Original Research Paper Association between the physical stability of flurbiprofen suspension and the interaction of HPMC/SDS
Hongyu Wng,Yiwei Sun,Bixue Yng,Snming Li,*
henyang Pharmaceutical University,No.103,Wenhua Road,Shenyang 110016,China
inyi University,Middle section of Shuang Ling Road,Lanshan District,Linyi,Shandong 276005,China
1. Introduction
As we all know,drug delivery systems(DDS)are composed of active pharmaceutical ingredient(API)and excipient.In most cases,a pharmaceutical formulation is designed by more than one excipient,among which polymer and surfactant are frequently used.Polymers can be classified as natural and synthetic polymer and surfactants mainly include anionic surfactant,cationic and nonionic surfactant in terms of their electrification.And polymer/surfactant mixtures can also be used in the field such as food industry,personal care products,paints,pesticides,etc[1].
The interaction between surfactant and polymer can be mainly classified as the following aspects.First,the surfactant blended with the polymer could cause a change of its critical micelle concentration(CMC),which is deemed as the critical aggregation concentration(CAC)in polymer/surfactant system.The lower CAC values will result in the greater solubilizing capacity of surfactant[2,3].Second,the rheology behavior of the polymer will be changed when the surfactants coexist,which can be characterized by the viscosity and sol–gel transition[4,5].Third,there are synergetic effects between polymer and surfactant on promoting adsorption on the surface of solid[6–8].In addition,the polymer/surfactant interaction has a vital role in the wettability of hydrophobicity materials[9],and so on.
The interaction between polymer and surfactant is benef icial to many applications in pharmaceutics,such as the dissolution promotion of tablets[10],the diffusional behavior adjustment of the drug in gels[11],crystal form[12,13],as well as the recovery and release enhancement of API in nanoparticles forms[14].As for suspensions,there are many articles about taking advantage of polymer/surfactant complex to improve the physical stability and drug release behavior[9,15–17],which have placed emphasis on the formulation optimization or the change of the preparation’s nature.Besides,when pharmaceutic adjuvants were incorporated into the formulation,the inf l uence of additive agent on the property of API was also studied[18–20].However,the association between the properties of formulations and the interaction of adjuvants has not been investigated yet.
This research focused on the association among the suspension stability and the interaction of polymer/surfactant.In this work,f l urbiprofen(FBP)was used as a model drug and nonionic polymer hydroxypropylmethyl cellulose(HPMC,K4M)was selected as suspending agent to prepare suspensions.According to previous studies[21–23],anionic surfactant sodium dodecylsulfate(SDS)with a series of concentration from 0.0 mM to 12.0 mM was introduced to improve the physical stability of the FBP suspensions.The participation of SDS contributed to form suspensions with uniform dispersion,and eliminate the f l oating particles as well as ensure that the dispersion is more persistent.Known from others’work,to better understand the mechanism responsible for the differences in function caused by SDS,the interaction between 0.2%HPMC and SDS with a series of concentration was studied in detail.Interestingly,the distribution of drug concentration and the redispersions time of FBP suspensions were associated with the interaction of HPMC/SDS.And it laid the foundation for controlling the properties of pharmaceutical formulation by adjusting the interaction among excipients.
2. Materials and methods
2.1. Materials
Flurbiprofen was purchased from Shandong Lukang Pharmaceutical Group Co.,Ltd.China.Hydroxypropylmethylcellulose,HPMC,(trade name Methocel,K4M,methoxyl content 22.7%,hydroxypropyl content 8.9%),pharmaceutical grade,was obtained from Colorcon Ltd.,England.Sodium dodecylsulfate(SDS,with 91.0%)was purchased fromTianjin Bodi Chemical Limited by Share Ltd,China.Ethanol(95%)was purchased fromTianjin Fuyu Fine Chemical Co.,Ltd.China.Redistilled water was made in our laboratory.
2.2. Preparation of FBP suspensions
According to the preliminary trial results,the optimal concentration of HPMC was fixed at 0.2%considering the settling velocity and dispersity.According to the anti-solvent precipitation method[24],accurately weighed amounts of FBP were dissolved in ethanol to form a clear solution.Then under vigorous agitation 100μl drug solutions was added to 10 ml HPMC or HPMC/SDS dispersions separately.The stirring could not stop until they were mixed thoroughly and followed by transferred equivalent to a 10 ml graduated test tube.
2.3. Content of FBP in suspensions
Because not all the suspensions have obvious interface for calculating sedimentation volume ratio,the drug concentration in different sites was compared for quantifying the dispersion of drug among different suspensions.After the suspensions stood for half hour,an aliquot of 40μl was withdrawn from the tube at 0.2 cm,0.5 cm,1 cm and 1.5 cm(the total height of 10 ml suspensions was about 6.5 cm)under the liquid level.And then the aliquot was dissolved by a certain amount of ethanol adequately.After that the absorbance of FBP was tested by UV spectrophotometer(UV-1750)at 247 nm and the content was calculated.
2.4. The characteristic of FBP suspensions
2.4.1. The morphology of the suspended particles
To obtain the dispersion state of the suspended particles in suspensions,the particle morphology was observed by polarizing microscope(LW100P)at 100×magnification and captured by camera(THDCE-20S).
2.4.2. Re-dispersion time
Re-dispersion time is defined as the seconds required to make the precipitate from the bottom of the container to go back to dispersions and get a uniform suspension again.When the test of drug concentration was completed,the suspensions were transferred into labeled vials with lid and stood in an upright position for 24 h.Then the times required for redispersion of FBP suspensions were measured by inverting the bottles to 180°at a uniform speed[25].
2.4.3. Zeta potential and the particle size
Several FBP suspensions were chosen to represent the change regulation of zeta potential and particle size.Malvern Zetasizer nano ZS90(Malvern Instruments,UK)and NICOMPTM 380 ZLS were used to obtain the zeta potential in the suspensions and the particle size of suspended particles respectively.
2.5. The interaction between HPMC and SDS
2.5.1. Conductivity
The interaction between polymer and surfactant can be divided into four different regions by the concentration of surfactant.The first demarcation point is the minimum surfactant concentration when polymer began to interact with surfactant,namely the critical association concentration(CAC),the second is the critical micelle concentration(CMC)of the surfactant,and the last corresponds to the surfactant concentration of which the polymer chains become saturated by surfactant molecules regarded as the polymer saturation point(PSP).The three valuable breaking points for HPMC/SDS complex should be found firstly by electrical conductivity[26,27].Conductivity measurements were carried out at 25°C in a jacketed vial by adding the proper volume of solutions.The conductance of the solution was measured and recorded after the electrical resistance reached a stable value,and each sample was measured for three times.
2.5.2. Viscosity
Viscosity measurements of the solutions above were performed on an Ubbelhode capillary viscometer,which was immersed in a grant thermostat.All samples were allowed to equilibrate for 15 min before the measurements.Three viscosity measurements were taken at least and average values were calculated.The results were expressed as Specific viscosity in the following equation:
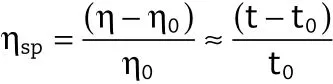
Where η and η0are the solution and pure solvent viscosities,respectively,t and t0are the f l ow times in the viscometer of the solution and pure solvent respectively.
2.5.3. Solubilization
Solubilization experiment was carried out under the conditions of imitating the operation of suspension preparation named the short time solubilization.Brief l y excessive drug powder was added to the dispersions during agitation and kept on 10 min,then followed by extracting the supernatant after standing for 30 min.Then,the non-solubilized fraction was removed by filtrating with 0.45μm nylon membrane.A certain amount of subsequent filtrate was removed exactly and diluted by ethanol.After that the absorbance of FBP was tested by UV spectrophotometer as mentioned above and the solubility of FBP was calculated.Meanwhile excess drug powder was added in the corresponding dispersions,which were located in the isothermal oscillation at 25°C for three days and finally processed in the same way.
2.5.4. Wettability
Wettability can be evaluated by determining the contact angle.The higher value of contact angle means the less degree of wetting.The equilibrium contact angles were determined by using the method reported previously[28].The tangent method was chosen to compute the contact angles between the baseline of the drop and the tangent at the drop boundary[29].
3. Results and discussion
3.1. The appearance of the suspensions
Floater was found on the surface of FBP suspension dispersed by 0.2%(w/v)HPMC alone.What is more,its distribution was not uniform with a majority of precipitation centered on the lower half of the tube and cake precipitation took place in the bottom.Amazingly,the f l oater disappeared gradually,f l oc appeared and the suspensions became more and more uniform along with the addition of SDS,which indicated that the presenceofSDSwasconducivetotheuniformityofFBPsuspensions.
3.2. The content of FBP in suspensions
Because the liquid samples were not filtered,the concentration should be considered as an apparent concentration that was a total effect of dissolved FBP and suspended particles together.It illustrated that the apparent concentration can represent the distribution of drug and the dispersion degree of suspensions more exactly.The content was expressed by calculating the percent of extracted weight of FBP in the total addition amount.And the drug content as a function of SDS concentration was shown in Fig.1.Obviously,the drug content was higher when SDS presented,which indicated that the participation of SDS was good for the uniform dispersion of FBP suspensions.These results also suggested that the concentration of SDS at 6.0 mM that is just around the PSP as shown in Fig.6 was a turning point.When the concentration of SDS was below PSP,the contents in the same suspension from different sites were very similar meaning that these suspensions were welldistributed.The drug content in the upper half of the suspensions was very small after PSP indicating that the terrible homogeneity of the suspension.In addition,the difference of drug content from the bottom to top sites was more obvious after PSP,which might be due to the increase of settling velocity as a result of enough f l occulation in these systems[30].
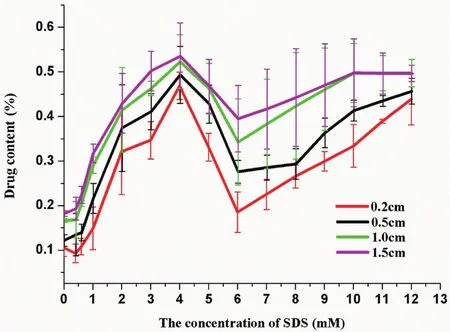
Fig.1–The content of f l urbiprofen from different sites in various suspensions(means±SD,n=3).

Fig.2–The morphology of suspended particles in suspensions dispersed by 0.2%HPMC:(a)with 0.0 mM SDS(b)with 2.0 mM SDS(c)with 3.0 mM SDS(d)with 5.0 mM SDS(e)with 6.0 mM SDS(f)with 8.0 mM SDS(g)with 10.0 mM SDS(h)with 12.0 mM SDS.
3.3. The characteristic of FBP suspensions
3.3.1. The particle morphology
The morphology of particles in the suspensions was presented in Fig.2.It was obvious that when the suspension was suspended by HPMC alone,agglomerate could be observed.With the addition of SDS,the agglomerate scattered and f l occules appeared slowly.It needed to be noted that def l occulation would appear when the concentration of SDS reached 10.0 mM.
3.3.2. The re-dispersion time

Fig.3–The re-dispersion times of the suspensions(means±SD,n=3).
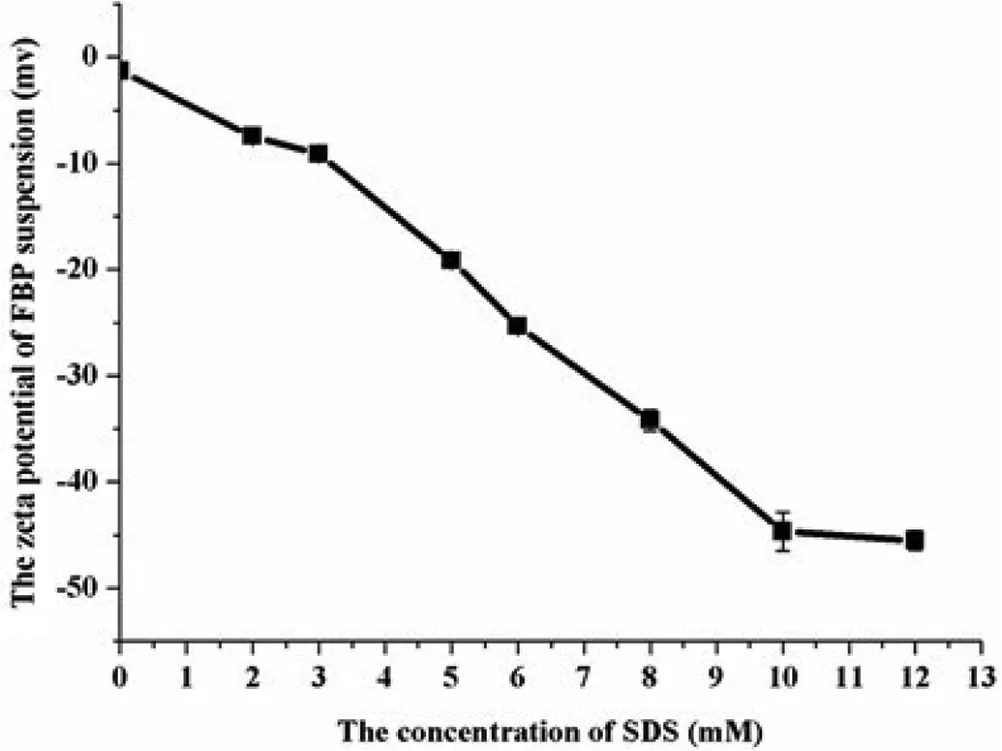
Fig.4–The zeta potential of FBP suspensions(means±SD,n=3).
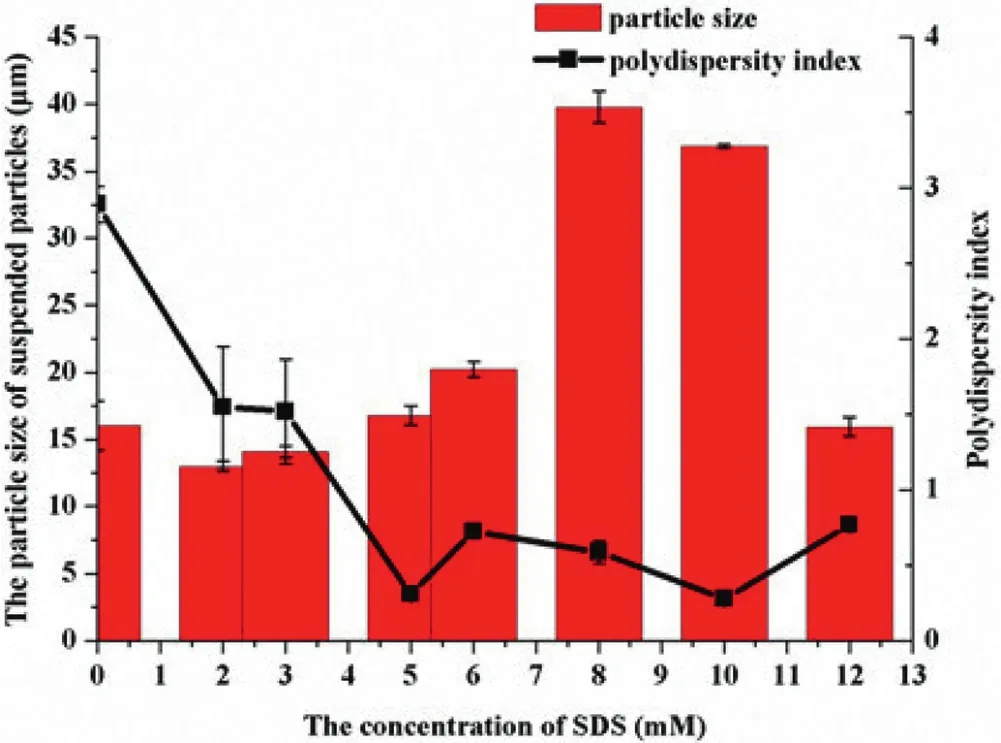
Fig.5–The particle size and polydispersity index(PDI)of suspended particles in FBP suspensions(means±SD,n=3).
The re-dispersion time of all the suspensions was shown in Fig.3.The longest time needed for redispersion was the suspension containing 3.0 mM SDS,which just happened at CAC as showed in Fig.6.The redispersion time of FBP suspensions with SDS was longer than that without SDS except for the suspensions with the concentration of higher than 8.0 mM of SDS.This might be because a complete adsorbed film around the suspending solid formed with enough SDS.And when the SDS was insufficient to form complete adsorbed monolayer,it would bridge the particles resulting in a longer re-dispersion time[30].It should be noted that,when the concentration of SDS was greater than 5.0 mM the time decreased little by little,which should be ascribed to the formation of f l occulation[31–33].
3.3.3. The zeta potential and particle size
The results of zeta potential were shown in Fig.4.The zeta potential of suspension suspended by HPMC alone was near zero,which resulted from the adsorption of neutral polymer on the surface of electronegative parties[34],and it was not sufficient to induce electrostatic stabilization[35].Magically,with SDS added into the system the absolute value of the zeta potential increased deeply,which likely contributed to the stability through electrostatic repulsion[9].And when the concentrations of SDS above 8.0 mM,the zeta potential suggested that the suspensions were considered as a physical stable system[35,36].The intensity weighted mean diameter of the dispersed particle was shown in Fig.5.The particle size first increased and then decreased with the addition of SDS implying there was a process from f l occulation to def l occulation in the suspensions with the addition of SDS.Similar results was found by the photomicrograph(Fig.2)as well.It was obvious that,the polydispersity index(PDI)became smaller when SDS existed,meaning that the suspensions were more uniform[37,38].
3.4. The interaction between HPMC and SDS
3.4.1. Conductivity
The conductivity of a series of SDS solutions with or without 0.2%HPMC was shown in Fig.6.In the pure surfactant solutions,the electrical conductance shows two different linear regimes as a function of surfactant concentration,corresponding to the concentration below and above the critical micelle concentration(CMC).From these data,the CMC has been calculated yielding 4.8 mM by the intersection of the data regression lines method[39],which was incomplete agreement with the values reported in literature[40].This might be ascribed to the existence of more impurity.In the presence of HPMC another two transition points were observed like other ionic surfactantnonionic polymer systems[41,42].They were CAC and PSP,in this article their values were 2.9 mM and 6.2 mM respectively.In the ionic surfactant-nonionic polymer solutions before the concentration of surfactant reached the CAC,there have scarcely any interactions between the polymer and surfactant.It was obvious that the CAC is lower than the CMC,which because of the formation of surfactant-polymer complex is easier than the formation of micelles[43,44].It is worth noting that there was an obvious decrease in the slope after reaching CAC owing to the reduction of free ions of the surfactant in solutions,either by adsorption on or by forming cluster with the polymer[45].When the concentration of SDS exceeded the PSP,the slope with or without HPMC were very similar,suggesting that micellization took place[46].
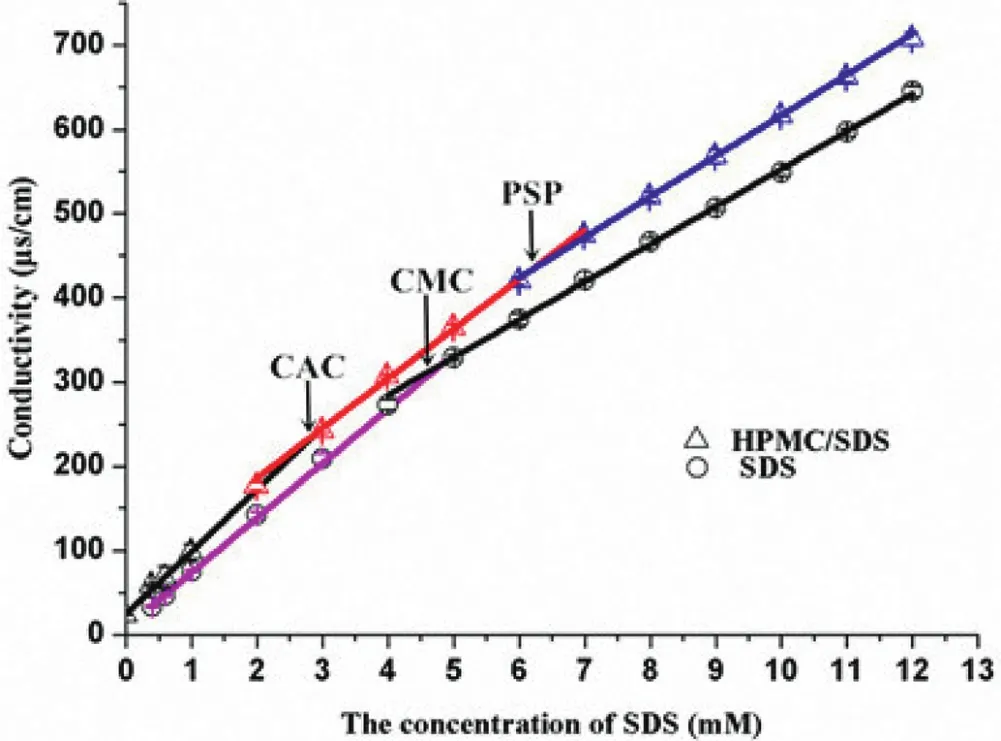
Fig.6–The conductivity of SDS solutions with and without 0.2%HPMC(means±SD,n=3).
3.4.2. Viscosity measurement
As shown in Fig.7,the specific viscosity increased at lower SDS concentrations and then decreased gradually at higher SDS concentrations,which clearly demonstrated that SDS had a significant role on the viscosity of HPMC/SDS solution.At the concentration of SDS around CAC the specific viscosity increased more obviously and reached maximum at the SDS concentration of 5.0 mM,which was very similar to the concentration of CMC[47].After that the specific viscosity reduced gradually.On the one hand,with relatively few SDS in the system,one bonded micelle was shared by more than one polymer molecule to form a three dimensional network resulting in the increase of viscosity.The viscosity decreased at higher surfactant concentrations as a consequence of the solubilization of hydrophobic sites of neighboring polymer chains and the gradually loss of polymer-networking[45,48].On the other hand,the micelles bound to the polymer chain at the beginning caused the HPMC molecular size increased due to the electrostatic repulsion.The specific viscosity reached its maximum value when HPMC molecule expanded as large as possible.Then the appearance of electrostatic screening would happen by adding more surfactants,which resulted in the weakness of expansion and the reduction of viscosity[47,49].
It is noteworthy that,the change of viscosity in HPMC/SDS dispersions and the drug content variation in suspensions were very similar before the concentration of SDS was below CMC.According to Stokes equation,there was an inverse proportion between the rate of sedimentation and the viscosity of the medium[50].Hence it was concluded that when the concentration of SDS was below CMC,the viscosity of the medium contributed to the dispersion of suspensions more uniformly and durably.However after that point the content did not decline with the viscosity as expected,which was probably affected by other factors.After many trials the longest time needed for redispersion was the suspension prepared by HPMC combined with 3.0 mM SDS while the peak of viscosity appeared in the mixture of HPMC with 5.0 mM SDS.It indicated that the variation of re-dispersion time of suspensions was not in accordance with the changing of viscosity absolutely,which mainly depended on the state of precipitate when the concentration of suspending agent was constant[31].
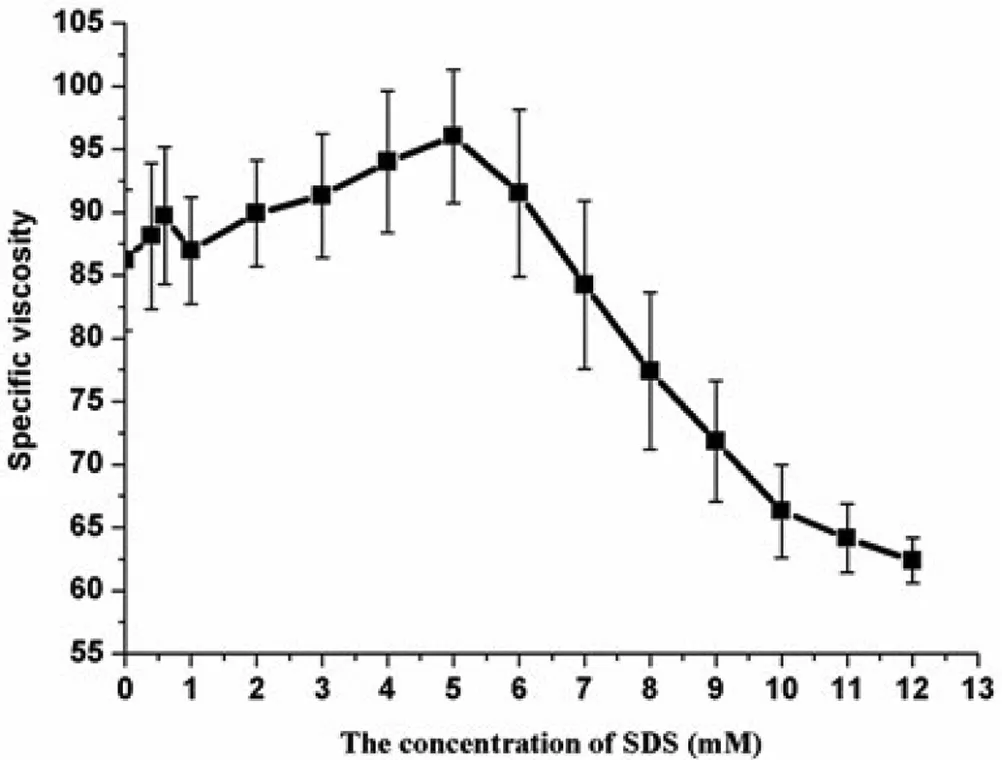
Fig.7–The viscosity of 0.2%HPMC solution with and without a series concentration of SDS(means±SD,n=3).
3.4.3. The solubilization study
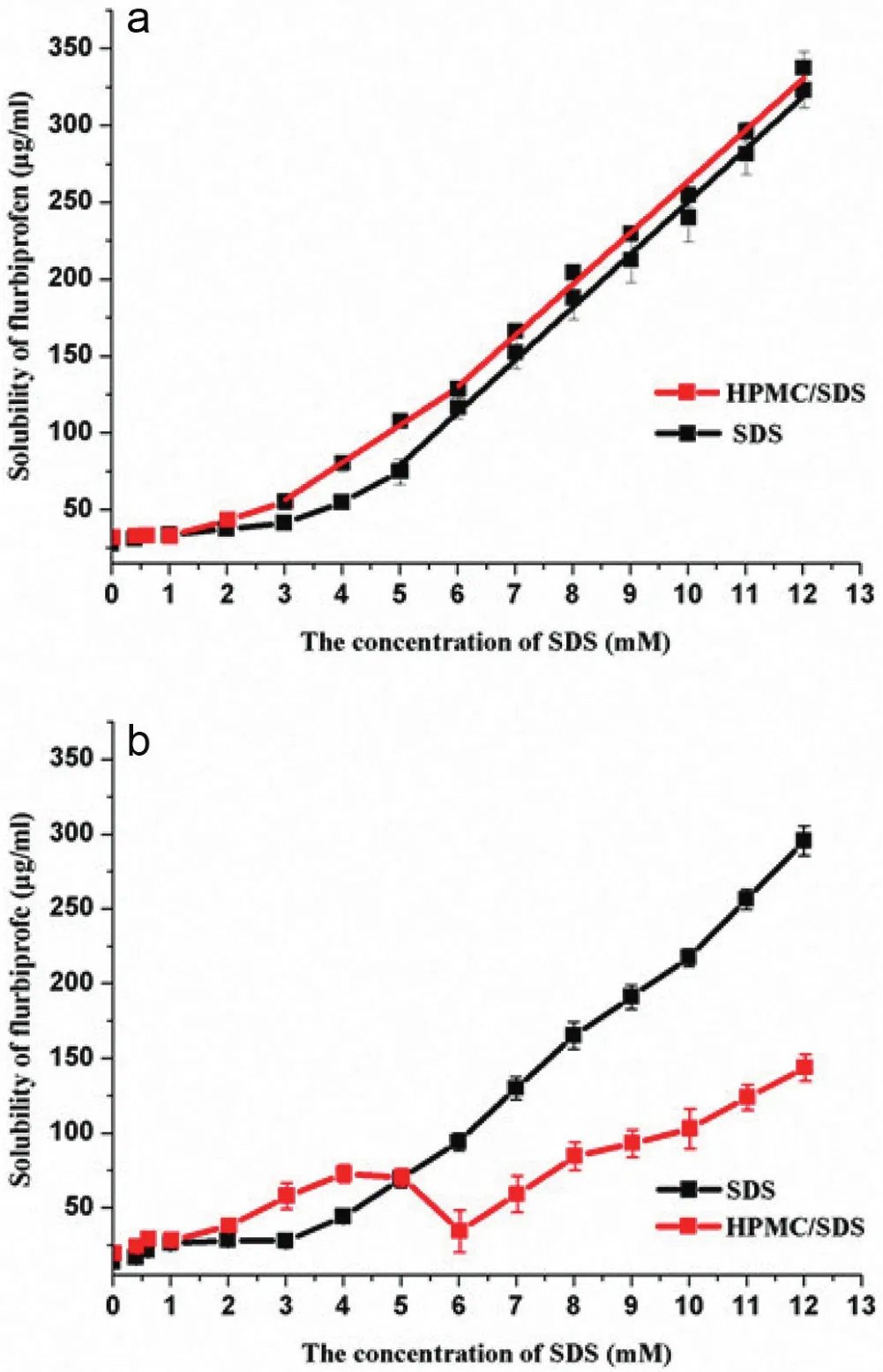
Fig.8–a.The solubilization of a series concentration of SDS solutions with and without 0.2%HPMC for 3 days(means±SD,n=3).b.The short time solubilization of a series concentration of SDS solutions with and without 0.2%HPMC(means±SD,n=3).
Fig.8a and Fig.8b showed the 3-days solubilization and the short time solubilization of FBP in SDS and HPMC/SDS solutions respectively.In pure SDS solutions,solubilization began once the CMC was exceeded.In contrast,as for HPMC/SDS mixture,solubilization began appearing at concentration about CAC well below the CMC and that was in good agreement with previously published study[51].It was interesting to find that the variation tendency of the drug content in suspensions was very similar to that of the short time solubilization in HPMC/SDS system except the samples containing SDS from 10.0 mM to 12.0 mM.And it was predicted that the same consequence would be observed between the drug content for 3 days and the 3-day solubilization.It suggested that the solubility of FBP played an essential role in the drug content in suspensions.Although there was a peak value of viscosity in HPMC/SDS system containing 5.0 mM SDS,the drug content still declined because of the smaller solubility than the former dispersion.Both the viscosity and solubility reduced around PSP when the micelles were occupied by hydrophobic sites of HPMC molecules,resulting in the appearance of a valley of drug content.The viscosity played a dominant role compared to the solubilization in the drug content when the concentration of SDS above 10.0 mM,which caused the content in suspensions did not increase with the addition of more SDS.
Although the dissolution of FBP was improved,there was no significant distinction between the solubility of FBP in SDS solutions and that in HPMC/SDS solutions before CAC,which attributed to the absence of synergistic effect between HPMC and SDS in solubilization.It further highlighted the fact that HPMC and SDS have no interaction before CAC and some scholars even defined CAC as the concentration when solubilization started[52].And surprisingly,after PSP similar slopes of solubility curves in Fig.8a(34.28 and 33.40)were found,which confirmed that the HPMC molecules were saturated with SDS and micellization occurred.
3.4.4. Wettability
In view of the fact that in the absence of enough SDS,there was f l oater in the suspensions.It is necessary to discuss the inf l uence of SDS and HPMC/SDS solutions on the wettability of FBP,which can be ref l ected on the value of contact angle.And the consequence was showed in Fig.9.In pure SDS solutions the contact angle of compact declined with the increase of SDS.In the binary solutions,when the concentration of SDS below CMC,the contact angles were obviously smaller than the former,which suggested that there existed synergistic effect between HPMC and SDS.The conclusions were in agreement with the previous studies[9,53].While the concentration of SDS between CMC and PSP the contact angle almost unchanged in the mixture system.And the result displayed that the solution of 0.2%HPMC can reduce the contact angle as well,which confirmed the fact that HPMC is an amphipathic polymer with surface activity[45,46].
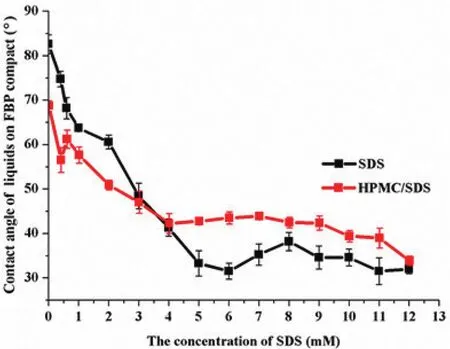
Fig.9–The contact angle of a series concentration of SDS solutions with and without 0.2%HPMC on the FBP compacts(means±SD,n=3).
4. Conclusion
In this study the physical stability of FBP suspensions has been associated with the interaction of HPMC/SDS.As the concentration of SDS below CAC,the interaction of HPMC/SDS was not significant.When the concentration of SDS was CMC,the specific viscosity was the maximum among the HPMC/SDS systems and f l occulation began to take effect in the suspensions.The dispersion of FBP concentration in suspensions mainly depended on the viscosity or the solubilization ability of the dispersions when the concentration of SDS was lower or higher than CMC respectively.When the concentration of SDS was between CAC and PSP,the concentration of drug in suspensions was more uniform.However,after the PSP,the homogeneity of the suspension became worse and worse until the SDS was enough to form a def l occulation state.And interestingly,the re-dispersion time of suspensions were shorter by shorter when the concentration of SDS above CMC,and the longest re-dispersion time of suspensions appeared at the concentration of SDS around CAC.It is concluded that,it is possible to control the properties of pharmaceutical preparations by adjusting the interaction between pharmaceutic adjuvants.
Conf l icts of interest
The authors report no conf l icts of interest.The authors alone are responsible for the content and writing of this article.
This work was financially supported by National Natural Science Foundation of China(No.81473161 and No.81273445).
[1]Goddard ED.Polymer/surfactant interaction:interfacial aspects.J Colloid Interface Sci 2002;256:228–235.
[2]Werawatganone P,Wurster DE.Determination of the hydrolysis kinetics of alpha-naphthyl acetate in micellar systems and the effect of HPMC(catalyst present).J Pharm Sci 2007;96:448–458.
[3]Barreiro-Iglesias R.Poly(acrylic acid)microgels(carbopol?934)/surfactant interactions in aqueous media Part II:ionic surfactants.Int J Pharm 2003;258:179–191.
[4]Masrat R,Maswal M,Chat OA,et al.A rheological investigation of sol–gel transition of hydroxypropyl cellulose with nonionic surfactant sorbitan monopalmitate:modulation of gel strength by UV irradiation.Colloids Surf A Physicochem Eng Asp 2016;489:113–121.
[5]Rodr?guez R,Alvarez-Lorenzo C,Concheiro A.Inf l uence of cationic cellulose structure on its interactions with sodium dodecylsulfate:implications on the properties of the aqueous dispersions and hydrogels.Eur J Pharm Biopharm 2003;56:133–142.
[6]Miguel MG.Association of surfactants and polymers studied by luminescence techniques.Adv Colloid Interface Sci 2001;89–90:1–23.
[7]Grza?dka E,Wis′niewska M,Gun’ko VM,et al.Adsorption,electrokinetic and stabilizing properties of the guar gum/surfactant/alumina system.J Surfactants Deterg 2015;18:445–453.
[8]Grza?dka E.Inf l uence of surfactants on the adsorption and elektrokinetic properties of the system:guar gum/manganese dioxide.Cellulose 2013;20:1313–1328.
[9]Nakach M,Authelin JR,Tadros T,et al.Engineering of nanocrystalline drug suspensions:employing a physicochemistry based stabilizer selection methodology or approach.Int J Pharm 2014;476:277–288.
[10]Knoos P,Onder S,Pedersen L,et al.Surfactants modify the release from tablets made of hydrophobically modified poly(acrylic acid).Results Pharma Sci 2013;3:7–14.
[11]Barreiro-Iglesias R,Alvarez-Lorenzo C,Concheiro A.Incorporation of small quantities of surfactants as a way to improve the rheological and diffusional behavior of carbopol gels.J Control Release 2001;77:59–75.
[12]Wei H.Crystallization habit of calcium carbonate in presence of sodium dodecyl sulfate and/or polypyrrolidone.J Cryst Growth 2004;260:545–550.
[13]Zhu W,Romanski FS,Dalvi SV,et al.Atomistic simulations of aqueous griseofulvin crystals in the presence of individual and multiple additives.Chem Eng Sci 2012;73:218–230.
[14]Bhakay A,Azad M,Vizzotti E,et al.Enhanced recovery and dissolution of griseofulvin nanoparticles from surfactantfree nanocomposite microparticles incorporating wet-milled swellable dispersants.Drug Dev Ind Pharm 2014;40:1509–1522.
[15]Ramirez JC,Herrera-Ordonez J.Kinetic aspects of styrene minisuspension polymerization using a mixture PVA–SDS as stabilizer:effect of the time of addition of SDS.Eur Polym J 2007;43:3819–3825.
[16]Xiao C,Gao L,Lu M,et al.Effect of polyvinylpyrrolidone on rheology of aqueous SiC suspensions with polyethylene glycol as binder.Colloids Surf A Physicochem Eng Asp 2010;368:53–57.
[17]Terayama H,Funakoshi M,Torigoe K,et al.Kinetics of formation of drug particles in the presence of surfactant/polymer.Colloids Surf B Biointerfaces 2002;23:65–71.
[18]Otsuka N,Ueda K,Ohyagi N,et al.An insight into different stabilization mechanisms of phenytoin derivatives supersaturation by HPMC and PVP.J Pharm Sci 2015;104:2574–2582.
[19]Kamalgharibi M,Hormozi F,Zamzamian SAH,et al.Experimental studies on the stability of CuO nanoparticles dispersed in different base f l uids:inf l uence of stirring,sonication and surface active agents.Heat Mass Transf 2015;52:55–62.
[20]Wanawongthai C,Pongpeerapat A,Higashi K,et al.Nanoparticle formation from probucol/PVP/sodium alkyl sulfate co-ground mixture.Int J Pharm 2009;376:169–175.
[21]Meng X,Chen Y,Chowdhury SR,et al.Stabilizing dispersions of hydrophobic drug molecules using cellulose ethers during anti-solvent synthesis of micro-particulates.Colloids Surf B Biointerfaces 2009;70:7.
[22]Cerdeira AM,Mazzotti M,Gander B.Formulation and drying of miconazole and itraconazole nanosuspensions.Int J Pharm 2013;443:209.
[23]Knieke C,Azad MA,Davé RN,et al.A study of the physical stability of wet media-milled fenofibrate suspensions using dynamic equilibrium curves.Chem Eng Res Des 2013;91:1245–1258.
[24]Verma S,Gokhale R,Burgess DJ.A comparative study of topdown and bottom-up approaches for the preparation of micro/nanosuspensions.Int J Pharm 2009;380:216–222.
[25]Mac Donald R,Camargo SS,Meyre-Silva C,et al.Development of an oral suspension containing dry extract of Aleurites moluccanus with anti-inf l ammatory activity.Rev Bras Farmacognosia 2016;26:68–76.
[26]Kamenka N,Burgaud I,Zana R,et al.Electrical conductivity,self-diffusion,and f l uorescence probe investigations of the interaction between sodium dodecyl sulfate and Ethyl(hydroxyethyl)cellulose.J Phys Chem 1994;98:274–282.
[27]Sovilj V.Conductometric and potentiometric investigations of ionic surfactant–gelatin interaction.Colloid Polym Sci 1998;276:328–334.
[28]Babu K,Pal N,Bera A,et al.Studies on interfacial tension and contact angle of synthesized surfactant and polymeric from castor oil for enhanced oil recovery.Appl Surf Sci 2015;353:1126–1136.
[29]Yang B,Xu L,Wang Q,et al.Modulation of the wettability of excipients by surfactant and its impacts on the disintegration and release of tablets.Drug Dev Ind Pharm 2016;42:1945–1955.
[30]Petzold G,Mende M,Lunkwitz K,et al.Higher efficiency in the f l occulation of clay suspensions by using combinations of oppositely charged polyelectrolytes.Colloids Surf A Physicochem Eng Asp 2003;218:47–57.
[31]Zatz JL,Schnitzer L,Sarpotdar P.Flocculation of sulfamerazine suspensions by a cationic polymer.J Pharm Sci 1979;68:1491.
[32]Tempio JS,Zatz JL.Flocculation effect of xanthan gum in pharmaceutical suspensions.J Pharm Sci 1980;69:1209–1214.[33]Zatz JL,Sarpotdar P,Gergich G,et al.Effect of surfactants on the f l occulation of magnesium carbonate suspensions by xanthan gum.Int J Pharm 1981;9:315–319.
[34]Duro R,Alvarez C,Martínezpacheco R,et al.The adsorption of cellulose ethers in aqueous suspensions of pyrantel pamoate:effects on zeta potential and stability.Eur J Pharm Biopharm 1998;45:181–188.
[35]Jacobs C,Müller RH.Production and characterization of a budesonide nanosuspension for pulmonary administration.Pharm Res 2002;19:189–194.
[36]Vega E,Egea MA,Valls O,et al.Flurbiprofen loaded biodegradable nanoparticles for ophtalmic administration.J Pharm Sci 2006;95:2393–2405.
[37]Ali HS,York P,Blagden N.Preparation of hydrocortisone nanosuspension through a bottom-up nanoprecipitation technique using microf l uidic reactors.Int J Pharm 2009;375:107–113.
[38]Patravale VB,Date AA,Kulkarni RM.Nanosuspensions:a promising drug delivery strategy.J Pharm Pharmacol 2004;56:827–840.
[39]Ribeiro ACF,Lobo VMM,Valente AJM,et al.Transport properties of alkyltrimethylammonium bromide surfactants in aqueous solutions.Colloid Polym Sci 2004;283:277–283.
[40]Marcolongo JP,Mirenda M.Thermodynamics of Sodium Dodecyl Sulfate(SDS)micellization:an undergraduate laboratory experiment.J Chem Educ 2011;88:629–633.
[41]Minatti E,Zanette D.Salt effects on the interaction of poly(ethylene oxide)and sodium dodecyl sulfate measured by conductivity.Colloids Surf A Physicochem Eng Asp 1996;113:237–246.
[42]Zanette D,Soldi V,Romani AP,et al.The role of the carboxylate head group in the interaction of sodium dodecanoate with poly(ethylene oxide)investigated by electrical conductivity,viscosity,and aggregation number measurements.J Colloid Interface Sci 2002;246:387–392.
[43]Avranas A,Iliou P.Interaction between hydroxypropylmethylcellulose and the anionic surfactants hexane-,octane-,and decanesulfonic acid sodium salts,as studied by dynamic surface tension measurements.J Colloid Interface Sci 2003;258:102–109.
[44]Li Y,Dubin PL.Polymer–Surfactant Complexes;1994.
[45]Sovilj VJ,Petrovic′LB.Inf l uence of hydroxypropylmethyl cellulose–sodium dodecylsulfate interaction on the solution conductivity and viscosity and emulsion stability.Carbohydr Polym 2006;64:41–49.
[46]Silva SMC,Antunes FE,Sousa JJS,et al.New insights on the interaction between hydroxypropylmethyl cellulose and sodium dodecyl sulfate.Carbohydr Polym 2011;86:35–44.
[47]Ahn S,Ahn SW,Song S-C.Thermosensitive amphiphilic polyphosphazenes and their interaction with ionic surfactants.Colloids Surf A Physicochem Eng Asp 2008;330:184–192.
[48]Scherlund M,Brodin A,Malmsten M.Nonionic cellulose ethers as potential drug delivery systems for periodontal anesthesia.J Colloid Interface Sci 2000;229:365–374.
[49]Hormnirun P,Sirivat A,Jamieson AM.Complex formation between hydroxypropylcellulose and hexadecyltrimethylamonium bromide as studied by light scattering and viscometry.Polymer(Guildf)2000;41:2127–2132.
[50]Meng X,Chen Y,Chowdhury SR,et al.Stabilizing dispersions of hydrophobic drug molecules using cellulose ethers during anti-solvent synthesis of micro-particulates.Colloids Surf B Biointerfaces 2009;70:7–14.
[51]Kilau HW,Voltz JI.Synergistic wetting of coal by aqueous solutions of anionic surfactant and polyethylene oxide polymer.Colloids Surf 1991;57:17–39.
[52]Ridell A,Evertsson H,Nilsson S.Inf l uence of counterion on the interaction of dodecyl sulfates and cellulose ethers.J Colloid Interface Sci 2002;247:381–388.
[53]Persson B,Nilsson S,Sundel?f LO.On the characterization principles of some technically important water-soluble nonionic cellulose derivatives.Part II:surface tension and interaction with a surfactant.Carbohydr Polym 1996;29:119–127.
 Asian Journal of Pharmacentical Sciences2018年1期
Asian Journal of Pharmacentical Sciences2018年1期
- Asian Journal of Pharmacentical Sciences的其它文章
- GUIDE FOR AUTHORS
- Original Research Paper Pulmonary delivery of liposomal dry powder inhaler formulation for effective treatment of idiopathic pulmonary fibrosis
- Original Research Paper Preparation of poly(lactide-co-glycolide)microspheres and evaluation of pharmacokinetics and tissue distribution of BDMC-PLGA-MS in rats
- Original Research Paper Intranasal administration of carbamazepineloaded carboxymethyl chitosan nanoparticles for drug delivery to the brain
- Original Research Paper Validation of kinetic modeling of progesterone release from polymeric membranes
- Original Research Paper The accelerated blood clearance phenomenon of PEGylated nanoemulsion upon cross administration with nanoemulsions modified with polyglycerin
