Quantitative determination of metaxalone in human plasma by LC-MS and its application in a pharmacokinetic study
China Pharmaceutical University,No.24,Tongjiaxiang,Nanjing 210009,China
Short Communication
Quantitative determination of metaxalone in human plasma by LC-MS and its application in a pharmacokinetic study
Lanting Zhao,Qian Li,Chang Shu,Keli Wang,Li Ding*
China Pharmaceutical University,No.24,Tongjiaxiang,Nanjing 210009,China
A R T I C L EI N F O
Article history:
Received 10 November 2015
Received in revised form 31
December 2015
Accepted 12 January 2016
Available online 28 January 2016
Metaxalone
A simple and rapid method using liquid chromatography–mass spectrometry(LC-MS)for the determination of metaxalone in human plasma has been developed and validated. Letrozole was used as the internal standard(IS).The plasma samples were simply treated with acetonitrile which allowed the precipitation of plasma proteins.The chromatographic separation was achieved on a Sapphire C18(2.1 mm×150 mm,5 μm,Newark,USA) column using the mobile phase(5 mM ammonium acetate containing 0.01%formic acid: acetonitrile(45:55,v/v))at a fow rate of 0.3 ml/min.The selected ion monitoring(SIM)in the positive mode was used for the determination of[M+H]+m/z 222.1 and 286.1 for metaxalone and letrozole,respectively.The standard curve obtained was linear(r2≥0.99) over the concentration range of 30.24?5040 ng/ml.Meanwhile,no interfering peaks or matrix effect was observed.The method established was simple and successfully applied to a pharmacokinetic study of metaxalone in healthy Chinese volunteers after a single oral dose administration of 800 mg metaxalone.The main pharmacokinetic parameters of metaxalone were as follow:Cmax,(1664±1208)ng/ml and(2063±907)ng/ml;AUC0?36,(13925±6590)ng/ ml h and(18620±5717)ng/ml h;t1/2,(13.6±7.7)h and(20.3±7.7)h for the reference and test tablets,respectively.These pharmacokinetic parameters of metaxalone in healthy Chinese volunteers were reported for the frst time.
?2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
1.Introduction
Metaxalone,5-[(3,5-dimethylphenoxy)methyl]-2-oxazolidinone, is a skeletal muscle relaxant and clinically used for relieving the local muscle spasmodic pain caused by infammation,trauma and other musculoskeletal conditions[1].Recently,some formulations of metaxalone tablets were developed in China. However,there is no pharmacokinetic study of metaxalone in Chinese volunteers reported.It is meaningful to develop andvalidate a method for the determination of metaxalone in human plasma which can be applied to the pharmacokinetic study.
By now,several analytical methods,such as reversed phasehigh performance liquid chromatography(RP-HPLC)[2]and [3],UV-spectroscopy[4]and reversed phase–ultra performance liquid chromatography(RP-UPLC)[5]have been reported and used for the determination of metaxalone in bulk or solid oral dosage-forms.Nirogi et al.[6]reported a liquid–liquid extraction technique and Goswami et al.[7]reported a solid phase extraction method for the determination of metaxalone in human plasma by using LC-MS/MS.The sample preparations of these two methods are liquid–liquid extraction and solid phase extraction,which are time-consuming,and the extraction recoveries are relatively low.The aim of this study was to develop and validate a time-saving method with simple sample preparation procedures,and which can be applied to the pharmacokinetic study of metaxalone in healthy Chinese volunteers.
2.Materials and methods
2.1.Chemicals and reagents
Metaxalone reference standard(purity,99.3%)was provided by Frontage laboratories,Inc.(Suzhou,PR China).Letrozole(purity, 100.0%)was purchased from the National Institutes for Food and Drug Control(Beijing,PR China).Acetonitrile and methanol were of HPLC grade(Merck,Germany).Formic acid and ammonium acetate were of analytical grade purity and were purchased from Nanjing Chemical Reagent Co.,Ltd.(Nanjing, PR China).Ultra-pure water was made by Millipore water purifcation system(Millipore,Molsheim,France).Reference tablets (SKELAXIN?,800 mg)were purchased from King Pharmaceuticals Inc.(USA)and test tablets(800 mg)were provided byACTH Pharma Tech(Shanghai)Co.,Ltd.(Shanghai,PR China),which were stored at 4°C until use.
2.2.Instruments and analytical conditions
Chromatographic separation was performed using an Agilent 1100 Series system(Agilent Technologies,Palo Alto,CA,USA), which includes an Agilent 1100 binary pump(model G1312A), an Agilent 1100 auto-sampler(model G1067A)and a thermostat column compartment(model G1330B).Chromatographic separation was achieved on a Sapphire C18(2.1 mm×150 mm, 5 μm,Newark,USA)column using the mobile phase(5 mM ammonium acetate containing 0.01%formic acid:acetonitrile (45:55,v/v))at a fow rate of 0.3 ml/min.The auto-sampler temperature was kept at 10°C,and 2 μl supernatant was injected into LC-MS system.
The mass detection was achieved by using an Agilent Technologies Series LC/MSD SL(Agilent Technologies,USA) equipped with an electrospray ionization source.High purity nitrogen was used as the nebulizer and auxiliary gases.The detection ions in the positive ionization mode for metaxalone and letrozole were[M+H]+m/z 222.1 and 286.1,respectively.Fragment voltages of metaxalone and letrozole were set at 155V and 120V,respectively.
2.3.Preparation of stock solution,calibration standards and quality control(QC)samples
Metaxalone and letrozole stock solutions were prepared in methanol and were stored in?20°C refrigerator.The working solutions of metaxalone ranged 0.6048–100.8 μg/ml were prepared by diluting the stock solution with methanol-water(50:50, v/v)solution.The IS working solution(49.80 μg/ml)was prepared with methanol.All working solutions were stored at?20°C until use.Calibration standards(30.24–5040 ng/ml)and quality control (QC)samples(50.40,504.0,4032 ng/ml)were prepared by spiking 200 μl blank plasma samples with different volumes of working solutions.
2.4.Sample preparation
Frozen human plasma samples were thawed at ambient temperature.Aliquot of 200 μl plasma sample was added to 1.5 ml polypropylene tube which containing 20 μl IS working solution(49.80 μg/ml)and vortex-mixed for 10 s.600 μl aliquot of acetonitrile was added into the tube and vortexed for 3 min followed by a centrifugation at 15600 rpm for 5 min.2 μl of supernatant of each sample was injected into the LC-MS system.
2.5.Method validation
The method was validated according to the newest FDA guidance for industry bioanalytical method validation,which was validated through selectivity,linearity,sensitivity,accuracy and precision,matrix effect,and recovery.Selectivity was assessed by analyzing at least six different sources of blank human plasma samples.
Linearity was verifed in the plasma concentration range of 30.24–5040 ng/ml,and it was determined by weighted(1/C2, r≥0.99)least squares linear regression of calibration curve with seven calibration standards.Sensitivity is defned as the lowest analytical concentration which can be measured with acceptable accuracy,precision and signal to noise ratio(S/N>10).Interday precisions and accuracy were determined by analyzing 5 replicates of LLOQ and QC samples on three consecutive days (n=15).The recovery and matrix effect were evaluated at three QC concentration levels.The extraction recoveries were determined by comparing peak areas of metaxalone in plasma samples at 3 QC levels(n=5)which were spiked with the analyte prior to extraction with those of samples to which the corresponding solution was added after extraction.Matrix effect was studied by calculating peak area ratios of metaxalone and letrozole with matrix and without matrix.Matrix effect(ME)was accepted if the coeffcient variation(CV)of the peak area ratios of metaxalone and letrozole did not exceed 15%.Stability of metaxalone was evaluated with three replicates at concentration levels of 50.40,4032 ng/ml.The stability tests of room temperature stability(RTS),auto-sampler stability(ASS),freezethaw stability(FTS)and long-term stability(LTS)were evaluated. Short-term stability was performed by placing samples at room temperature for 6 h.Long-term stability was assessed by freezing samples at?20°C for 30 d.Freeze-thaw stability was also evaluated after three freeze-thaw cycles(room temperature to?20°C),and post-preparation stability of processed samples was tested by placing it in an auto-sampler at 10°C for 24 h.
2.6.Pharmacokinetic study
The established and validated method was successfully applied to a pharmacokinetic study of metaxalone in Chinese volunteers.Twelve healthy male Chinese volunteers aged 20–45 without history of cardiovascular,endocrine,gastrointestinal,hematologic,hepatic,renal,and neuropsychosis were recruited after a thorough medical,biochemical and physical examination.All volunteers were given written informed consent before participating in the study according to the principles of the Declaration of Helsinki.This study was designed as a randomized,crossover trial.Twelve healthy volunteers were randomly assigned to take either reference formulation or test formulation on the frst day,and the other formulation was taken after 7 d washout period.Volunteers were fasted for 10 h before administering metaxalone tablets(800 mg)with 240 ml water.Water intake was allowed at 2 h post-dose,and standard meals were provided at 4 h and 10 h post-dose.Blood samples(3 ml)were collected into heparin tubes at 0,1,2,2.5,3,3.5,4,4.5,5,5.5,6,7,8,12,16,24 and 36 h after dosing.The plasma samples were obtained by centrifuging heparin tubes at 4000 rpm for 5 min and frozen at?20°C until analysis.
3.Results and discussion
3.1.Method development
The sample preparation procedures of liquid–liquid extraction[6]and solid phase extraction[7]for the determination of metaxalone in human plasma are time-consuming and the extraction recoveries are relatively low.It is meaningful to develop a better method with simple sample handling procedure and high extraction recovery to research pharmacokinetic characteristics of metaxalone in Chinese volunteers. Plasma sample was simply treated with acetonitrile.In order to enhance the sensitivity and avoid matrix effect,the composition of the mobile phase and the injection volume were optimized.In order to achieve higher sensitivity and better peak shape,different concentrations of formic acid(0.01%, 0.05%,0.1%,)in water portion of the mobile phase were optimized.It was found that 0.01%formic acid produced highest sensitivity.After trying different concentrations of ammonium acetate,5 mM ammonium acetate solution containing 0.01%formic acid produced higher response and better peak shape.Matrix interference was inexistence when the injection volume was set at 2 μl.Finally,the chromatographic conditions of optimized mobile phase(5 mM ammonium acetate solution containing 0.01%formic acid:acetonitrile(45:55,v/v))and 2 μl injection volume were adopted. The mass spectrum conditions were then optimized and it was found that the best detection was produced when the parameters of drying gas fow,nebulizer pressure,drying gas temperature,and capillary voltage,were set at 12 l/min, 50 psi,350°C,and 4000 V,respectively.The full-scan ion mass spectra(Fig.1)shows two peak signals[M+H]+m/z 222.1 and 286.1 of metaxalone and letrozole,respectively, which are selected as detection ions.
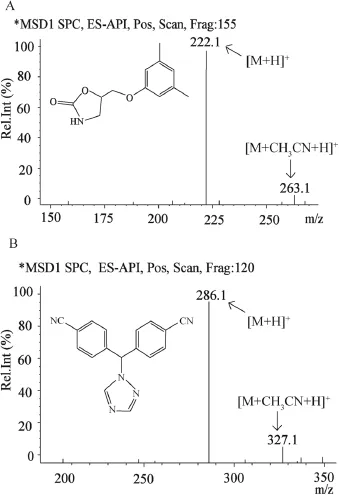
Fig.1–Full-scan mass spectra of(A)metaxalone and (B)letrozole.
3.2.Assay validation
3.2.1.Selectivity
The selectivity of the method was proved by comparing SIM chromatograms of the blank plasma sample,blank plasma sample spiked with metaxalone and the IS and a plasma sample obtained from volunteers after oral administration of metaxalone tablets.The corresponding chromatograms are showed in Fig.2.The retention time of metaxalone and the IS were 2.9 and 2.8 min,respectively.No interference was found in all plasma samples.
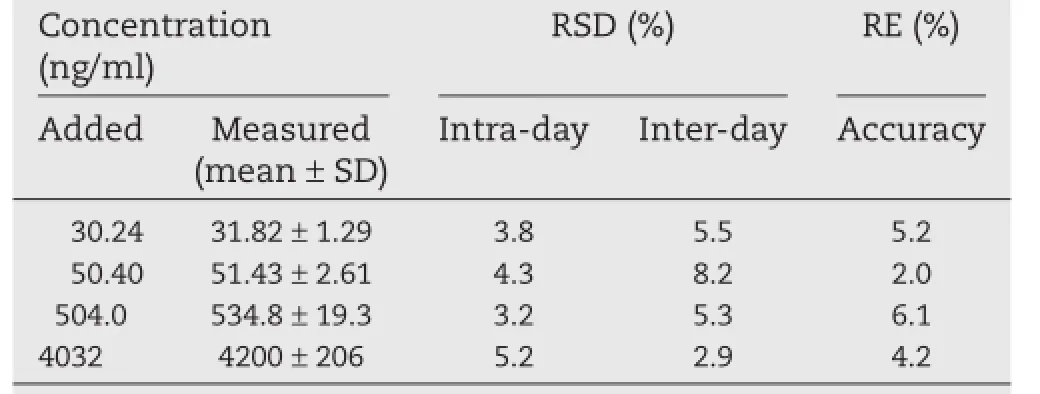
Table 1–Intra-day and inter-day precision and accuracy of metaxalone in human plasma(n=5).
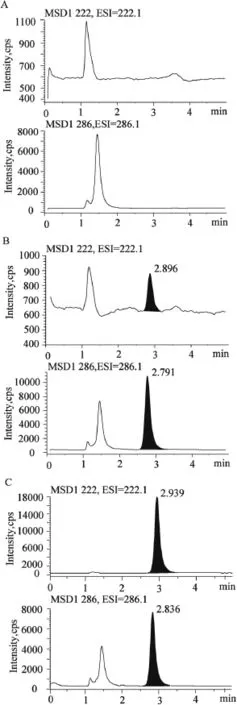
Fig.2–Typical chromatograms of metaxalone and the IS in human plasma samples.(A)Blank plasma sample; (B)a blank plasma sample spiked with metaxalone (30.24 ng/ml)and the IS(49.80 μg/ml);and(C)a plasma sample obtained from a volunteer at 2 h after oral administration one SKELAXIN?tablet(800 mg).
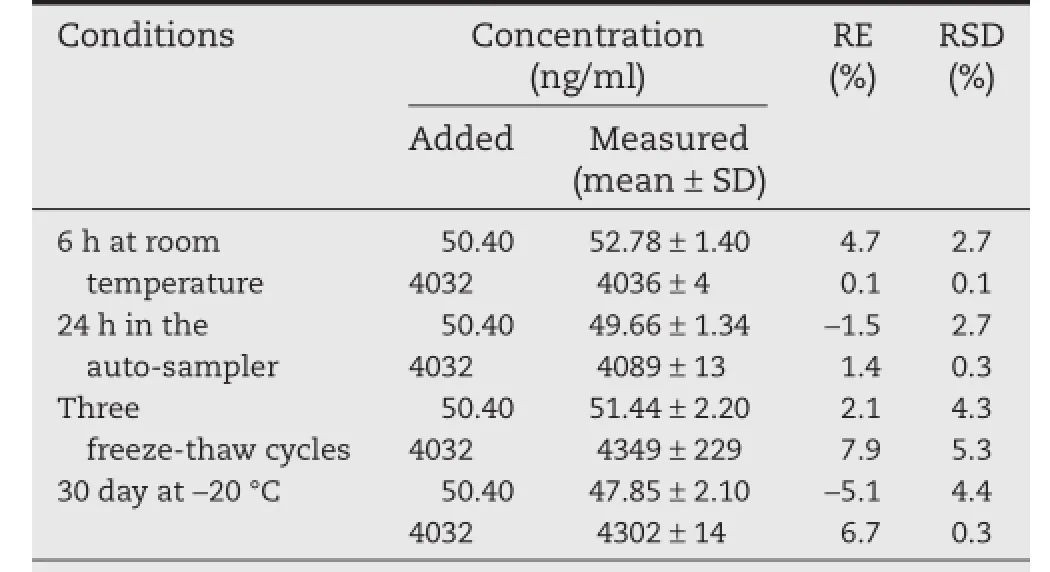
Table 2–Stability of metaxalone in human plasma (n=3).
3.2.2.Linearity,LLOQ,accuracy and precision
Calibration curves over the concentration range of 30.24 to 5040 ng/ml were linear with correlation coeffcient r≥0.99.The typical regression equation of the calibration curve was f=0.0008·C?0.0034.The intra-day and inter-day results of precision and accuracy are summarized in Table 1.The data demonstrate that the precision and accuracy values are within the acceptable criteria.
3.2.3.Extraction recovery and matrix effect
The mean extraction recoveries at three QC levels(50.40,504.0, 4032 ng/ml)were as follows:98.6%±4.4%,93.4%±2.7%, 96.1%±1.5%,respectively.The mean recovery of the IS was 96.3%±1.9%.Matrix effects of the six different sources of blank plasma on metaxalone at three QC levels(50.40,504.0, 4032 ng/ml)were as follows:98.7%±4.7%,105.1%±2.1%, 105.6%±2.2%,respectively.
3.2.4.Stability
The stability investigations of room temperature stability(RTS), auto-sampler stability(ASS),freeze–thaw stability(FTS)and long-term stability(LTS)were performed,and the results are summarized in Table 2.The data indicate that metaxalone is stable enough during sample preparation and storage conditions.
3.2.5.Pharmacokinetic study
The developed and validated method was successfully applied to the pharmacokinetic study of metaxalone in twelve healthy male Chinese volunteers after oral administrating metaxalone tablets(800 mg).The pharmacokinetic parameters were calculated by DAS 3.2.5.The mean plasma concentration-time curves of the reference tablets and test tablets are shown in Fig.3.The values of the main pharmacokinetic parameters(Cmax,AUC0?36,AUC0?∞,t1/2,Tmax,)are given in Table 3.All the reported pharmacokinetic parameters of metaxalone for the reference tablets in humans are listed in Table 4.The main pharmacokinetic parameters of metaxalone in Chinese volunteers obtained in this study(see Table 3)are almost consistent with those in American after oral administrating SKELAXIN?tablets(800 mg)under fasted conditions[8].Compared to the high fat conditions[7]and[8],the measured values of pharmacokinetic parameters (Cmax,AUC0?36,AUC0?∞)are relatively lower under the fasting condition,which implied that the pharmacokinetic characteristics of metaxalone are signifcantly affected by food.
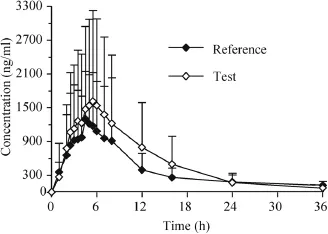
Fig.3–Mean plasma concentrations-time profle of metaxalone in Chinese healthy volunteers after oral administration metaxatone tablets(800 mg).Data are means±SD,n=12.
4.Conclusion
In summary,a new simple and reproducible LC-MS method for the determination of metaxalone in human plasma has been validated.It was successfully applied to the pharmacokinetic study in healthy male Chinese volunteers.The developed method has advantages of simple sample preparation procedure and high extraction recovery.The pharmacokinetic parameters of metaxalone in healthy Chinese volunteers were reported for the frst time.
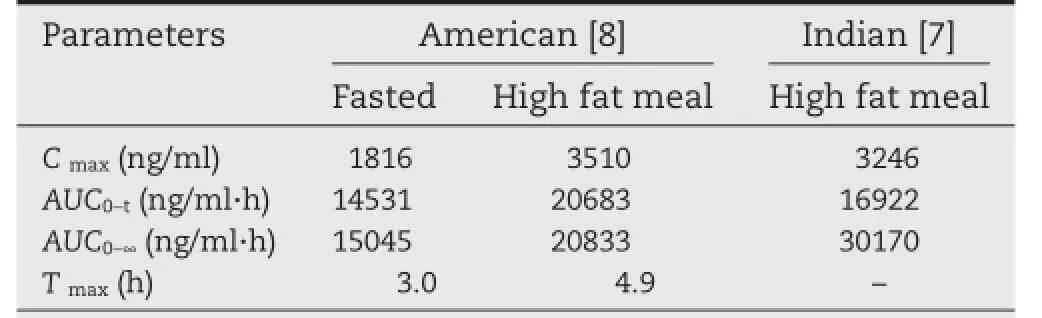
Table 4–Reported pharmacokinetic parameters of metaxalone in human after oral dose administration of the reference tablets(SKELAXIN?,800 mg).
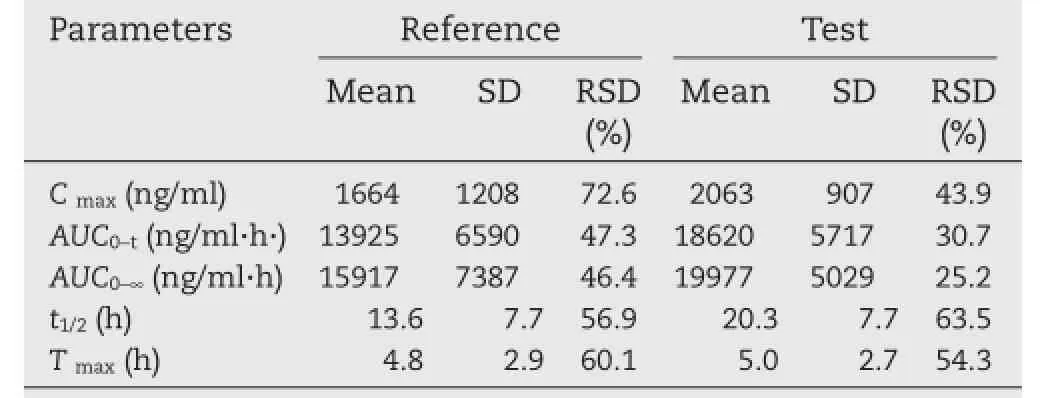
Table 3–Pharmacokinetic parameters of metaxalone measured in Chinese volunteers.
R E F E R E N C E S
[1]Moore KA,Levine B,Fowler D.A fatality involving metaxalone.Forensic Sci Int 2005;149:249–251.
[2]Sahu PK,Annapurna MM,Kumar SD.Development and validation of stability indicating RP-HPLC method for the determination of metaxalone in bulk and its pharmaceutical formulations.J Chem 2011;8(S1):S439–S447.
[3]Panda SS,Patanaik D,Kumar BVVR.New stability-indicating RP-HPLC method for determination of diclofenac potassium and metaxalone from their combined dosage form.Sci Pharm 2012;80:127–137.
[4]Reddy NV,Ishaq BM,Rajan VS,et al.Analytical method development and validation of Metaxalone on bulk and its pharmaceutical formulation by UV spectroscopic method.Int Res J Pharm 2013;4(3):149–152.
[5]Trivedi RK,Patel MC.Development of a stability-indicating RP-UPLC method for rapid determination of metaxalone and its degradation products in solid oral dosage form.Sci Pharm 2012;80:353–366.
[6]Nirogi RVS,Kandikere VN,Shukla M,et al.Quantifcation of metaxalone in human plasma by liquid chromatography coupled to tandem mass spectrometry.J Anal Toxicol 2006;30(4):245–251.
[7]Goswami D,Saha A,Gurule S,et al.Metaxalone estimation in biological matrix using high-throughput LC–MS/MS bioanalytical method.J Chromatogr B Analyt Technol Biomed Life Sci 2012;902:132–136.
[8]FDA.SKELAXIN?(Metaxalone)Tablets(Initial U.S.Approval: April 2008).<http://www.accessdata.fda.gov/drugsatfda_docs/ label/2008/013217s053lbl.pdf>.
*< class="emphasis_italic">Corresponding author.
.China Pharmaceutical University,No.24,Tongjiaxiang,Nanjing 210009,China.Tel.:+86 25 83271485;fax:+86 25 83271485.
E-mail address:dinglihg@sina.com(L.Ding).
http://dx.doi.org/10.1016/j.ajps.2016.01.002
1818-0876/?2016 Production and hosting by Elsevier B.V.on behalf of Shenyang Pharmaceutical University.This is an open access article under the CC BY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
LC-MS
Pharmacokinetics
 Asian Journal of Pharmacentical Sciences2016年5期
Asian Journal of Pharmacentical Sciences2016年5期
- Asian Journal of Pharmacentical Sciences的其它文章
- Effects of duration of phenytoin administration on mRNA expression of cytochrome P450 and P-glycoprotein in the liver and small intestine of rats
- Effect of surface ligand density on cytotoxicity and pharmacokinetic profle of docetaxel loaded liposomes
- Stability of freeze-dried pH-responsive dextrin nanogels containing doxorubicin
- Evaluation of pharmacokinetics underlies the collaborated usage of lamivudine and oxymatrine in beagle dogs
- A hydrophobic peptide fraction that enhances the water dispersibility of curcumin
- An intravenous clarithromycin lipid emulsion with a high drug loading,H-bonding and a hydrogen-bonded ion pair complex exhibiting excellent antibacterial activity
