Evaluation of pharmacokinetics underlies the collaborated usage of lamivudine and oxymatrine in beagle dogs
Zhenbo Li,Lei Shng,Chng Liu,Pnqin M,Chunnun Wu, Wenjun Zhng,Yinghu Sun,Yongjun Wng,Xiohong Liu,*,Jin Sun,e,*
aSchool of Pharmacy,Shenyang Pharmaceutical University,No.103 Wenhua Road,Shenyang 110016,China
bSchool of Pharmacy,China Medical University,No.77 Puhe Road,Shenyang North New Area,Shenyang 110122,China
cKangya of Ningxia Pharmaceuticals Co.Ltd,No.57 Fuan West Lane,Yinchuan 750002,China
dTianjin Medical University Cancer Hospital,Tianjin 300060,China
eMunicipal Key Laboratory of Biopharmaceutics,School of Pharmacy,Shenyang Pharmaceutical University,No. 103 Wenhua Road,Shenyang 110016,China
Evaluation of pharmacokinetics underlies the collaborated usage of lamivudine and oxymatrine in beagle dogs
Zhenbao Lia,1,Lei Shangb,1,Chang Liua,Panqin Mac,Chunnuan Wud, Wenjuan Zhanga,Yinghua Suna,Yongjun Wanga,Xiaohong Liua,*,Jin Suna,e,*
aSchool of Pharmacy,Shenyang Pharmaceutical University,No.103 Wenhua Road,Shenyang 110016,China
bSchool of Pharmacy,China Medical University,No.77 Puhe Road,Shenyang North New Area,Shenyang 110122,China
cKangya of Ningxia Pharmaceuticals Co.Ltd,No.57 Fuan West Lane,Yinchuan 750002,China
dTianjin Medical University Cancer Hospital,Tianjin 300060,China
eMunicipal Key Laboratory of Biopharmaceutics,School of Pharmacy,Shenyang Pharmaceutical University,No. 103 Wenhua Road,Shenyang 110016,China
A R T I C L EI N F O
Article history:
Received 3 December 2015
Received in revised form 1 January 2016
Accepted 5 February 2016
Available online 16 February 2016
Oxymatrine
Lamivudine
Matrine
Beagle dog
Intravenous/oral regime
Combinational therapy of lamivudine and oxymatrine has been employed in the battle against hepatitis B virus in clinical setting.However,the pharmacokinetic behavior of the drug or active metabolism in intravenous/oral co-administration regime is poorly investigated.Herein, we evaluated the pharmacokinetic characteristic through a tailor-designed 3 way crossover-Latin square experiment in adult male beagle dogs.Six dogs were randomly treated by intravenous administration of lamivudine(2.5 mg/kg),oxymatrine(15 mg/kg)and combinational dosage,named as intravenous regime.Meanwhile the other six dogs were orally administrated with lamivudine(2.5 mg/kg),oxymatrine(15 mg/kg)and combinational dosage, named as oral regime.The pharmacokinetic feature in simultaneous oral treatment appeared to have no signifcant difference when compared with individual administration, even including matrine,the active metabolite of oxymatrine.In intravenous regime,the main pharmacokinetic parameters of simultaneous administration were nearly consistent with intravenous regime remedy.The collaborated application of lamivudine and oxymatrine contributed to non-distinctive pharmacokinetic fuctuations of beagle dogs in intravenous/ oral regime,compared with individual employment,which established a vital base for the clinical co-administration against hepatitis B.Furthermore,the present study demonstrated that the determination of pharmacokinetics between combinational and individual therapy might assist in the development of drug compatibility in clinical therapy.
?2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
More than 240 million individuals around the world have suffered from chronic hepatitis B virus(HBV)infection,which is a DNA virus characterized by its reverse transcription for replication in infected hepatocytes[1–4].At present,the therapy options mainly depend on nucleotide analogues(NA)and interferon alpha(INF-α)[5].Lamivudine(3TC,Fig.1A),possessing anti-HBV replicated function,was the frst approved oral NA. However,3TC-resistance phenomena,such as viral drugresistance and dose-dependent side effects[6],will be likely to emerge in the long-term treatment when using 3TC alone due to the emergence of drug resistance mutations in polymerase protein.To deal with the challenge,co-administrated therapeutic strategy combining 3TC with other antiviral drugs appeared[7–9].Furthermore,the collaborated employment of lamivudine and oxymatrine could signifcantly decrease the conversion rate of HBVDNA and hepatitis B e antigen(HBeAg) in patients when compared with mono-therapy of 3TC because of the ability of oxymatrine to inhabit the development of drug resistance to 3TC[6,10–13].
Oxymatrine(OM,Fig.1B)is a major quinolizidine alkaloid from the Chinese herbSophora alopecuraides L.,Sophora subprostrataandSophora favescens Ait.,and has been extensively used as liver protecting drugs in the treatment of liver ailments in traditional Chinese medicines(TCM)[14].OM and its active metabolite matrine(M,Fig.1C)have been proven to have an active antiviral effect on HBV infection in the clinical trials[6,8,11,15,16].And Xiaoyan Cui reported the superiority of the combination of 3TC with oxymatrine or matrine over 3TC alone[6].
Although the single use of 3TC to treat HBV has largely been replaced by more effective combination with OM,which has been demonstrated previously in the literature,the impact of combination therapy on the pharmacokinetics of each drug or its active metabolite is largely unknown[6].Herein,the present study is carried out to investigate the effects of OM on the pharmacokinetics of 3TC and to characterize the pharmacokinetic behavior of OM and 3TC during co-administration to beagle dogs following intravenous or oral administration.Whether or not there are signifcant pharmacokinetic interactions,the results of which will assist in the development of this twodrug combination in clinical therapy strategy.
2.Materials and methods
2.1.Chemicals
3TC(content>98.5%)was purchased from Hefei Scenery Chemical Co.,Ltd.(Hefei,Anhui,China).Standard OM,M and famotidine(internal standard)were supplied by the National Institute for the Control of Pharmaceutical and Biological Products(Beijing,China).Bulk OM(content>98.5%)was purchased from Ningxia Bo-er-tai-li Pharmaceutical Co.Ltd.(Yinchuan, Ningxia,China).Acetonitrile was of HPLC grade and other chemicals were of analytical grade.
2.2.Animals
Twelve healthy adult male beagle dogs(Laboratory Animal Center of Shenyang Pharmaceutical University,Shenyang,Liaoning,China)weighing 10±1.3 kg(mean±standard error)were used for the pharmacokinetic study.The animal experimental protocols described below were approved by theAnimal Care and Use Committee at Shenyang Pharmaceutical University.
2.3.Pharmacokinetic experiments
A randomized 3-way crossover-Latin square experiment with a washout period of 1 week was designed.Dogs(n=6)were randomly assigned to groups to characterize the pharmacokinetics and interaction of 3TC(2.5 mg/kg)and OM(15 mg/ kg)given intravenously alone and in combination.Another six dogs were used to characterize the pharmacokinetics and interaction of 3TC(5.0 mg/kg)and OM(30 mg/kg)given orally alone and in combination.The dogs were housed in standard stainless-steel cages under a 12 h light/dark cycle with access to water and standard laboratory diet.The drugs were administered to all dogs following an overnight fast,and access to food was restored 4 h after dosing.In the intravenous administration study,blood samples were collected prior to drug administration and at 2,5,10,15,30,45,60,90,120,150,180, 240,360 and 480 min after dosing through an intravenous catheter placed in the opposite foreleg vein.In the oral administration study,blood samples were collected prior to drug administration and at 10,20,30,45,60,90,120,150,180,240, 300,360,540 and 720 min after dosing through an intrave-nous catheter placed in the foreleg vein.The blood samples were centrifuged immediately,and plasma was stored at–20°C until analysis.
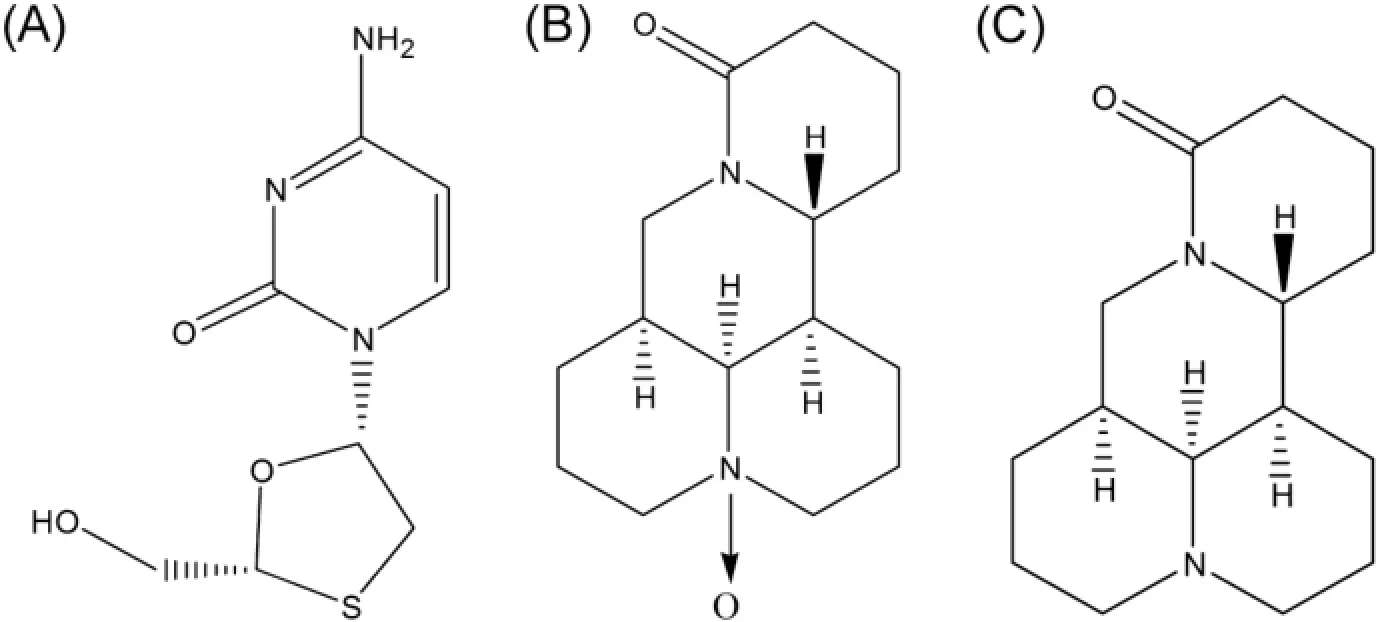
Fig.1–Structures of 3TC,OM and M(A-3TC;B-OM;C-M).
2.4.Assay of 3TC,OM and M
The 3TC,OM and M concentrations in dog plasma were determined by means of the HPLC-UV method reported.Briefy, the analytes and internal standard were simultaneously extracted from plasma samples with OASIS?HLB Extraction Cartridges(1 cc,30 mg,Waters,Corp.),and separated on a C18column with an isocratic mobile phase consisting of 13%acetonitrile in 5 mmol/l sodium heptanesulfonate with the pH adjusted to 3.2 with phosphoric acid.The UV absorbance was monitored at 220 nm,and the column temperature was maintained at 40°C.The linear calibration curves of the analytes were obtained in the concentration range of 0.1~40 mg/l (r2>0.999).The lower limit of quantifcation of the method was 0.1 mg/l for these analytes.The method was evaluated in terms of recovery,accuracy and precision.The recoveries extraction of 3TC,OM and M was≥70%.Accuracy and precision for the determination fell well within the limits of acceptability(<15%).
2.5.Pharmacokinetic analysis
The maximum concentration(Cmax)and corresponding peak time(Tmax)were determined by the inspection of the individual drug plasma concentration–time profles.The elimination rate constant(ke)was obtained from the least-squares ftted terminal log-linear portion of the plasma concentration–time profle.The elimination half-life(t1/2)was calculated from 0.693/ke.The area under the curve from zero to infnity(AUC0~∞) was calculated using the trapezoidal rule with extrapolation to infnity withke.The mean residence time from zero to infnity(MRT)was estimated by moment analysis.The total clearance from plasma(CL)was calculated as dose/AUC0~∞.The steady-state volume of distribution(Vss)was calculated using (dose×AUMC0~∞)/(AUC0~∞)2.The relative bioavailability(Fr)was calculated as AUCco-administration/AUCalone.The oral bioavailability (F)was calculated using(AUCp.o.×dosei.v.)/(AUCi.v.×dosep.o.).
2.6.Statistical analysis
Data are expressed as the mean±standard error(SE).Comparisons between co-administration and single-administration groups were performed using the pairwise Student’s two onesidedt-test,and differences were considered statistically signifcant when P<0.05.
3.Results and discussion
3.1.Pharmacokinetics of 3TC
The plasma concentrations of 3TC were measured by the validated UPLC method as reported in our previous study.Fig.2 shows the mean plasma concentration–time profles for 3TC after either oral or intravenous administrations of 3TC,alone and in combination with OM.The pharmacokinetic parameters for 3TC are demonstrated in Table 1.The absorbed fractions of 3TC,a ratio of the AUCs for single oral or cocombination of OM oral administration to single intravenous administrations,were 80.5%and 73.3%,respectively.There were no statistically signifcant differences in the main pharmacokinetic parameters of 3TC between mono-therapy and coadministration with OM after either oral or intravenous administrations,despite the appearance of a 13.4%increase in the intravenousAUC0~∞(from 6.35±1.62 to 5.50±0.68 μg·h/ ml)(P>0.05).The results indicated no pharmacokinetic changes in 3TC following oral or intravenous administration when in combination with OM to beagle dogs.
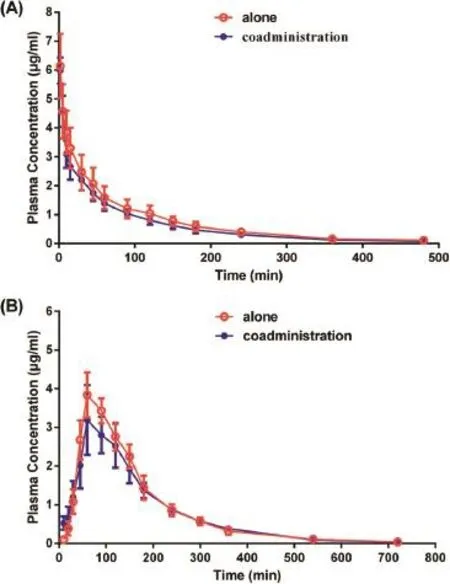
Fig.2–(A)Mean plasma 3TC concentration–time curves following two intravenous regimens involving a single dose of 2.5 mg/kg 3TC to beagle dogs∶alone(red line)and in combination with a single dose of 15 mg/kg OM(blue line).(B)Mean plasma 3TC concentration–time curves following two oral regimens involving a single dose of 5.0 mg/kg 3TC to beagle dogs∶alone(red line)and in combination with a single dose of 30 mg/kg OM(blue line). Each value represents the mean±SE(n=6).
3.2.Pharmacokinetics of OM
The plasma concentrations of OM were measured by the validated UPLC method as reported by Piao et al.[17].The mean pharmacokinetic parameters of OM are summarized in Table 2 and the corresponding mean plasma concentration–time profles for OM are illustrated in Fig.3.In the case of the pharmacokinetics of OM following intravenous mono-andco-administration with 3TC,the OM concentrations declined in a bi-exponential manner.Furthermore,the fractions of OM orally absorbed,determined by the ratio of AUCs,with and without oral 3TC,to intravenous administration alone were 30.9%and 26.3%,respectively.In spite of the approximate 10% differences illustrated in main pharmacokinetic parameters, no statistically signifcant pharmacokinetic interactions were observed following intravenous or oral co-administration of OM between mono-and co-administration(P>0.05).
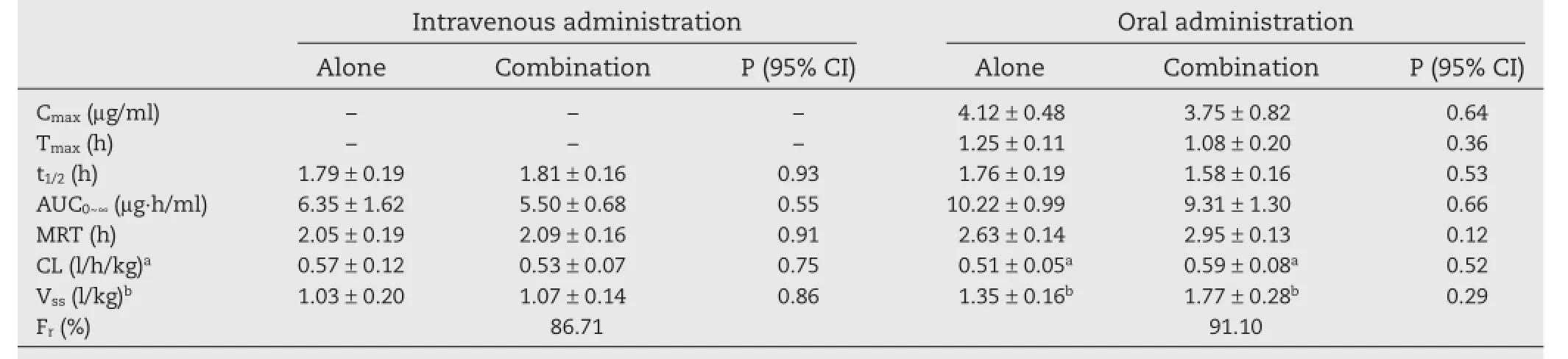
Table 1–Pharmacokinetics of 3TC following intravenous and oral administration alone and in combination with OM.
3.3.Pharmacokinetics of M
In the case of the active metabolite of OM,Fig.4 shows the mean plasma concentration–time profles of M after administration of OM alone and in combination with 3TC[18].The pharmacokinetic parameters for M are presented in Table 3. Following intravenous administration,the concentrations of M in plasma were generally lower than those of its parent compound.After oral administration,the Cmax(12.30±4.10 and 10.06±2.88 μmol/l)and AUC0~∞(56.31±19.13 and 50.64±8.25 μmol·h/l)were approximately four-fold higher than the intravenous Cmax(2.87±0.85 and 2.59±0.16 μmol/l)and AUC0~∞(6.64±1.59 and 7.90±2.23 μmol·h/l).As illustrated in Fig.4A, OM was immediately biotransformed into M,as the Cmaxwas reached in the frst collected sample.In oral regimen of OM alone or concomitantly with 3TC,the Tmaxvalues of M were 3.75±0.36 and 4.50±0.22 h,respectively,and the concentrations of M in plasma were higher than those of OM in the following time-point.The oral biotransformation rates of OM were 49.8%and 51.2%following single and combinational therapy,respectively.On the basis of the equimolecular transformation of OM into M[8,13],the absorbed total fractions of OM,calculated by the ratio of the AUCs of OM plus M for single oral and 3TC-containing oral administration to single intravenous administrations,were 57.4%and 50.3%,respectively. The difference in these pharmacokinetic parameters was evaluated to be statistically insignifcant(P>0.05),indicating that there were no pharmacokinetic interactions involving the metabolism of OM following both oral and intravenous administration in combination with 3TC.
3TC is not signifcantly metabolized and is eliminated primarily as unchanged drug via the kidneys.Approximately 5% to 10%of the parent compound is metabolized to the pharmacologically inactive trans-sulfoxide metabolites after a single oral dose[11].However,M,the metabolite of OM,is bioactive and represents a primary reduction pathway by intestinal bacteria and liver[12,13].So it is necessary to investigate simultaneously the pharmacokinetic behavior of M and OM after OM administration.
After mono-administration in this study,3TC was rapidly eliminated after both intravenous and oral administration,and had excellent oral bioavailability(Tmax=1.25 h,F=80.5%). However,compared with the fndings of Hussey et al.(CL=0.36 l/h/kg)[10],the higher values observed in our study(0.57±0.12 l/h/kg)are probably due to the exclusion of samples taken 8 h after intravenous dosing alone.Furthermore,the CL andVssfol-lowing intravenous mono-administration of 3TC were consistent with the allometric estimation according to CL,Vssand species body weight(W):CL=0.74×W0.76,Vss=1.09×W0.94.The estimates of CL andVss(0.48 l/h/kg and 0.95 l/kg,respectively)were similar to the observed values for dogs in this study(0.57±0.12 l/h/kg and 1.03±0.20 l/kg,respectively).
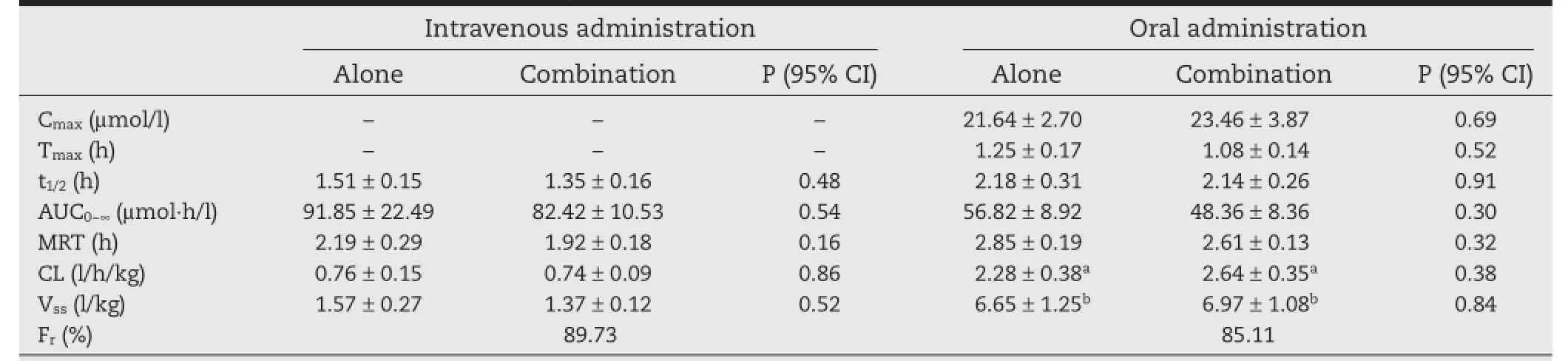
Table 2–Pharmacokinetics of OM following intravenous and oral administration alone and in combination with 3TC.
In our study,following intravenous administration of OM alone,theVssof 1.57±0.27 l/kg is slightly greater than the total body fuid of dogs,and larger than the value of 3TC(1.03 l/kg) in dogs.This suggests that OM may undergo intracellular distribution and freely penetrate tissues beyond the systemic circulation,and that the concentration of OM distributing in tissues might be higher than that of blood vessels.Meanwhile,the phenomenon of biotransformation from OM to M was also observed in our investigation,which was consistent with previous studies showing that OM and its metabolite M, after intramuscular injection,undergo wide tissue distribution in mice or rats,including the kidney,liver,lung,bone marrow,spleen,and heart[12].Additionally,OM is rapidly eliminated(t1/2=1.51±0.15 h)after intravenous administration and the oral bioavailability of OM plus M for single oral administration to single intravenous administration was about 57.4% in our investigation.
It was seen in the oral regimen that the estimate of OM transformed into M(49.8%)was in conformity with that obtained previously from a single-dose study with dogs(56.5%) [18].In addition,the bioconversion of M after intravenous administration of OM was less than 10%.The ratio of the AUC of M after intravenous dosing to oral dosing is roughly regarded as refection of the metabolism by liver compared to the sum of the metabolism by liver and gastrointestinal bacteria.Therefore,the results suggested that the reduction metabolism of OM by gastrointestinal bacteria was four times higher than that of liver and gastrointestinal bacteria is the main pathway of OM metabolism.In this study,it was also indicated that the relatively large individual variance of pharmacokinetic parameters of OM and that of M emerged. This may be attributable to the facts that OM is metabolized via hepatic and intestinal bacteria pathways,and that the hepatic function and the fora distribution are individualized.

Fig.3–(A)Mean plasma OM concentration–time curves following two intravenous regimens involving a single dose of 15 mg/kg OM to beagle dogs∶alone(red line)and in combination with a single dose of 2.5 mg/kg 3TC(blue line).(B)Mean plasma OM concentration–time curves following two oral regimens involving a single dose of 30 mg/kg to beagle dogs∶alone(red line)and in combination with a single dose of 5.0 mg/kg 3TC(blue line).Each value represents the mean±SE(n=6).
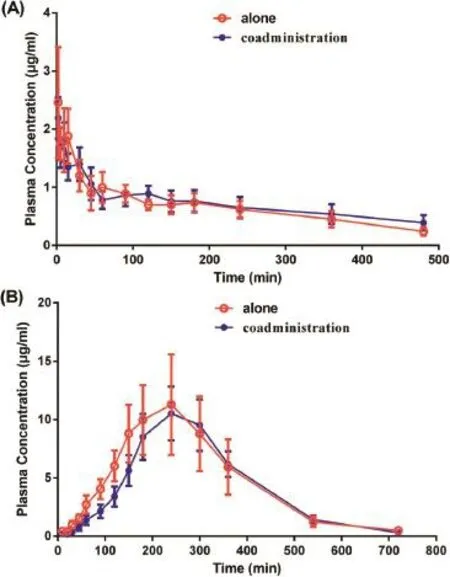
Fig.4–(A)Mean plasma M concentration–time curves following two intravenous regimens involving a single dose of 15 mg/kg OM to beagle dogs∶alone(red line)and in combination with a single dose of 2.5 mg/kg 3TC(blue line).(B)Mean plasma M concentration–time curves following two oral regimens involving a single dose of 30 mg/kg to beagle dogs∶alone(red line)and in combination with a single dose of 5.0 mg/kg 3TC(blue line).Each value represents the mean±SE(n=6).
The pharmacokinetic parameters of 3TC obtained from coadministration in this study with beagle dogs are consistentwith those obtained from mono-administration(P>0.05). Changes in certain issues,such as protein binding,absorption,distribution,metabolism and excretion,are the basis of many drug interactions.Changes in protein binding and metabolism would not signifcantly affect the distribution and elimination of 3TC,because its protein binding is low(<36%) and metabolism represents only a minor route of elimination.Approximately 5%to 10%of the parent compound is metabolized to the pharmacologically inactive metabolite[11,19]. 3TC is a highly soluble and permeable drug with a rapid dissolution rate.As a consequence,oral doses are rapidly absorbed by passive diffusion across the intestinal membrane[14].Furthermore,its relatively low molecular weight(229 D)and low plasma protein binding result in a wide distribution and free penetration into tissues beyond the systemic circulation[11]. OM and M are unlikely to be able to affect these passive and free penetration processes.Moreover,3TC is eliminated primarily unchanged drug via the kidneys by fltration and active renal tubular secretion,in part via the renal organic cation transport system[11].Although OM and M are eliminated partially via the kidney,this does not signifcantly affect either the extent of absorption or the elimination of 3TC,as indicated by an absence of signifcant changes in the AUC0~∞and t1/2of 3TC (P>0.05).These fndings agree with published results of drug interaction studies involving 3TC[11,12].
Furthermore,the pharmacokinetics of OM is not signifcantly affected by co-administration of 3TC.The low potential of OM for drug–drug interactions is similar to that of 3TC, because the properties of OM are fairly similar to those of 3TC, such as a low protein binding(29.36±4.17%),relatively low molecular weight(264 D),highly water soluble(more than 150 mg/ ml)character.In our laboratory,it has been shown that OM and M mainly undergo passive diffusion across Caco-2 cell membrane.Hence,it is little likely that there will be an interaction involving interference with the process of absorption.This assumption is also demonstrated by the Tmaxvalues of OM and 3TC(1.25 h for the single-regimen and 1.08 h for the coregimen,respectively)after oral administration.3TC did not markedly affect either the extent of distribution or the elimination of OM,as shown by a lack of any signifcant change in the Vssand CL of OM,respectively(P>0.05).
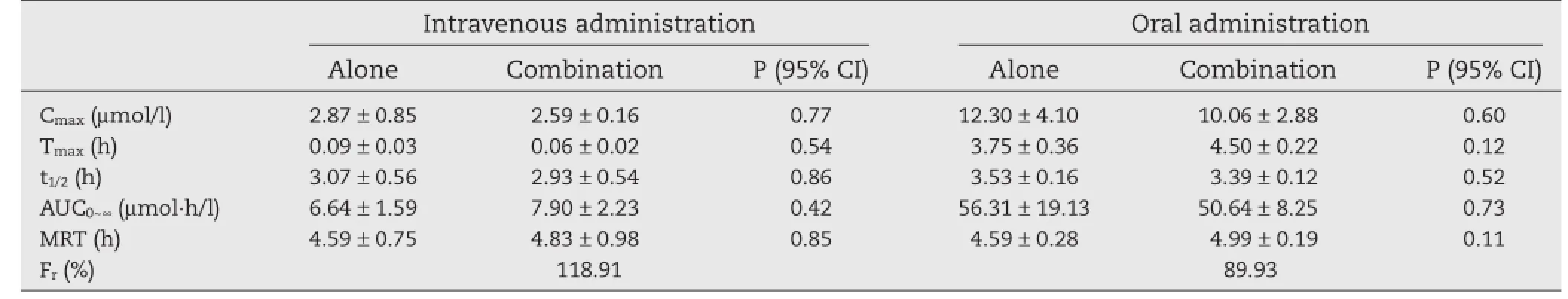
Table 3–Pharmacokinetics of M following OM intravenous and oral administration alone and in combination with 3TC.
4.Conclusion
There is no pharmacokinetic interaction when 3TC and OM are co-administrated,either intravenously or orally,and the potential for 3TC to interact with its metabolite M has been examined to be low.And this study focused on the pharmacokinetic interactions in dogs to account for the collaborated employment of lamivudine and oxymatrine.However,the clinical pharmacokinetics remains to be studied for confrming this conclusion.Notwithstanding this limitation,the study does provides thein vivopharmacokinetic basis for supporting the recent clinical practices that no dosage adjustment is needed and serious adverse events are absent when lamivudine and oxymatrine are co-administrated,which demonstrated that the determination of pharmacokinetics between combinational and individual therapy might assist the development of drug compatibility in clinical therapies.
Acknowledgments
We thank fnancial support from the National Natural Science Foundation of China(Nos.30901996,81173009 and 81302722). We also thank fnancial support from the General Project in Education Department of Liaoning Province(No.L2013390)and the Specifc Science Foundation of Shenyang Pharmaceutical University(Nos.ZCJJ2014409 and ZCJJ2013402).
R E F E R E N C E S
[1]Hou JL,Xu D,Shi G,et al.Long-term telbivudine treatment results in resolution of liver infammation and fbrosis in patients with chronic hepatitis B.Adv Ther 2015;32:727–741.
[2]Hagiwara S,Nishida N,Kudo M.Antiviral therapy for chronic hepatitis B:combination of nucleoside analogs and interferon.World J Hepatol 2015;7:2427–2431.
[3]Chung SM,Byoun YS,Kim HS,et al.The feasibility of discontinuing lamivudine in lamivudine-resistant chronic hepatitis B patients on lamivudine and adefovir combination therapy.Intervirology 2014;57:337–343.
[4]Lee HJ,Kim DY,Keam B,et al.Lamivudine prophylaxis for hepatitis B virus carrier patients with breast cancer during adjuvant chemotherapy.Breast Cancer 2014;21:387–393.
[5]WHO.Hepatitis E vaccine:WHO position paper,May 2015–Recommendations.Vaccine,2015.
[6]Cui X,Wang Y,Kokudo N,et al.Traditional Chinese medicine and related active compounds against hepatitis B virus infection.Biosci Trends 2010;4:39–47.
[7]Poortahmasebi V,Malekzadeh R,Montazeri G,et al. Lamivudine resistance and precore variants in Iranian patients with chronic hepatitis B:correlation with virological and clinical features.Jundishapur J Microbiol 2015;8:e20262.
[8]Lau DT,Khokhar MF,Doo E,et al.Long-term therapy of chronic hepatitis B with lamivudine.Hepatology 2000;32:828–834.
[9]Ma ZJ,Li Q,Wang JB,et al.Combining oxymatrine or matrine with lamivudine increased its antireplication effect against the hepatitis B virus in vitro.Evid Based Complement Alternat Med 2013;2013: 186573.
[10]Hussey EK,Donn KH,Daniel MJ,et al.Interspecies scaling and pharmacokinetic parameters of 3TC in humans.J Clin Pharmacol 1994;34:975–977.
[11]Johnson MA,Moore KH,Yuen GJ,et al.Clinical pharmacokinetics of lamivudine.Clin Pharmacokinet 1999;36:41–66.
[12]Song YL,Dong QL,Chen HY,et al.[The metabolism of 3H-oxymatrine in mice and rats].Zhongguo Yi Xue Ke Xue Yuan Xue Bao 1986;8:261–265.
[13]Wang ML,Zhou QL,Wang BX.[Studies on metabolism of oxymatrine by human intestinal bacteria].Zhongguo Zhong Yao Za Zhi 2001;26:272–274.
[14]Sun J,Luo C,Wang Y,et al.The holistic 3M modality of drug delivery nanosystems for cancer therapy.Nanoscale 2013;5:845–859.
[15]Lu LG,Zeng MD,Mao YM,et al.Oxymatrine therapy for chronic hepatitis B:a randomized double-blind and placebocontrolled multi-center trial.World J Gastroenterol 2003;9:2480–2483.
[16]Sokal EM,Roberts EA,Mieli-Vergani G,et al.A dose ranging study of the pharmacokinetics,safety,and preliminary effcacy of lamivudine in children and adolescents with chronic hepatitis B.Antimicrob Agents Chemother 2000;44:590–597.
[17]Piao H,Liu S,Piao H,et al.Development of an osmoticallydriven pellet coated with acrylic copolymers(Eudragit(R)RS 30 D)for the sustained release of oxymatrine,a freely water soluble drug used to treat stress ulcers(I):in vitro and in vivo evaluation in rabbits.Drug Dev Ind Pharm 2013;39:1230–1237.
[18]Wang S,Wang G,Li X,et al.Simultaneous determination of oxymatrine and its active metabolite matrine in dog plasma by liquid chromatography-mass spectrometry and its application to pharmacokinetic studies.J Chromatogr B Analyt Technol Biomed Life Sci 2005;817:319–325.
[19]Moore KH,Yuen GJ,Raasch RH,et al.Pharmacokinetics of lamivudine administered alone and with trimethoprimsulfamethoxazole.Clin Pharmacol Ther 1996;59:550–558.
*< class="emphasis_italic">Corresponding authors.
s.School of Pharmacy,Shenyang Pharmaceutical University,No.103 Wenhua Road,Shenyang 110016,China.Tel.: +86 24 23986325;fax:+86 24 23986325.
E-mail addresses:lvj221@163.com(X.Liu);sunjin66@21cn.com(J.Sun).1Contributed equally to this work.
http://dx.doi.org/10.1016/j.ajps.2016.02.007
1818-0876/?2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
 Asian Journal of Pharmacentical Sciences2016年5期
Asian Journal of Pharmacentical Sciences2016年5期
- Asian Journal of Pharmacentical Sciences的其它文章
- Quantitative determination of metaxalone in human plasma by LC-MS and its application in a pharmacokinetic study
- Effects of duration of phenytoin administration on mRNA expression of cytochrome P450 and P-glycoprotein in the liver and small intestine of rats
- Effect of surface ligand density on cytotoxicity and pharmacokinetic profle of docetaxel loaded liposomes
- Stability of freeze-dried pH-responsive dextrin nanogels containing doxorubicin
- A hydrophobic peptide fraction that enhances the water dispersibility of curcumin
- An intravenous clarithromycin lipid emulsion with a high drug loading,H-bonding and a hydrogen-bonded ion pair complex exhibiting excellent antibacterial activity
