic grade.
2.2.Degradation of clarithromycin in different oils
Clarithromycin was dissolved in MCT,LCT and an MCT–LCT mixture(1:1)by agitation in a water bath maintained at 80°C.Then,the oils containing 0.2%CLA were sealed in vials and incubated at 80°C in a thermostatically controlled water bath.The samples were then withdrawn at intervals of 0,1,2,4,6,8,12,and 24 h and cooled to room temperature immediately to terminate the reaction.The drug content was determined by HPLC as illustrated in Section 2.4,and the percentage CLA degradation was calculated by the changes in CLA content.
2.3.Preparation of CLE
The clarithromycin lipid emulsion with a concentration of 5.0 mg/ml was prepared by a thin-flm dispersed homogenization method.CLA,CHEMS and 2.0%(w/v)PL-100M were dissolved in an appropriate amount of anhydrous ethanol at 60°C.Then,the mixture was transferred to a round bottom fask and the organic reagent removed in a rotary evaporator(N-1001,EYELA,Japan).The aqueous phase containing 2.5%(w/ v)glycerol was adjusted to pH 8.0 with 0.05 mol/l NaOH and heated at 60°C.After removal of ethanol,the aqueous phase was added to the round bottom fask.The system was stirred at 60°C until the lecithin had uniformly dispersed,giving the drug in the aqueous phase.The oil phase(MCT,15%w/v,preheated at 60°C)was added slowly to the aqueous phase containing the drug under high shear mixing(ULTRATURRAX?T18 basic,IKA?WORKS,Germany)at 10,000 r/min over 3 min to prepare the coarse emulsion,and the fnal volume was adjusted to 100 ml with water for injection.The fnal emulsion was prepared using a high-pressure homogenizer(pharmaceutical ultra-high pressure homogenizer of AH100D,ATS Engineering Inc.,China)operated at 600 bar for 8 cycles with cooling water circulating around the equipment.The pH of the fnal emulsion was adjusted to 8.0 with 1 mol/l NaOH.Finally, the emulsion was sealed in vials in an atmosphere of nitrogen and autoclaved at 121°C for 10 min.After thermal sterilization,the emulsion was immediately cooled to 25°C. Formulation investigations of CLE were conducted with a singlefactor test.Also,microscopic examination was carried out using a Motic DMBA450 microscope(MoticChina Group Co.Ltd., Beijing,China)to check if there were any particles more than 5 micron in the system.
2.4.Characterization of CLE
The particle size distribution(PSD)and zeta potential of CLE were measured by photon correlation spectroscopy(PCS)and electrophoretic light scattering(ELS)respectively,using a NicompTM380 Particle Sizing system(Zeta Potential/Particle Sizer NicompTM380ZLS,Santa Barbara,California,USA).Samples were immediately diluted 1:5000 with double distilled water to obtain an optimum scattering intensity before measurement at 25°C.
A reverse phase HPLC analytical method was used for the drug analysis[9].The method was validated in terms of its selectivity,linearity,accuracy and precision.
The interfacial drug-loading was evaluated using the ultracentrifugation technique.The CLE sample was centrifuged at 50,000 r/min for 2 h using CS120GXL(Hitachi Koki Co.,Ltd., Tokyo,Japan).The resulting oil phase and water phase were collected and their CLA content was assayed by HPLC.The total amount of CLA in CLE was determined after disrupting the emulsion with ethanol.The interfacial drug-loading content (%)was calculated based on the following equation:
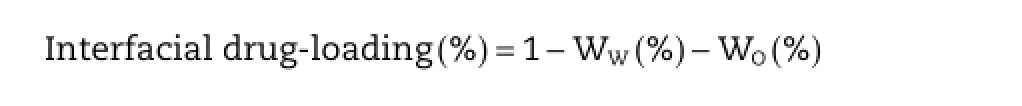
whereWW(%)andWO(%)are the drug contents(%)in the water phase and the oil phase,respectively.
2.5.Possible interaction between CLA and CHEMS
2.5.1.Preparation of the CLA–CHEMS complex and physical mixtures
Different molar ratios of CLA and CHEMS were dissolved in anhydrous ethanol under gentle stirring at 60°C for 30 min.Then, the organic phase was removed under vacuum at 60°C.The resulting product was the CLA–CHEMS ion-pair complex.
CLA–CHEMS physical mixtures were prepared by blending the two ingredients in different molar ratios in a porcelain mortar for 30 min.
All the powders mentioned above were collected and sealed in tight glass vials at 4°C until required for use.
2.5.2.Characterization of CLA–CHEMS complex
Samples(3–5 mg)were sealed in aluminum pans ftted with perforated lids,using an empty pan as control.All samples were heated at a rate of 10°C/min from 25°C to 250°C in a nitrogen atmosphere(DSC-1,Mettler-Toledo,Switzerland).
FT-IR spectra were recorded on a BRUKER IFS 5S FI-IR system between 4000 and 400 cm?1using the KBr disk method.
Samples were dissolved in deuterated dimethyl sulfoxide (DMSO-d6)and then transferred to NMR tubes for analysis.NMR spectra were recorded on an Advance Bruker instrument(1H at 600 MHz).The chemical shifts were relative to the tetramethylsilane(TMS)signal at 0 ppm for1H NMR.
2.6.Stability study
2.6.1.Thermal sterilization test
The fnal emulsion of clarithromycin was prepared according to Section 2.3.Then,several thermal sterilization methods were selected and used to optimize the sterilization conditions,including(i)in a 100°C rotating water bath for 30 min;(ii)in a 100°C rotating water bath for 45 min;(iii)autoclaving at 100°C for 45 min;(iv)autoclaving at 115°C for 30 min;(v)autoclaving at 121°C for 8 min;and(vi)autoclaving at 121°C for 10 min. Different indicators were used to evaluate the stability of the samples.
2.6.2.Freeze–thaw stability test
CLE samples were frozen in a freezer at?20°C for 12 h and then thawed at ambient temperature for another 12 h.The freeze–thaw cycle was repeated 3 times.The stability of CLE was evaluated by its physical appearance and PSD after each cycle.
2.7.Microbiology test
2.7.1.Susceptibility test
Minimal inhibitory concentrations(MICs)of CLA,CLA–CHEMS and CLE were determined by the agar dilution method as proposed by the National Committee for Clinical Laboratory Standards[18].Agar plates were prepared by serial twofold dilution.The inocula were adjusted to give a fnal cell density of approximately 104CFU/ml,and the determined concentration of antimicrobial drug ranged from 0.031 to 256 mg/l.CLA and drug-free lipid emulsions were used as positive and negative controls,respectively.The MIC values(mg/l)were evaluated according to the actual CLA concentration in each sample.
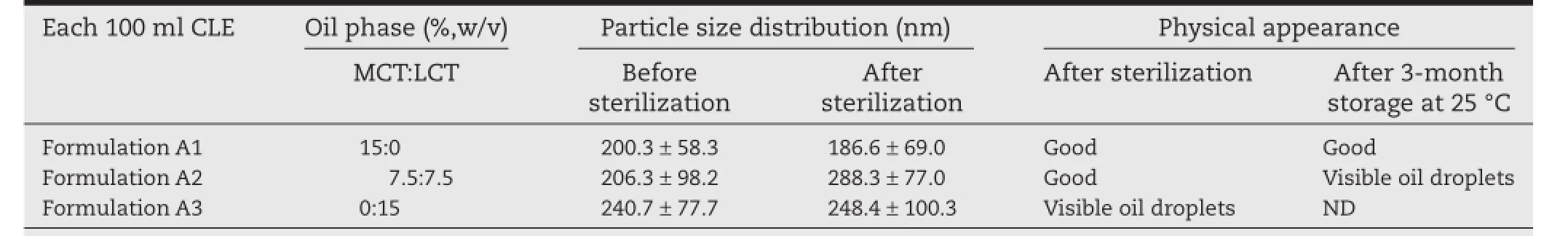
Table 1–The effect of oil phase composition on the characterization of CLE.
The medium and incubation conditions were as follows: Mueller-Hinton agar(MHA)with 5%sheep blood forStreptococcus pneumoniae,35±2°C,5%CO2,20 to 24 h;Haemophilus test medium(HTM)forHaemophilus infuenzae,35±2°C,5%CO2, 20 to 24 h;and Mueller-Hinton agar(MHA)for all other strains and incubated for 16 to 18 h at 35±2°C in ambient air.
2.7.2.Mouse model of sepsis
Isolates ofStaphylococcus aureusandStreptococcus pneumoniawere obtained from clinical sources.The medium and incubation conditions for the organisms were the same,as illustrated in Section 2.7.1.The single bacterial colonies were grown overnight at 37°C in appropriate broth and then diluted to the minimum lethal dose(MLD)with 5%dry yeast.The following experiments on mice with septicemia caused by the two strains were conducted separately[19].
Kunming mice of SPF grade weighing 18~22 g were kindly provided by the Experimental Animal Center of Academy of Military Medical Sciences.All animal experiments were carried with the permission of the local animal ethical committee,and conformed to the Guide for Care and Use of LaboratoryAnimals. In this test,mice were randomly divided into eighteen groups each of ten animals(fve males and fve females per group)and fasted overnight prior to the experiment.Mice were infected intraperitoneally with 0.5 ml of the bacterial suspension.At 1 h after infection,seven groups of mice each were treated intragastrically with different doses of CLA,and seven groups intravenously with CLE.Percentages of animals receiving each dose surviving to day 7 after infection were recorded,and the 50%effective doses and 95%confdence limits were calculated by the BLISS method.
3.Results and discussion
3.1.Preparation investigations of the CLE
3.1.1.Investigation of oil phase composition
The composition of the oil phase has a great infuence on the physicochemical stability of lipid emulsions.LCT and MCT have been mainly used for many years as the oil phase in clinical applications.
Accordingly,a 15%(w/v)oil phase of LCT and MCT,in the ratio of 7.5:7.5,was employed to evaluate the effect of the oil composition on the physicochemical properties of the CLE.The particle size distribution and physical appearance were chosen as the main parameters for evaluation,as shown in Table 1. Referring to the formulation A3,it was obvious that when using LCT as the oil phase,there were visible oil droplets on the surface of the CLE.Oil droplets appeared in formulationA2 after 3 months of storage at 25°C.This could be explained by the increased interfacial tension in the presence of the continuous phase caused by the relatively longer hydrocarbon chain of LCT[20],which adversely affected the strength of the oil flm, making it too weak to withstand sterilization or long-term storage.
Furthermore,the degradation profles of CLA in different oils were evaluated at 80°C,by monitoring the change in CLA concentration over 24 h.As shown in Fig.1,CLA underwent a marked degradation in LCT over 24 h with a concentration reduction of about 20%.However,there was very little drug degradation in the case of MCT.The reason for the difference in CLA chemical stability between LCT and MCT was considered to be the saturation level of the fatty acids,the unsaturated fatty acids in LCT being more active than the inert saturated fatty acids in MCT,allowing the release of radicals and peroxides at a high temperature[21].However,the peroxy radical produced during the oxidization process would accelerate the peroxidation of CLA while the hydrogen peroxide produced was liable to be degraded into low molecular weight substances containing carbonyl groups,and then undergo further oxidation to carboxylic acids leading to the degradation of CLA.Therefore,the addition of MCT to the oil phase of the CLE would reduce the degradation of CLA.Compared with LCT,MCT had a better immune-system profle since it is not involved in prostaglandin and leukotriene metabolism[22].In addition,MCTcould be a source of energy due to rapid hydrolysis and oxidation[23].Moreover,using MCT as the oil phase could reduce the viscosity of the system,which would undoubtedly beneft the large scale production.
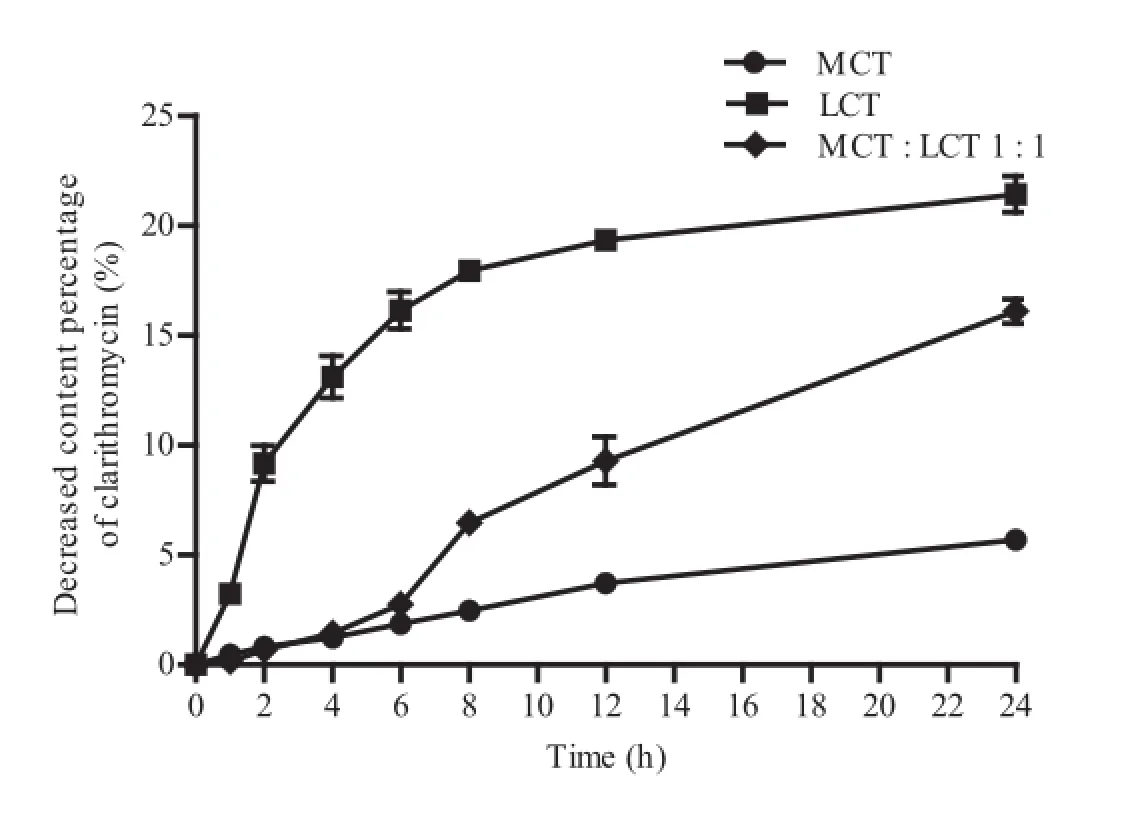
Fig.1–The degradation of CLA in different oils at 60°C within 24 h.
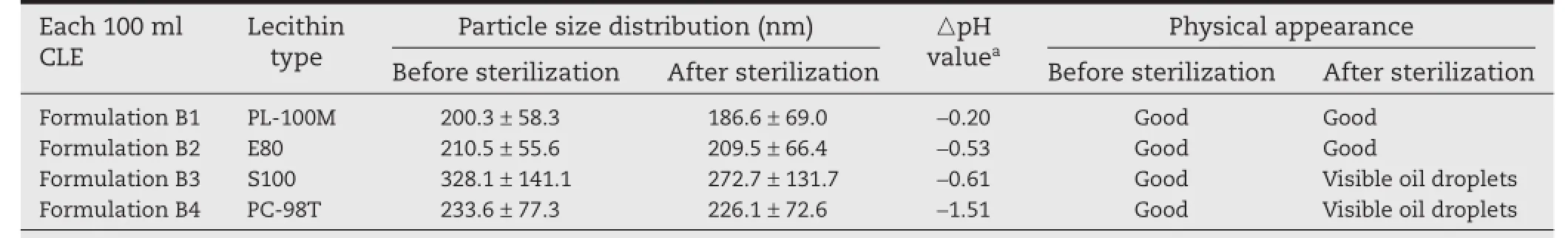
Table 2–The effect of types of lecithin on the characterization of CLE.
3.1.2.Investigation of different kinds of lecithin
It is well known that the emulsifcation process,which mainly depends on the emulsifers used,plays a critical role in the stability of lipid emulsions.Natural lecithin obtained from animal (egg yolk)or plant(soybean)sources is commonly used as an emulsifer in the preparation of lipid emulsions,exhibiting excellent biocompatibility.Infuence of the lecithin type on the lipid emulsion was investigated using formulations B1–B4 prepared according to Section 2.3(Table 2).
Natural lecithin contains a variety of components like phosphatidic acid(PA),phosphatidylinositol(PI),phosphatidylserine (PS)and some other ionic components.The content of these components is usually low but they can increase the heatstress and storage stability of the emulsion through increasing the ζ-potential of the oil droplets[24].The phosphatidylcholine(PC)content(%)of the natural lecithin used in this test was PC-98T(98.6%)>S100(96%)>E80(82.1%)>PL-100M(80.1%). Formulation B3 and B4 with S100 and PC-98T produced unstable lipid emulsions with visible oil droplets appearing after storage at 60°C for 5 d,and this result was consistent with the components in the different types of lecithin illustrated above. Formulation B2 with E80 showed good physical stability as refected by PSD and its appearance,whereas the pH after sterilization decreased markedly.Moreover,the content of CLA in the CLE decreased drastically after a month of storage at room temperature(data not shown).This could be explained by the fact that E80 is hydrolyzed in an alkaline and high temperature environment especially in the presence of electrolytes. Hydrolysis of E80 would produce free fatty acids,which would then trigger the chemical degradation of CLA,since CLA is very unstable in acid[1].Formulation B1 with PL-100M exhibited good chemical and physical stability during autoclaving and storage processes.The excellent emulsifying effects could be attributed to the minor components in PL-100M,including sphingomyelin,cholesterol,and lysophosphatidylcholine.In addition,PL-100M is stable in alkaline conditions,which would prevent CLA from further degradation and ensure the storage stability of CLE at a fnal pH of 8.0.
3.1.3.Investigations of membrane stabilizers in the CLE
The choice of membrane stabilizer also plays an important role in the preparation of a lipid emulsion.A mixture of emulsifers can form a close-packed complex interfacial flm that is less susceptible to breakdown during sterilization.
Cholesterol(CHO)is a fundamental component of the cell membrane,and it can stabilize the cell membrane by modifying the mobility and order of the phospholipids.Also, hydrogen bonds can be formed between CHO and drugs with an ester group to prevent drug leakage[25,26].As shown in Table 3,formulation C1 with a single emulsifer of PL-100M was unable to form a stable interfacial flm and so there was precipitation after sterilization.Also,no substantial improvement in the stability of formulation C2 was produced by the addition of CHO.This phenomenon could be explained by the fact that the interaction between CLA and CHO was not strong enough to maintain CLA in the interfacial lecithin layer of the lipid emulsion.Above all,one prerequisite for localizing the drug effciently in the interfacial flm is a strong interaction between the drug and excipient used in the formulation.
Since the dimethylamino group of CLA can interact with substances containing carboxyl groups,like hexyl acid through a lipophilic ion-pair[7,8],cholesteryl hemisuccinate(CHEMS),consisting of succinic acid esterifed to the β-hydroxyl group of CHO,was also investigated in the CLE.The effect of adding different amounts of CHEMS in the preparation of CLE was evaluated and the results are shown in Table 3.Addition of CLA and CHEMS at a molar ratio at 1:2(formulation C5) markedly improved the stability of CLE.Also,formulations C3 and C4 with less CHEMS added exhibited poor stability during autoclaving or storage,due to breakdown of the interfacial flm and precipitation of the drug from the phospholipid bilayer.Based on these results,CHEMS(CLA:CHEMS,M ratio 1:2)was judged to be suitable and essential for the preparation of CLE.
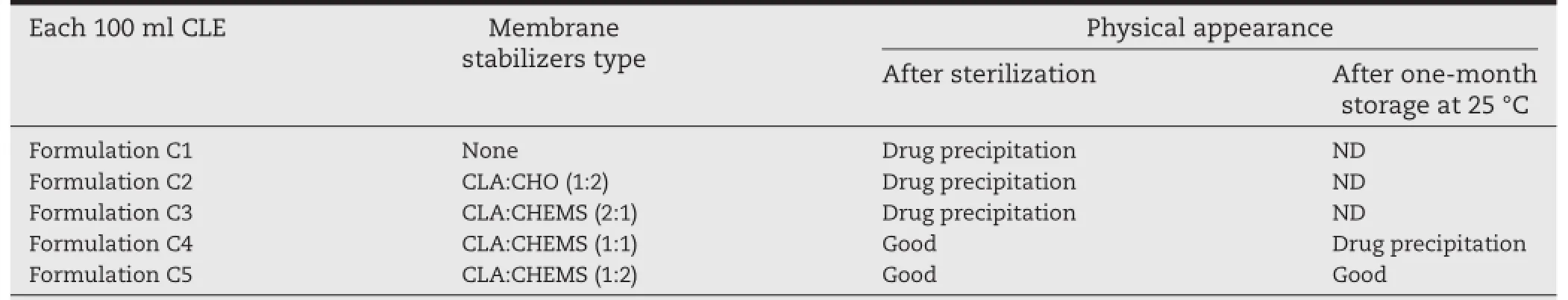
Table 3–The effect of types of membrane stabilizers on the characterization of CLE.
In addition,Pluronic F68 is commonly used as a co-emulsifer in lipid emulsions since it can rapidly stabilize the newly created interface[27].The effect of F68 addition was also investigated.Lipid emulsions stabilized by F68(0.2%,w/v),PL-100M and CHEMS(CLA:CHEMS,M ratio 1:2)displayed almost the same behavior as the formulation without F68 during a threemonth storage at 25°C(data not shown),whereas coalescence and cracking appeared after a six-month storage.Also,it was found that addition of excess co-emulsifer interfered with the formation of the interfacial flm.Moreover,it has been reported that parenteral administration of F68-based formulations induce non-IgE-mediated hypersensitivity reactions in some individuals[28,29].So,the use of F68 should be limited since a pliable close-packed interfacial flm could already be formed by PL-100M and CHEMS.
3.2.Verifcation of interaction between CLA and CHEMS
To confrm the interaction between CLA and CHEMS,DSC and FT-IR analyses were carried out.In addition,the possible formation mechanism was also considered.
3.2.1.DSC analysis
Fig.2Ashows the DSC curves of CLA,CHEMS,CLA–CHEMS physical mixture(PM)and the equivalent molar complex(CP).Pure CLA and CHEMS samples exhibited sharp endothermic peaks around 231°C and 186°C,which were attributed to their respective melting temperatures.The calorimetric analysis of the physical mixture and complex in different molar ratios showed an endothermic signal at about 157°C,which was not present in the endothermic peaks of the two starting compounds,indicating the formation of a new chemical entity.The DSC profles of PM suggested that CLA and CHEMS could interact and form a new complex simply by trituration without any solvent.
3.2.2.FT-IR analysis
In order to further investigate the possible mechanism of the interaction between CLA and CHEMS,pure CLA,CHEMS and CLA–CHEMS physical mixture(PM)and complex(CP)at different molar ratios were subjected to FT-IR analysis(Fig.2B). The spectrum of CLA showed characteristic absorption bands of the hydroxyl group,and ketone and lactone carbonyl groups of CLA around 3468 cm?1,1691 cm?1,and 1733 cm?1. The signal of the lactone carbonyl group of CHEMS was obtained around 1731 cm?1,which overlapped that of CLA and, also,the absorption peak of the carboxylic acid carbonyl group at 1706 cm?1was observed.The IR spectra of the 1:2 CLA and CHEMS complex showed some differences compared with other samples and confrmed the formation of a new complex.Signals around 1635 cm?1and 1573 cm?1were attributed to the asymmetrical stretching vibration of the carboxylic salt bonds and the bending vibration of an ammonium ion,respectively,which indicated the occurrence of proton translocation between the carboxyl and amino groups or,in other words,the formation of HIP.This interaction would increase the lipophilicity of CLA and,thus,help the localization of the drug in the phospholipidrich phase.Moreover,the broadening of the hydroxyl band and the disappearance of the ketone band in the spectrum of the complex compared with its physical mixture indicated the formation of a hydrogen bond between CLA and CHEMS. Consequently,it was concluded that for CLE,the H-bonding and hydrogen-bonded ion pair interaction between CLA and CHEMS(Fig.3)effectively“l(fā)ocked”CLA in the interfacial flm, thereby reducing the injection pain by decreasing the amount of free drug in the aqueous phase.Therefore,it is feasible to increase the CLA stability in the lipid emulsion by H-bonding and formation of a CLA–CHEMS hydrogen-bonded ion pair complex.These results confrm the optimal formulation composition of CLE.
3.2.3.1H NMR analysis
The1H NMR spectra of CLA,CHEMS and CLA–CHEMS complex (molar ratio 1:2)are shown in Fig.4.The signal of the proton from the hydroxyl group of CHEMS(12.27 ppm)was shifted upfeld in the CLA–CHEMS complex(9.32 ppm),which indicated the formation of N-H.These data suggested coordinate bond formation between CLA and CHEMS,which offered further evidence for the formation of HIP after FT-IR spectroscopy.
3.2.4.Possible formation mechanism of H-bonding and HIP CLA–CHEMS complex(HHIPC)
Different from the other studies,the CLA–CHEMS ion pair complex was prepared in anhydrous ethanol,which is a solvent with low toxicity and a high dielectric constant.It is well known that solvents with a low dielectric constant can contribute to the formation of ion-pair,since in solvents with a high dielectric constant,the interionic attraction between ion pair might be weakened due to the formation of hydrogen bonds with solvent molecules.However,results showed that the interaction between CLA and CHEMS was strong enough to form a hydrogen-bonded ion pair and keep it stable in a solvent with a high dielectric constant.Ion pairing is a chemical approach to make ionized drugs and counter ions interact with each other in solvents to form a distinct chemical entity through Coulomb attraction.The electrostatic force is described by Coulomb’s law as follows:
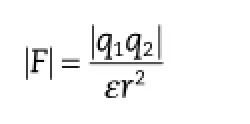
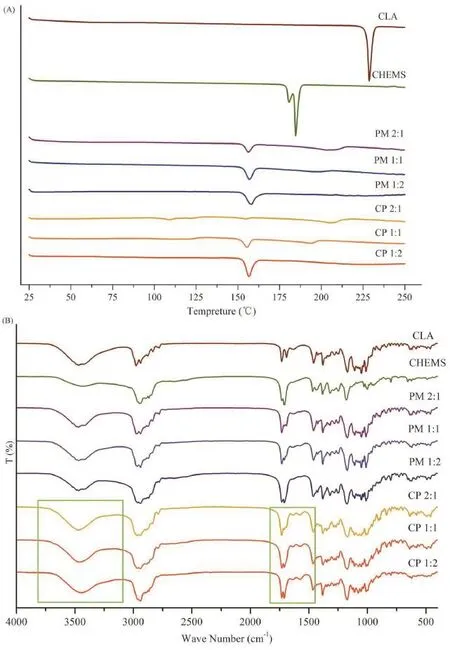
Fig.2–DSC thermograms(A)of CLA,CHEMS,different molar radios of physical mixture(PM)and complex(CP).FI-IR spectra (B)of CLA,CHEMS,different molar radios of physical mixture(PM)and complex(CP).
where F is the force of attraction,q1and q2are the magnitudes of the electrical charges,ε is the dielectric constant of the medium,and r is the distance between the ions.It could be speculated that with regard to the CLA–CHEMS ion pair complex,q1,q2,and ε were constant,while the distance between the cation and anion was variable.It might be that in theprocess of ion pair complex preparation,the attraction force increased when the distance between CLA and CHEMS was reduced.As shown in Fig.5A,when CLA and CHEMS were dissolved in anhydrous ethanol,both ions underwent complete solvation.Then,with the evaporation of the solvent,CLA and CHEMS became closer and shared about one molecule of solvent.Finally,CLA and CHEMS interacted with each other to form an HHIPC.The whole preparation process of CLE is described in Fig.5B.Similarly,in the case of CLE,CLA,CHEMS and PL-100M were dissolved in anhydrous ethanol,and an HHIPC with improved lipophilicity was possibly formed and intercalated between the acyl hydrocarbon chains of the phospholipids after removal of the solvent(Fig.5A).When adding oil to the drug-containing water phase,the lipid bilayer is converted to a monolayer which stabilizes the formed oil-inwater(o/w)structure.After high pressure homogenization and thermal sterilization,a re-emulsifying and re-constituting lipid emulsion is obtained.No globules in the micron range were found in this system following microscopic observations in more than 20 felds,indicating that the prepared CLE was safe for intravenous administration.Consequently,CHEMS (CLA:CHEMS,M ratio 1:2)was suitable for the preparation of a stable CLE.
To verify whether the CLA–CHEMS ion-pair was located in the lecithin-rich interfacial flm,ultracentrifugation was used to separate different phases in the CLE.By analyzing the CLA content(%)in the oil phase and water phase,the CLA content (%)in the interfacial flm was obtained by subtracting them from the total CLA content.Due to the formation of HHIPC, the CLA contents in the oil phase or water phase were less than 2%and about 10%,respectively,indicating that approximately 88%CLA was located in the interfacial layer. Simultaneously,a small quantity of CLA with improved lipophilicity could also distribute in the oil phase during the process of high speed shearing and high pressure homogenization.
3.3.Stability test
3.3.1.Thermal sterilization test
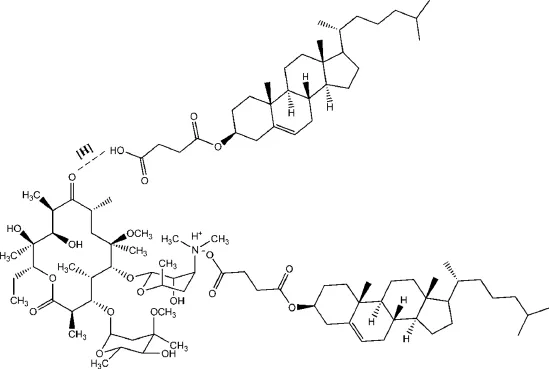
Fig.3–The schematic representation of H-bonding and HIP CLA–CHEMS complex formation.
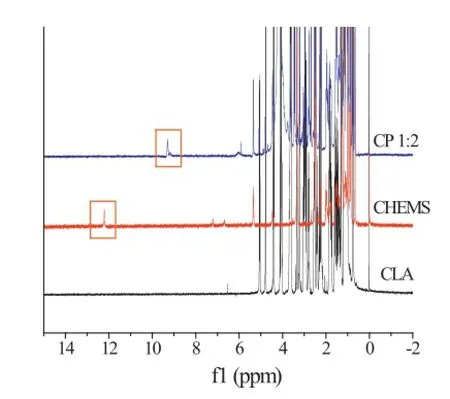
Fig.4–The1H NMR spectra of CLA,CHEMS and complex (CP,molar ratio 1∶2).
The physical stability of emulsions is the primary concern during terminal autoclaving,because it is a prerequisite for developing stable formulations[30].In general,the methods of sterilization are mainly dependent on the nature and excipients of the preparation,and for lipid emulsions,the preferred and reliable sterilization process is steam sterilization.As for thermal sterilization,temperature and time play important rolesin the destruction of microorganisms by heat.Also,it has been reported that thermal sterilization might cause irreversible redistribution of emulsifer compounds and more rapid Brownian motion,which affects the physical and chemical stability of lipid emulsions[31].So,the PSD,polydispersity index(PI),drug loading content,pH value,and physical appearance of lipid emulsions before and after sterilization were chosen as the main parameters for evaluation.
The infuence of the sterilization methods on the properties of the lipid emulsion is presented in Table 4.From these results it can be clearly seen that sterilization in a 100°C rotating water bath for 30 min and 45 min,and autoclaving at 115°C for 30 min,led to a markedly wider PI compared with the pristine lipid emulsions.The dramatic changes in particle size indicated droplet aggregation during these sterilization processes.Autoclaving at 115°C for 30 min even led to a marked reduction in drug content.It should be noted that heating would also induce the degradation of phospholipids,producing free fatty acids or lysophosphatides,which might cause toxicity and reduce the pH.As shown in Table 4,there was a decrease in pH in emulsions sterilized by methods ii–iv.Since CLA is liable to degrade in an acidic environment,it is necessary to keep the pH within an acceptable range.Also,these preparations displayed poor stability with visible oil droplets appearing after10 d of storage at 25°C.However,the formulation exhibited good stability when autoclaved at 121°C for 8 min and 10 min,indicating the superiority of autoclaving at 121°C over other sterilization methods.It is well known that a higher sterilization temperature and a shorter sterilization time are preferable when the sterilizing effects are similar on account of the lower activation energy of drug degradation.To ensure a better sterilization effect,autoclaving at 121°C for 10 min was selected as the optical sterilization method.
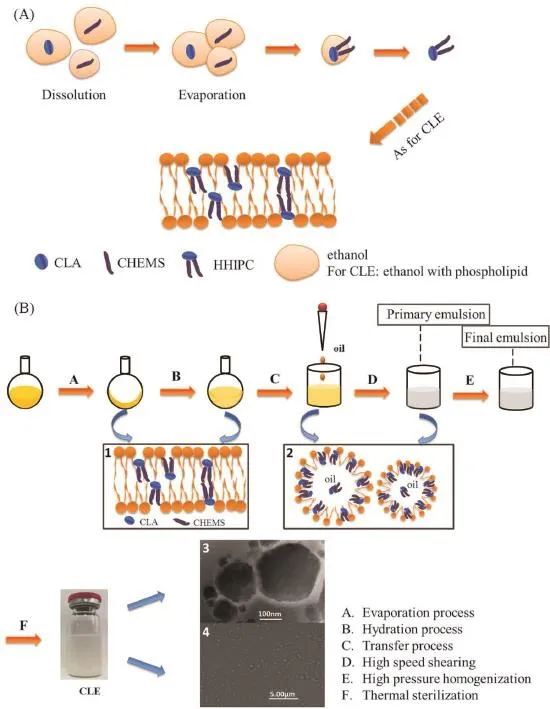
Fig.5–Possible formation mechanism(A)of H-bonding and HIP CLA–CHEMS complex.The whole preparation process(B)of clarithromycin lipid emulsion(CLE).(1)Possible structure of CLA,CHEMS and phospholipid after evaporation or in water phase.(2)Possible structure of CLE.(3)TEM image of CLE.(4)Microscopic observations of CLE.
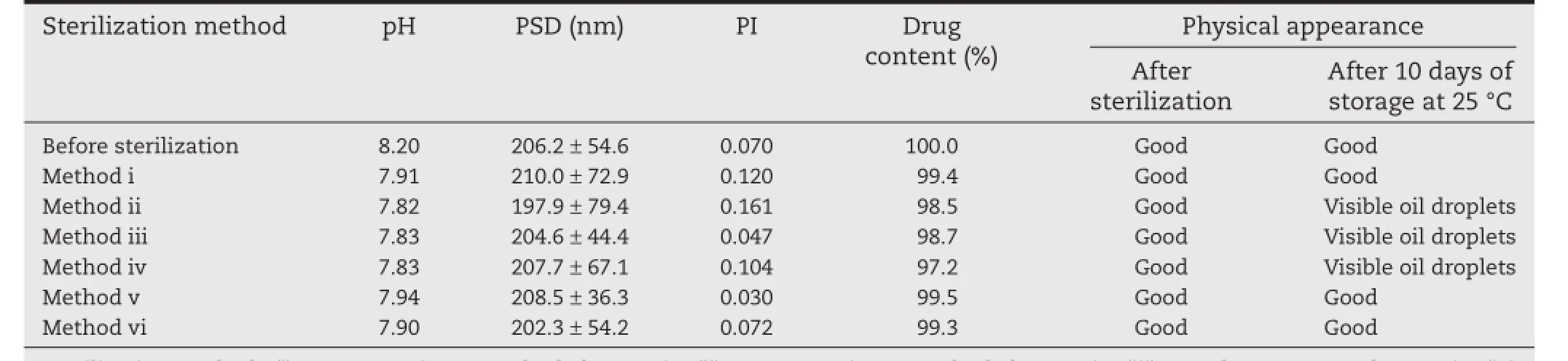
Table 4–The effect of different sterilization methods on the characterization of CLE.
3.3.2.Freeze–thaw stability test
The effect of environmental stresses,such as freeze–thawing treatment,was studied.For lipid emulsions,crystallization of the oil or aqueous phase and droplet coalescence might occur during freezing and thawing[32].The PSD and physical appearance were recorded after each freeze–thawing cycle to characterize the stability of the CLE.No marked change in particle size was observed after the process of freeze–thawing (Fig.6),indicating the good freeze–thawing stability of the CLE, which was ascribed to the thick interfacial membrane formed by PL-100M and CHEMS that was able to protect the lipid emulsion droplets from coalescence under extreme conditions. Hence,a formulation of 1:2 CLA and CHEMS with 2.0%PL-10M seems to be an attractive formulation with satisfactory properties.
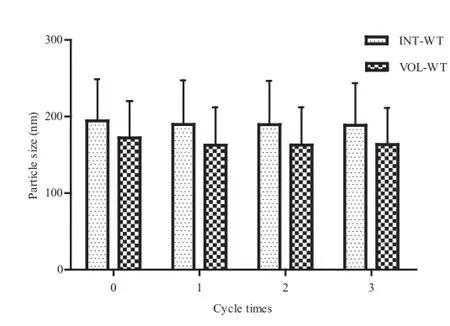
Fig.6–Particle size with INT-WT and VOL-WT after each freeze–thawing cycle.INT-WT∶intensity weighting;VOLWT∶volume weighting.
3.4.Microbiology testing
3.4.1.Susceptibility test
Thein vitroantibacterial activity of the CLE and HHIPC was tested by the MIC method,and the MICs of the CLE and HHIPC were compared with that of the parent drug and drug-free lipid emulsions againstStaphylococcus aureus,Streptococcus pneumoniae,Haemophilus infuenzae,andPseudomonas aeruginosa.As expected,blank lipid emulsions exhibited no antibacterial activity against all the tested strains.The results showed that the MICs of the formulations tested were similiar(Table 5),which suggested that HHIPC and CLE exhibited similiar activity to the parent drug against different bacterial strains,either sensitive or resistant to CLA,while HHIPC appeared to be more active than CLA againstHaemophilus infuenzae.From these results it could be confrmed that the formation of HHIPC and the preparation of the CLE did not infuence drug permeability and transport through biological membranes[33].Based on these results,further research onin vivoantibacterial activity was conducted.
3.4.2.Mouse model of sepsis
The results of CLA oral administration and CLE intravenous administration in sepsis studies are shown in Fig.7A and Fig.7B. ForStaphylococcus aureus,the 50%effective dose(ED50)of CLE was 1.929 mg/kg(95%confdence interval,1.310~2.843 mg/ kg),which was much more effcacious than CLA with an ED50of 12.941 mg/kg(95%confdence interval,8.296~20.187 mg/ kg).Similar observations were obtained in the sepsis mouse model of infection due toStreptococcus pneumoniae;the ED50of CLE was 0.678 mg/kg(95%confdence interval, 0.442~1.040 mg/kg),which was superior to that of CLA with an ED50of 2.406 mg/kg(95%confdence interval,1.709~3.387 mg/ kg).So,it appeared that forStreptococcus pneumoniae,CLE was 3 to 4 times more activein vivothan CLA,and forStaphylococcus aureus,CLE administrated intravenously was at least six-fold more potent than CLA given orally.The difference between CLA and CLE was determined by the BLISS method (P<0.05).
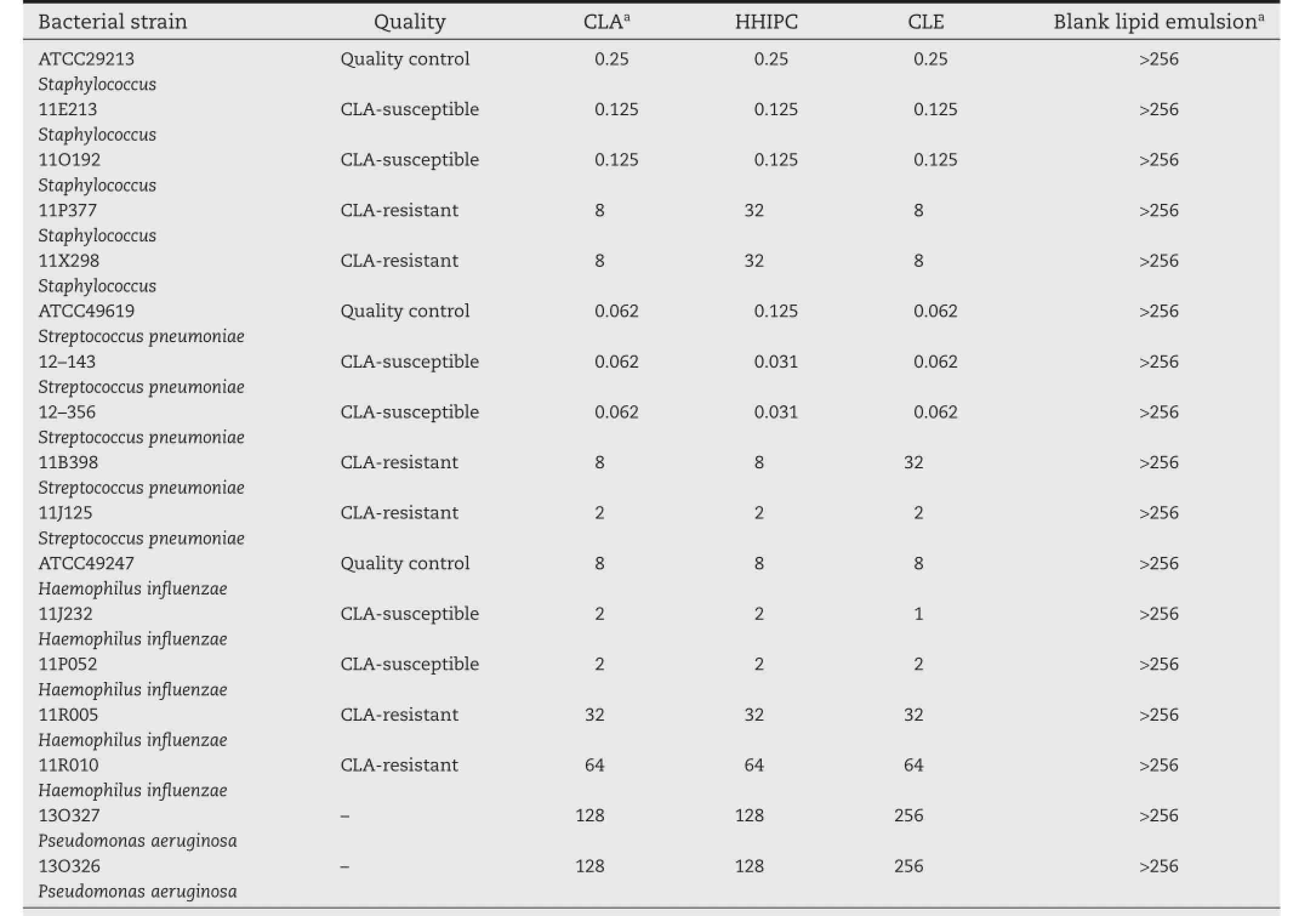
Table 5–In vitroantibacterial activities(MIC,mg/l).
Sepsis is a whole-body infammatory response commonly triggered by bacterial infection.It has also been reported that CLA exhibits a range of immunomodulatory and antiinfammatory properties[34].The effcacy of CLA in sepsis might be attributed to its ability to inhibit the formation of a bacterial bioflm on the airways,as well as the production of various infammatory mediators by alveolar macrophages and blood monocytes[35].Several studies have reported that following intravenous administration,lipid emulsions are readily taken by mononuclear phagocyte systems,and then preferentially distributed within infammatory areas or other peripheral tissues[36].After infecting different groups of mice with bacterial suspensions,mononuclear phagocyte cells showed a tropism to the vaccination site.Therefore,CLE carried by mononuclear phagocyte cells could exert an effective therapeutic potency.This could also explain why CLA administered orally displayed poor antibacterial activity on account of low bioavailability.From these results it can be concluded that the administration route is important for the effcacy of CLA in the treatment of sepsis.The experiments conducted in our study here have successfully encapsulated antibiotics into lipid emulsion delivery system by formation of H-bonding and HIP CLA–CHEMS complexes.
4.Conclusions
The present study was carried out to investigate the formulation andin vivoantibacterial activity of an intravenous clarithromycin lipid emulsion(CLE)prepared by a thin-flm dispersed homogenization method.An excess of CHEMS(CLA: CHEMS,M ratio 1:2)helped the formation of H-bonding and an HIP CLA–CHEMS complex and improved the lipophilicity of CLA.This allowed CLA to be localized in the interfacial flm with less free drug partitioning in an aqueous milieu,thus improving patient compliance by reducing the irritation of CLA following injection.Antibacterial testing showed that the CLE exhibited marked antibacterial activityin vivo,which confrms that CLA offers great promise in clinical applications.
Acknowledgments
We are grateful for assistance in the microbiology testing provided by Peking University First Hospital.Dr.David B Jack is gratefully thanked for correcting the English language of the manuscript.
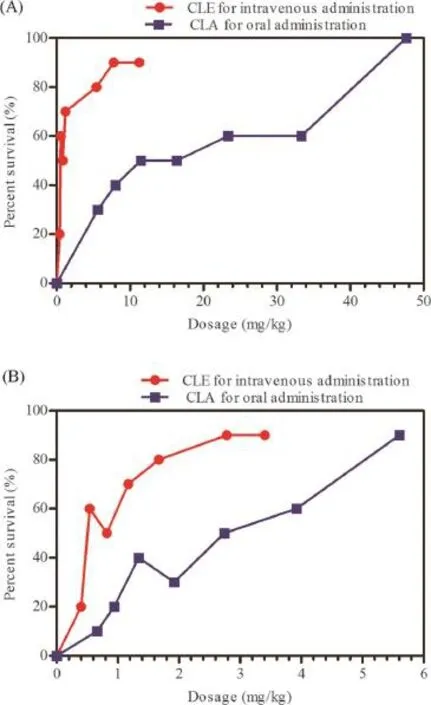
Fig.7–In vitroantibacterial activities(A)of CLE and CLA in a mouse model of sepsis caused byStreptococcus pneumoniae.In vivoantibacterial activities(B)of CLE and CLA in a mouse model of sepsis caused byStaphylococcus.
R E F E R E N C E S
[1]Nakagawa Y,Itai S,Yoshida T,et al.Physicochemical properties and stability in the acidic solution of a new macrolide antibiotic,clarithromycin,in comparison with erythromycin.Chem Pharm Bull 1992;40:725–728.
[2]Chaisson RE,Benson CA,Dube MP,et al.Clarithromycin therapy for bacteremicMycobacterium aviumcomplex disease.A randomized,double-blind,dose-ranging study in patients with AIDS.AIDS Clinical Trials Group Protocol 157 Study Team.Ann Intern Med 1994;121:905–911.
[3]Hewitt RG,Papandonatos GD,Shelton MJ,et al.Prevention of disseminatedMycobacterium aviumcomplex infection with reduced dose clarithromycin in patients with advanced HIV disease.AIDS 1999;13:1367–1372.
[4]Salvador A-E,Mark JE.The macrolides:erythromycin, clarithromycin,and azithromycin.Mayo Clin Proc 1999;74:613–634.
[5]Cannon JB,Williams NA,Papp KJ.Reduction of pain on intravenous infusion with bile salt formulations for a macrolide antibiotic.Int J Pharm 1995;114:65–74.
[6]Salem II,Düzgünes N.Effcacies of cyclodextrin-complexed and liposome-encapsulated clarithromycin againstMycobacterium aviumcomplex infection in human macrophages.Int J Pharm 2003;250:403–414.
[7]Liu X,Sun Y,Zhang Y,et al.Preparation andin vitro-in vivoevaluation of silybin lipid microspheres.Asian J Pharm Sci 2007;2:204–210.
[8]Lovell MW,Johnson HW,Hui HW,et al.Less-painful emulsion formulations for intravenous administration of clarithromycin.Int J Pharm 1994;109:45–57.
[9]Yan L,Wang YJ,Xing T.Formulation and thermal sterile stability of a less painful intravenous clarithromycin emulsion containing vitamin E.Int J Pharm 2008;346: 47–56.
[10]Jie L,Nie S,Yang X,et al.Optimization of tocol emulsions for the intravenous delivery of clarithromycin.Int J Pharm 2008;356:282–290.
[11]Mohammadi G,Nokhodchi A,Barzegar-Jalali M,et al. Physicochemical and anti-bacterial performance characterization of clarithromycin nanoparticles as colloidal drug delivery system.Colloids Surf B Biointerfaces 2011;88:39–44.
[12]Lee JM,Park KM,Lim SJ,et al.Microemulsion formulation of clonixic acid:solubility enhancement and pain reduction.J Pharm Pharmacol 2002;54:43–49.
[13]Larsen B,Beerhalter U,Biedler A,et al.Less pain on injection by a new formulation of propofol?A comparison with propofol LCT.Anaesthesist 2001;50:842–845.
[14]Castro GA,Coelho AL,Oliveira CA.Formation of ion pairing as an alternative to improve encapsulation and stability and to reduce skin irritation of retinoic acid loaded in solid lipid nanoparticles.Int J Pharm 2009;381:77–83.
[15]Zhang T.A novel submicron emulsion system loaded with vincristine-oleic acid ion-pair complex with improved anticancer effect:in vitroandin vivostudies.Int J Nanomedicine 2013;8:1185–1196.
[16]Pignatello R,Mangiafco A,Ruozi B,et al.Amphiphilic erythromycin-lipoamino acid ion pairs:characterization andin vitromicrobiological evaluation.AAPS PharmSciTech 2011;12:468–475.
[17]Ratajczak H.Charge-transfer properties of the hydrogen bond.I.Theory of the enhancement of dipole moment of hydrogen-bonded systems.J Phys Chem 1972;14:3000–3004.
[18]Wikler MA.Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically:approved standard. Wayne,PA:Clinical&Laboratory Standards Institute;2009.
[19]Griffth DC,Laurie H,Robert W,et al.In vivoantibacterial activity of RWJ-54428,a new cephalosporin with activity against gram-positive bacteria.Antimicrob Agents Chemother 2003;47:43–47.
[20]Fscoll D,Nehne J,Peterss H,et al.The infuence of mediumchain triglycerides on the stability of all-in-one formulations.Int J Pharm 2002;240:1–10.
[21]Klein RA.The detection of oxidation in liposome preparations.Biochim Biophys Acta 1970;210:486–489.
[22]Waitzberg DL,Lotierzo PH,Logullo AF,et al.Parenteral lipid emulsions and phagocytic systems.Br J Nutr 2002;87:S49–S57.
[23]Lima LA.Neonatal parenteral nutrition with medium-chain triglycerides:rationale for research.JPEN J Parenter Enteral Nutr 1989;13:312–317.
[24]Yamaguchi T,Nishizaki K,Itai S,et al.Physicochemical characterization of parenteral lipid emulsion:determination of Hamaker constants and activation energy of coalescence. Pharm Res 1995;12:342–347.
[25]Shao Y,Zhang C,Yao Q,et al.Improving cabazitaxel chemical stability in parenteral lipid emulsions using cholesterol.Eur J Pharm Sci 2014;52:1–11.
[26]Ma J,Teng H,Wang J,et al.A highly stable norcantharidin loaded lipid microspheres:preparation,biodistribution and targeting evaluation.Int J Pharm 2014;473:475–484.
[27]Floyd AG.Top ten considerations in the development of parenteral emulsions.Pharm Sci Technol Today 1999;2:134–143.
[28]Vercellotti GM,Hammerschmidt DE,Craddock PR,et al. Activation of plasma complement by perfuorocarbon artifcial blood:probable mechanism of adverse pulmonary reactions in treated patients and rationale for corticosteroids prophylaxis.Blood 1982;59:1299–1304.
[29]Ingram DA,Forman MB,Murray JJ.Activation of complement by Fluosol attributable to the pluronic detergent micelle structure.J Cardiovasc Pharmacol 1993;22:456–461.
[30]Chaturvedi PR,Patel NM,Lodhi SA.Effect of terminal heat sterilization on the stability of phospholipid-stabilized submicron emulsions.Acta Pharm Nord 1992;4:51–55.
[31]Herman CJ,Groves MJ.Hydrolysis kinetics of phospholipids in thermally stressed intravenous lipid emulsion formulations.J Pharm Pharmacol 1992;44: 539–542.
[32]Gu YS,Decker EA,McClements DJ.Application of multicomponent biopolymer layers to improve the freeze–thaw stability of oil-in-water emulsions:β-Lactoglobulin–ιcarrageenan–gelatin.J Food Eng 2007;80:1246–1254.
[33]Matschiner S,Neubert R,Wohlrab W,et al.Infuence of ion pairing onex vivopenetration of erythromycin into sebaceous follicles.Skin Pharmacol 1996;9:270–273.
[34]Tkalcevic VI,Bosnjak B,Pasalic I,et al.The antiinfammatory activity of clarithromycin inhibits TNFα production and prolongs survival following lipopolysaccharide administration in mice.Int J Antimicrob Agents 2008;32:195–196.
[35]Giamarellos-Bourboulis EJ.Immunomodulatory therapies for sepsis:unexpected effects with macrolides.Int J Antimicrob Agents 2008;32(Suppl.1):S39–S43.
[36]Sharma S,Shukla P,Misra A,et al.Chapter 8–interfacial and colloidal properties of emulsifed systems: pharmaceutical and biological perspective.In:Colloid and interface science in pharmaceutical research and development.Elsevier B.V.;2014.p.149–172.
*< class="emphasis_italic">Corresponding author.
.Shenyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China.Fax:+86 24 23911736.E-mail address:tanglab@126.com(X.Tang).
Abbreviations:CFU,colony-forming units;CHEMS,cholesteryl hemisuccinate;CHO,cholesterol;CLA,clarithromycin;CLE,clarithromycin lipid emulsion;CP,complex;DDW,double-distilled water;DMSO,deuterated dimethyl sulfoxide;ED50,50%effective dose;ELS,electrophoretic light scattering;HHIPC,H-bonding and HIP CLA–CHEMS complex;HIP,hydrogen-bonded ion pair;HTM,Haemophilus test medium; MAC,Mycobacterium aviumcomplex;MCT,medium chain triglyceride;MHA,Mueller-Hinton agar;MIC,minimal inhibitory concentration;MLD,minimum lethal dose;LCT,long chain triglyceride;PA,phosphatidic acid;PC,phosphatidylcholine;PCS,photon correlation spectroscopy;PI,phosphatidylinositol;PI,polydispersity index;PM,physical mixture;PS,phosphatidylserine;PSD,particle size distribution;TMS,tetramethylsilane;TS,tocopherol succinate.
http://dx.doi.org/10.1016/j.ajps.2016.04.002
1818-0876/?2016 Shenyang Pharmaceutical University.Production and hosting by Elsevier B.V.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年5期
Asian Journal of Pharmacentical Sciences2016年5期
- Asian Journal of Pharmacentical Sciences的其它文章
- Quantitative determination of metaxalone in human plasma by LC-MS and its application in a pharmacokinetic study
- Effects of duration of phenytoin administration on mRNA expression of cytochrome P450 and P-glycoprotein in the liver and small intestine of rats
- Effect of surface ligand density on cytotoxicity and pharmacokinetic profle of docetaxel loaded liposomes
- Stability of freeze-dried pH-responsive dextrin nanogels containing doxorubicin
- Evaluation of pharmacokinetics underlies the collaborated usage of lamivudine and oxymatrine in beagle dogs
- A hydrophobic peptide fraction that enhances the water dispersibility of curcumin
